Novel NiMo Catalysts Supported on Sol-Gel Nanosized HY Zeolite-Alumina Composites for Hydrodesulfurization of Diesel
2019-05-10YinHailiangLiuXinliangZhouTongnaLiuYunqi
Yin Hailiang; Liu Xinliang; Zhou Tongna; Liu Yunqi
(1. Academy of Science & Technology, China University of Petroleum (Huadong), Dongying 257061;
2. Environmental Protection Agency of Dongying, Dongying 257300;
3. State Key Laboratory of Heavy Oil Processing, China University of Petroleum (Huadong), Qingdao 266555)
Abstract: Composite materials (AZ-x) were prepared by mixing the nanosized HY zeolite and γ-Al2O3 using the sol-gel mixing method and were employed as the support for NiMo catalysts (NiMo/AZ-x). These composites and catalysts were characterized by XRD, BET, TPD, TPR, HRTEM and FT-IR spectroscopy. Compared with the NiMo catalyst supported on γ-Al2O3 (NiMo/γ-Al2O3), these NiMo/AZ-x catalysts show higher activity in the hydrodesulfurization (HDS) of FCC diesel,and the HDS activity increases with an increasing HY mass content in the composites, provided that the HY mass fraction does not exceed 20%. Compared with γ-Al2O3, the introduction of nanosized HY zeolite can improve the porous structure of the composites, which can benefit the mass transfer of the reactant molecules. Furthermore, the nanosized HY zeolite in the composites can modify their surface properties which would increase the ratio of metal species to be transformed into reducible phase in low temperature region and modify the morphology of the MoS2 phases to form more multi-stacked MoS2 active phases with increased dispersion of edges and corners of Mo atoms.
Key words: NiMo; hydrodesulfurization; sol-gel
1 Introduction
Nowadays, the removal of sulfur from diesel has gained much attention due to the declining diesel quality and increasingly stringent environmental regulations[1-2].To obtain the ultra-low sulfur diesel, it is necessary to eliminate highly refractory organic sulfides such as 4-methyldibenzothiophene (4-MDBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT) from the fuel[3-4]. The steric hindrance of alkyl groups in 4-MDBT and 4,6-DMDBT can be relieved via hydrogenation of phenyl ring or isomerization of methyl groups. An effective method to achieve this goal is the introduction of acidic microporous zeolites, such as HY[5-6], Beta[7]and ZSM-5[8], or mesoporous molecular sieves[9], into the γ-Al2O3support, and these catalysts supported on the resulting composites can exhibit high hydrogenation or isomerization ability for 4,6-DMDBT. However, these resulting composites were prepared unexceptionally by mechanically mixing the zeolite and γ-Al2O3, which would lead to the uneven dispersion of the two carrier components and this phenomenon is more serious when the nanosized zeolite is used, because the nanosized zeolite is mixed with γ-Al2O3after drying and calcination which would lead to serious agglomeration of the nanosized particles. The adoption of sol-gel mixing method makes the mixing process capable of avoiding the drying and calcination of nanosized zeolite to optimize the dispersion of the two carrier components.Furthermore, recent studies have shown that the sol-gel mixing method can result in modifying the morphology of MoS2phases and enhance the HDS activity of the metal sulfide catalysts[10]. Therefore, the design of composites for preparing the highly active metal sulfide catalysts should make use of the characteristics of sol-gel mixing process of nanosized zeolite and γ-Al2O3.
In this work, a series of composite materials (AZ-x)containing the nanosized HY zeolite and γ-Al2O3were prepared by the sol-gel mixing method, and were used as supports for NiMo catalysts (NiMo/AZ-x) to investigate its catalytic performance in the HDS of FCC diesel.And these composite materials and NiMo catalysts were characterized by XRD, BET, TPD, TPR, HRTEM, and FT-IR techniques. The activity test results show that the HDS activity of the NiMo/AZ-x catalysts is higher than that of the γ-Al2O3supported NiMo catalyst (NiMo/γ-Al2O3), and the activity increases with an increasing nanosized HY content in the AZ-x composites and declines when the zeolite content exceeds 20%. The improvement in the HDS activity of NiMo/AZ-x catalysts could be attributed to the fact that the introduction of nanosized HY zeolite can result in many advantages, such as better textural property, more acid amount, bigger ratio of metal species transformed into reducible phase in the low-temperature region, and higher dispersion of edges and corners in the Mo atoms.
2 Experimental
2.1 Preparation of nanosized HY zeolite-γ-Al2O3 composites
3.0 g of pseudo-boehmite powder were poured into 40 ml of water in a flask equipped with a reflux condenser and heated to the water boiling temperature for 1 hour with the steam condensed in the reflux condenser flowing back into the flask, in which a certain amount of nitric acid was added to meet the demand for comply with a H/Al molar ratio of 0.2.
The mixture was stirred for 3 hours at 373 K, and then was subject to ageing for 12 hours at room temperature,leading to the formation of AlOOH sol. The nanosized NaY zeolite was obtained by hydrothermal preparation method described elsewhere[5]. A zeolite emulsion was obtained by dispersing the prepared zeolite in water without drying and calcination treatment followed by sonication. The AlOOH sol was stirred in a beaker and a varying amount of nanosized NaY zeolite emulsion was added. The room temperature sol-gel technique using ammonia as the catalyst, which was then subject to drying at 373 K for 24 hours in air atmosphere, was applied to prepare the AlOOH-zeolite mixed oxides.
Nanosized HY zeolite-γ-Al2O3was prepared by ionexchange method, followed by calcination of the catalyst.The mixture of AlOOH-zeolite mixed oxides, NH4NO3,and water at a mass ratio of 1:1.5:20 was stirred at 363 K for 1 hour, and was then dried at 373 K for 12 hours. The above procedure was repeated 3 times. After the last step, the mixture was calcined in air at 823 K for 4 hours. The supports were prepared by mixing the above zeolite-γ-Al2O3and sesbania powder, followed by extruding to cylindrical form, drying overnight at 393 K,and calcination in air at 823 K for 4 hours. Composites containing the nanosized HY zeolite and γ-Al2O3with a definite ratio are denoted as AZ-x, where x is 1, 2, 3 and 4,corresponding to a HY mass fraction in the composites of 5%, 10%, 20%, and 30 %, respectively.
2.2 Preparation of catalysts
Various NiMo/AZ-x catalysts were prepared by coimpregnation of the above supports using the incipient wetness method with an aqueous solution containing an appropriate amount of Ni2(OH)2CO3, MoO3, and H3PO4.After the impregnation step, these catalysts were subject to ageing for 12 hours at room temperature, and were then dried at 393K for 12 hours. These dried NiMo catalysts were calcined at 773K for 4 hours. These catalysts had a nominal MoO3+NiO mass content of 24 %, with a Ni/(Mo+Ni) molar ratio of 0.26 and a P/MoO3mass ration of 0.063, separately.
2.3 Catalysts characterization
The XRD characterization was recorded on a Rigaku D/max-IIA diffractometer using a graphite-filter with Cu Kα radiation. The BET surface area, pore volume,and pore size for the catalysts were measured with a Micromeritics ASAP 2020 automated gas adsorption system using N2adsorption-desorption isotherms.The temperature-programmed reduction (TPR) with hydrogen and the temperature-programmed desorption of ammonia (TPD-NH3) experiments were carried out with a Micromeritics TPR/TPD 3000 apparatus equipped with a thermal conductivity detector (TCD) interfaced to a data station. The high resolution transmission electron microscopy (HRTEM) was carried out by a JEM 2100 microscope operating at a 200 kV of accelerating voltage and was fitted with an INCA X-SIGHT ED detector(Oxford Instruments). In order to obtain statistically reliable information, the slab length of ca. 300 particles was measured for each fresh sulfide catalyst. Moreover,the particle size distribution was evaluated by counting in micrographs taken for the same sample. The FT-IR spectra of pyridine adsorption were collected on a Nexus spectrometer (Nicolet, USA).
2.4 Catalytic activity measurement
The catalytic activity of sulfide catalysts was evaluated through HDS of diesel. The reaction took place in a highpressure fixed-bed microreactor. Prior to the catalytic activity experiments, 10 mL of NiMo oxide catalyst were presulfurized for 6 hours with 3% of CS2-toluene mixture under the conditions covering a temperature of 593 K,a total pressure of 4 MPa, a H2/toluene volume ratio of 300, and a LHSV of 2.0 h-1. The activity test was carried out at the steady state after 16 hours on-stream under the conditions covering a temperature of 623 K, a total pressure of 6 MPa, a H2/toluene volume ratio of 500, and a LHSV of 1.0 h-1. The diesel feedstock was a kind of FCC diesel containing 7216 μg/g of S. The total sulfur content in the feedstock and products was measured by an Analytikjena’s elemental analyzer (Mutli EA 3100) and the type of sulfur-containing compounds was measured by a Varian 3800 GC.
3 Results and Discussion
3.1 XRD of Composites and Catalysts
The XRD patterns of the synthesized composites are shown in Figure 1(A). The XRD pattern of the reference alumina (curve (a)) corresponds to the crystalline phase of γ-Al2O3(JCPDS card 29-0063). In the diffraction patterns of AZ-x composites, signals corresponding to the γ-Al2O3crystalline structure and the HY zeolite(JCPDS card 38-0239) can be observed. It is shown that,with the increase of HY content, the diffraction peak intensity of HY zeolite increases, while that of γ-Al2O3decreases. Being similar to our previous study[5], the HY diffraction peaks are weak because of the poor thermal stability of nanosized zeolite. The XRD patterns of NiMo oxide/AZ-x catalysts are shown in Figure 1(B).The diffraction signals of the corresponding supports are observed in the XRD patterns of the NiMo oxide catalysts except the signals of HY zeolite. In addition,several signals in the range of from 20oto 40o(2θ) are detected. These signals are attributed to the crystalline phase of MoO3( JCPDS card 76-1003)[11].

Figure 1 XRD patterns of supports, oxided and sulfided NiMo catalysts*—HY zeolite (JCPDS card 38-0239); &—γ-Al2O3 (JCPDS card 29-0063);#—MoS2 (JCPDS card 37-1492); +—MoO3 ( JCPDS card 76-1003)
It can be seen from Figure 1(B) that the MoO3diffraction peak intensity increases suddenly with the introduction of HY zeolite in AZ-x materials, implying that the introduction of nanosized HY zeolite is not favorable to the dispersion of active metal components. But,fortunately, the MoO3diffraction peak intensity decreases with the increase of HY zeolite content. This illustrates that the dispersion of active metal has been improved gradually when the HY zeolite content increases. Figure 1(C) shows that the diffraction peaks of MoS2(JCPDS 37-1492) are observed in these sulfide catalysts. The presence of the (002) peak at 2θ = 14 is representative of the stacking slabs along the c-axis in MoS2crystal. As revealed by the XRD patterns, the (002) peak intensity of the nanosized HY zeolite-containing catalysts is stronger than that of NiMo/γ-Al2O3catalyst. The result suggests that the procedure for preparation of sulfide catalysts using these composites as support is likely to form MoS2with more stacking slabs along the c-axis than NiMo/γ-Al2O3catalyst does. In addition, it can be seen that the (002) peak intensity of MoS2crystal in NiMo/AZ-x catalysts decreases when the HY zeolite content increases.This phenomenon is similar to the change of MoO3diffraction peak intensity in NiMo oxide/AZ-x catalysts,showing that the increase of HY content is in favor of the further dispersion of active metal.
3.2 Textural properties of catalysts
Textural characteristics of NiMo/AZ-x catalysts are shown in Table 1. The NiMo/γ-Al2O3catalyst has the lowest specific textural characteristics (surface area and pore diameter), whereas the NiMo/AZ-4 catalyst has the highest ones (surface area, total pore volume,and pore diameter). The total pore volume and pore diameter change irregularly, which can be attributed to the sol-gel method used for the preparation of AZ-x supports. However, a progressive increase can be noted in the specific surface area of the hybrid materials with the increase in proportion of HY zeolite in the materials. Figure 2 shows nitrogen adsorptiondesorption isotherms of the NiMo catalysts. According to the IUPAC classification, all the NiMo catalysts have a characteristic nitrogen adsorption-desorption isotherm of type IV with H2hysteresis loop. This hysteresis is attributed to different size of pore mouth and pore body (ink-bottle shaped pores)[12]. Poresize distributions of the NiMo catalysts are shown in Figure 3. It can be seen that, in general, the nanosized HY zeolite-containing catalyst has broader pore size distribution than NiMo/γ-Al2O3catalyst that can be attributed to their combined porosity. Also, a slight increase can be observed in the main pore size of NiMo/AZ-x catalysts in comparison with that of NiMo/γ-Al2O3counterparts. This can occur due to the solgel method employed in the synthesis of the hybrid supports. In this method, the alumina sol probably partially covers the pore mouths of HY and bigger pores are formed due to the accumulation of alumina and HY particles during this mixing procedure of alumina sol and zeolite slurry. This outcome can result in the disappearance of smaller pores in the adsorption pore size distribution shown in Figure 3. Some bigger pores can be observed in this Figure on the right side of the dashed lines corresponding to the pore size of NiMo/γ-Al2O3catalyst. The result is contrary to the literature report[13], and the reason is attributed to the different preparation methods of the support.

Table 1 Textural characteristics of NiMo catalysts

Figure 2 N2 adsorption-desorption isotherms of NiMocatalysts
3.3 TPD of Catalysts
Efficiency of these catalytic systems in HDS reaction should depend on their surface acidity[14], and the acidity of NiMo/AZ-x catalysts was investigated using NH3-TPD and Py-FTIR methods, respectively. Figure 4 shows the NH3-TPD profiles of the catalysts. The NH3-TPD profiles reveal one broader NH3desorption peaks from 423 K to 873 K, indicating the presence of two types of acid sites with different acid strengths.In general, the NH3desorption peak at 510 K reveals the existence of weak acid sites, whereas that at 680 K indicates the existence of medium and strong acid sites[15]. As the HY content increases, the lowertemperature peaks for NiMo/AZ-1 and NiMo/AZ-2 catalysts shift slightly to an much lower temperature,suggesting that the acidity of the weak acid sites is further weakened. When the HY content exceeds 20%,the peaks for NiMo/AZ-3 and NiMo/AZ-4 catalysts shift slightly to a higher temperature, suggesting that the acidity of the weak acid sites is strengthened.Compared with NiMo/γ-Al2O3catalyst, the intensity of the lower-temperature desorption peak of the catalysts(x = 1, 2, 3 and 4) also increases, indicating that the amount of weak acid sites increases. With an increasing HY content, the intensity of the higher-temperature desorption peak increases, denoting an increase in the amount of medium and strong acid sites. It is worthy of noting that the amount of medium and strong acid sites in the NiMo/AZ-3 catalyst exceeds that in the NiMo/AZ-4 catalyst.
To observe the changes in Brønsted acid sites (B acid) and Lewis acid sites (L acid) on the NiMo/AZ-x catalysts, the Py-FTIR spectroscopic analyses were performed. According to literature[16], the characteristic bands at 1 540 cm-1and 1 450 cm-1are assigned to pyridine adsorbed on the B acid sites and L acid sites,respectively. The results summarized in Table 2 and Figure 5 demonstrate that all NiMo catalysts have both L and B acid sites, with a considerable contribution of L acid sites. In addition, the amount of B acids, L acids and B + L acids, and B/L ratio increases with an increasing HY zeolite content. Therefore, the introduction of HY in the NiMo catalysts not only can affect the acid amount,but can also increase the B/L ratio.

Table 2 Amounts of the acid sites of NiMo catalysts
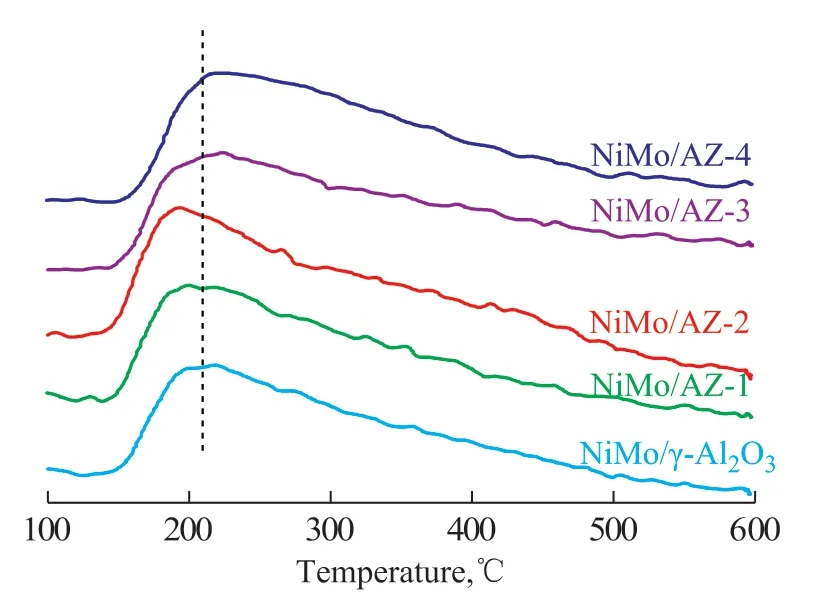
Figure 4 NH3-TPD profiles of NiMo catalysts
3.4 TPR of Catalysts
Results from the TPR characterization of the NiMo catalysts are shown in Figure 6. All the NiMo catalysts presented two reduction peaks in the low temperature region (673-973 K) and high temperature region(1073-1273 K). According to literature reports[17-18],H2consumption (573-773 K) can be attributed to the reduction of Mo6+to Mo4+of octahedral Mo oxide species with different degrees of agglomeration. And the reduction temperature increases with an increasing degree of agglomeration of octahedral Mo oxide species.The H2consumption (773-973 K) can be ascribed to the reduction from Mo4+to Mo0of octahedral Mo oxide species and to the reduction of small MoO3clusters.Finally, the signal observed in the high temperature region (973-1273 K) corresponds to the first step of reduction of tetrahedral Mo oxide species interacting strongly with the support. Therefore, the TPR results show that the catalysts have both types of Mo oxide species, octahedral with high agglomeration degree and tetrahedral, with a considerable contribution of the octahedral Mo oxide species reducible at a low temperature. In addition, the signal observed in the low temperature region (673-973 K) also indicates the existence of small MoO3clusters detected by XRD.Compared with NiMo/γ-Al2O3catalyst, the signals in the low temperature region of NiMo/AZ-x catalysts move to high temperature region indicating that the octahedral Mo oxide species are more difficult to be reduced. On the contrary, the peak areas of H2consumption in the low temperature region for all NiMo/AZ-x catalysts are obviously bigger than NiMo/γ-Al2O3catalyst. This phenomenon illustrates that the introduction of HY makes for the transformation of more metal species into the reducible phase. Among the NiMo/AZ-x catalysts, NiMo/AZ-3 catalyst has the lowest reduction temperature and the biggest reduction peak area in the low temperature region (673-973 K), indicating to its maximum reducibility.
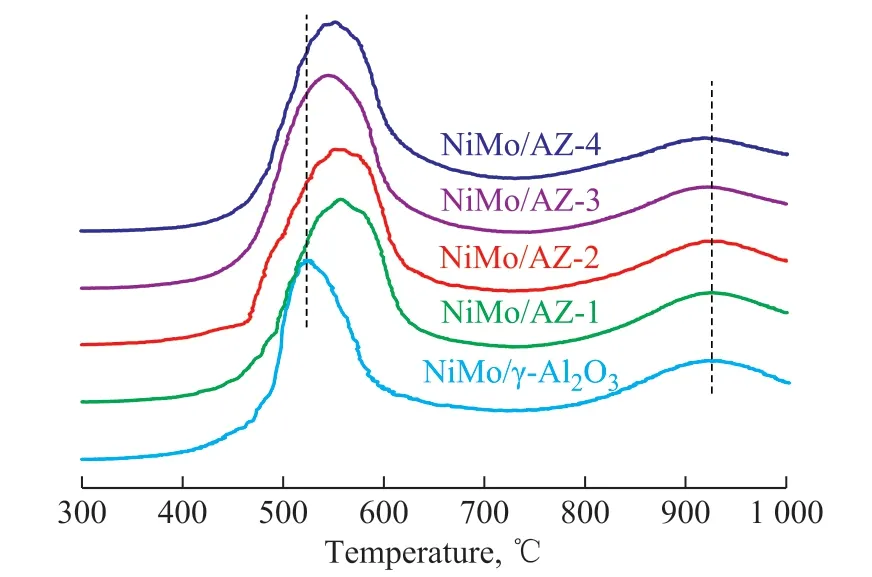
Figure 6 TPR profiles of NiMo catalysts
3.5 HRTEM of Catalysts
To obtain the detailed information about the dispersion of MoS2species, the HRTEM study of NiMo sulfide catalysts was performed. Figure 7 shows the HRTEM micrographs of these catalysts. The typical fringes of MoS2crystallites with a 0.61 nm on interplanar distance are observed in the images of all sulfide catalysts. However,some differences in the morphology of the sulfide MoS2phase are detected depending on the chemical composition of support. NiMo/γ-Al2O3catalyst shows the presence of MoS2particles formed by 1-5 layers with the crystal length ranging from 2 nm to 10 nm. In the case of NiMo/AZ-x catalysts, it is observed that they have longer and more stacked MoS2crystallites. The above HRTEM results were used to calculate the average length (L) and average stacking degree (average number of layers, N) of MoS2crystallites in different catalysts (Table 3), which were calculated by means of the equations described in the previous study[5]. And Figure 8 shows the layer number distribution in the different NiMo catalysts prepared in the present work. For NiMo/γ-Al2O3catalyst,the average slab length is 4.63 nm, and the average layer number is 3.3. Compared with the NiMo/γ-Al2O3catalyst,the NiMo/AZ-x catalysts exhibit a broader distribution in slab length (4.21-4.84 nm) and layer number (3.4-3.8).The introduction of nanosized HY zeolite increases the accumulation degree of MoS2particles, but it decreases with an increasing zeolite content. Similarly, the average slab length decreases with an increasing zeolite content,indicating that the increase of HY content can effectively increase the dispersion of active MoS2component on the surface of the catalyst.
On the basis of these results, the fraction of Mo atoms(fMo) located on the edge surface of MoS2crystallites was estimated for each catalyst. The fMowas calculated using equations (referred to in the literature[13]) assuming that the MoS2crystallites are perfect hexagons[19-20]. The results from Table 3 show that fMovalues for the NiMo catalysts are similar, which increase with an increasing zeolite content.
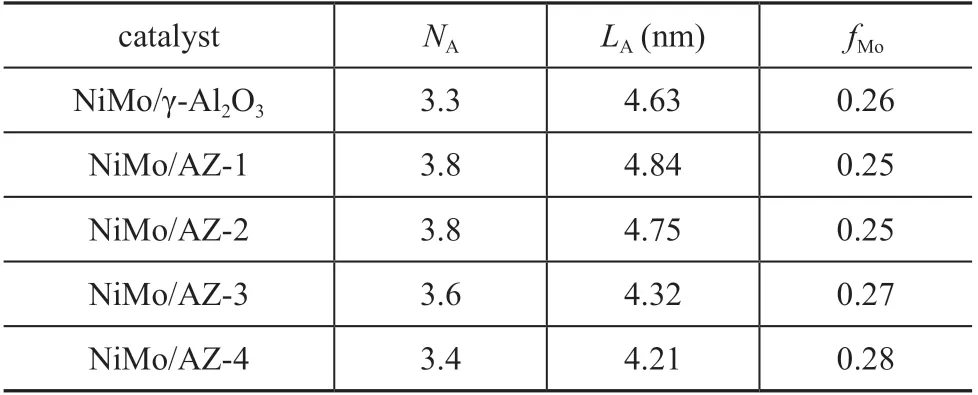
Table 3 Average stacking degree (NA), average slab length (LA) and fraction available Mo (fMo)
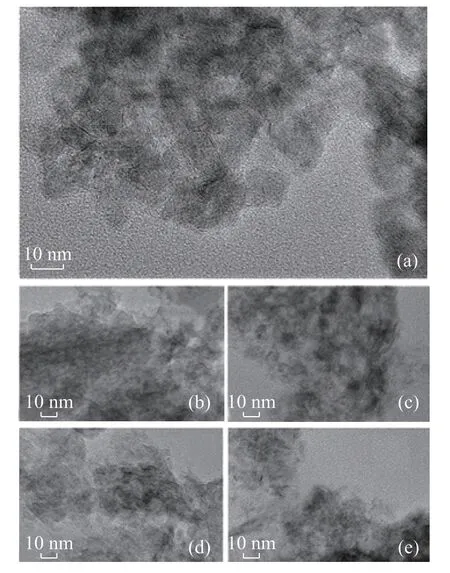
Figure 7 HRTEM images of sulfide catalysts:(a) NiMo/γ-Al2O3, (b) NiMo/AZ-1, (c) NiMo/AZ-2,(d) NiMo/AZ-3, (e) NiMo/AZ-4
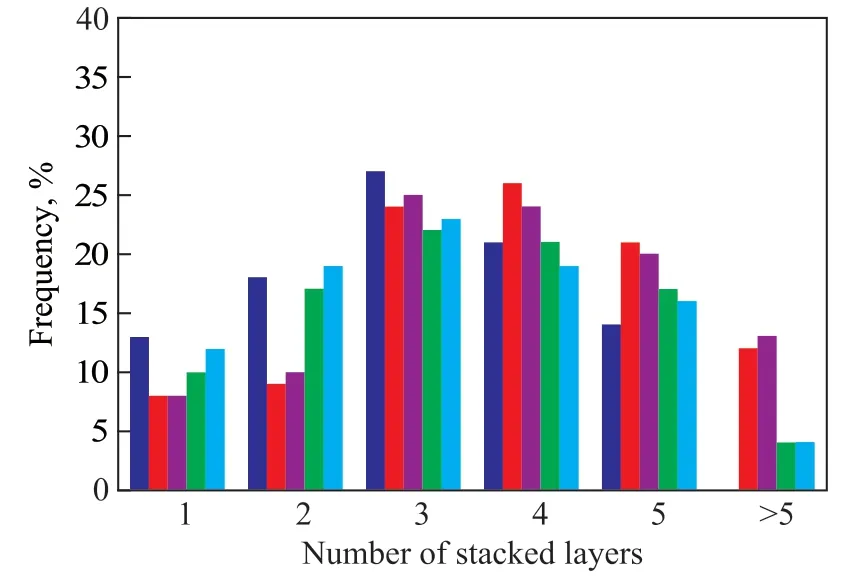
Figure 8 MoS2 stacking number distribution of the used catalysts■—NiMo/γ-Al2O3; ■—NiMo/AZ-1; ■—NiMo/AZ-3;■—NiMo/AZ-3; ■—NiMo/AZ-4
3.6 HDS activity evaluation
In the present work, the catalytic activity of the NiMo catalysts was tested in the HDS of diesel. It can be seen from Table 4 that all NiMo/AZ-x catalysts exhibit a higher HDS conversion than NiMo/γ-Al2O3catalyst, while the NiMo/AZ-3 catalyst demonstrates a highest HDS efficiency. The optimal HY content is 20% in the AZ-x support.
The hydrogenation performance of the catalyst is the main influencing factor for HDS activity. Because the corners and edges of the MoS2slabs are believed to be the active sites of hydrogenation, the more the corners and edges in the MoS2slabs, the higher the hydrogenation activity of the catalysts would be. With an increasing nanosized HY zeolite content, the NiMo/AZ-x catalysts bear shorter slab, which can provide more corners and edges leading to excellent hydrotreating activity, and this fact is also confirmed by the change in fMovalue.
The HDS performance of catalysts is the synergistic result of many factors, such as hydrogenation performance,acid property, textural properties, metal reducibility, etc.The NiMo/AZ-3 and NiMo/AZ-4 catalysts have higher and similar acid content, and their surface area, pore volume and fraction of available Mo are similar, so these two catalysts have high and similar HDS performance.The NiMo/AZ-3 catalyst shows the highest HDS efficiency and this performance may be related to its metal reducibility. This catalyst has the lowest reduction temperature and the biggest reduction peak area in the low temperature region (673-973 K), which can confirm its maximum reducibility.
4 Conclusions
The NiMo catalysts using nanosized HY zeolite-γ-Al2O3composites as the support prepared by the sol-gel method has higher HDS activity compared with the catalyst using γ-Al2O3as the support, and the catalyst containing 20%of HY zeolite shows the highest HDS activity. The solgel method is not conducive to the reducibility of metal phase, but the introduction of nanosized HY zeolite shows many advantages such as better textural property,more acid amount, and more metal species that can be transformed to reducible phase at low temperature and higher dispersion of edges and corners of Mo atoms,which can lead to good HDS performance of catalyst.

Table 4 Hydrotreating activity results of FCC diesel over different catalysts
Acknowledgement:This work was supported by the National Natural Science Foundation of China (Grant Nos. 21206197),the Shandong Provincial Natural Science Foundation under Grant 2016GSF117030, and the Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (BS2013CL021).
杂志排行
中国炼油与石油化工的其它文章
- Influence of Cr3+ Concentration on SO2 Removal over TiO2 Based Multi-walled Carbon Nanotubes
- Catalytic Cracking Characteristics of Plant Oil for Producing Light Olefins and Light Aromatics
- Polycrystalline Phase WO3/g-C3N4 as a High Efficient Catalyst for Removal of DBT in Model Oil
- Phosphorous-Modified Carbon Nanotube-Supported Pt Nanoparticles for Propane Dehydrogenation Reaction
- Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
- Kinetic Model of Hydrogenation for Removal of Trace Olefins from Alkylation Mixture Formed during Linear Alkylbenzene Synthesis
