Polycrystalline Phase WO3/g-C3N4 as a High Efficient Catalyst for Removal of DBT in Model Oil
2019-05-10LiXiupingZhaoRongxiangMaoChunfeng
Li Xiuping; Zhao Rongxiang; Mao Chunfeng
(College of Chemistry, Chemical Engineering and Environmental Engineering,
Liaoning Shihua University, Fushun 113001)
Abstract: The polycrystalline phase WO3/g-C3N4 was synthesized under stirring using tungstenic acid (H2WO4) and graphitic carbon nitride (g-C3N4) as raw materials. The catalyst was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), the Fourier transform infrared spectroscopy (FT-IR), and the Brunauer-Emmett-Teller analysis (BET). The polycrystalline phase WO3/g-C3N4 was determined by XRD technique. The oxidative desulfurization process was investigated using WO3/g-C3N4 as the catalyst, 30% hydrogen peroxide (H2O2) as the oxidant, and 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4) ionic liquids (ILs) as the extractant. The operating conditions, including H2WO4 amount, IL dose, H2O2 volume, temperature, catalyst dosage, and types of sulfur compounds,were systematically researched. The desulfurization rate could reach 98.46% for removing dibenzothiophene (DBT) from the model oil under optimal reaction conditions. In addition, the catalytic activity was slightly decreased after five recycles of catalysts. The reaction kinetics analysis shows that the oxidative desulfurization system was in accord with the first-order reaction kinetics equation. The mechanism of oxidative desulfurization was proposed.
Key words: oxidative desulfurization; WO3/g-C3N4; heterogeneous catalysis; extractant
1 Introduction
Rapid development of the automobile industry results in acid rain and smog[1]. These consequences have damaged human health[2]. Hydrodesulfurization (HDS) of liquid fuels is an effective and mature desulfurization method.However, the reaction conditions of HDS are very harsh,such as high temperature and high pressure[3]. Moreover,aromatic sulfur compounds such as thiophene (TH) and its derivatives (benzothiophene (BT), dibenzothiophene(DBT) and 4,6-dimethyl dibenzothiophene (4,6-DMDBT),etc.) can hardly be removed due to the steric hindrance[4-5]. Some non-HDS method[6-7]such as oxidative desulfurization (ODS), extractive desulfurization (EDS),biodesulfurization (BDS), and adsorptive desulfurization(ADS) have been extensively studied. Among them, the oxidative desulfurization has its unique advantages such as mild reaction condition, high desulfurization rate, and effective removal of thiophene and its derivatives.
In recent years, metal oxides have been widely used in the oxidative desulfurization of fuels[8]. Among these oxides the tungsten oxide is a better catalyst for desulfurization.But, its small specific surface area results in the shortage of active sites and low desulfurization rate. Therefore,it is very important to find a suitable support for the dispersion and immobilization of metal oxides, including the tungsten containing oxide[9]. Zhang, et al. reported the method for preparation of mesoporous W-MCM-41 material, with its catalytic efficiency evaluated for oxidative desulfurization reactions[10]. Li, et al.[11]showed that the desulfurization rate could reach 91.3% by using WO3-SBA-15 as the catalyst under mild reaction conditions. Jia, et al.[12]found that DBT and 4,6-DMDBT could be completely oxidized to their corresponding sulfones using 14% MoO3/γ-Al2O3as the catalyst under mild reaction conditions in 15 min. Li X., et al[13]also confirmed that WO3/SiO2was effective in removing BT and DBT. Moreover, it could be easily separated through using an external magnetic field. However, it still is a challenge in order to obtain the inexpensive raw materials and create a simple preparation process.
Graphitic carbon nitride (g-C3N4) with a layered structure is the most promising support because of its advantages such as high chemical and thermal stability, and unique electrical and functional properties[14-15]. For instance, Jing Ding, et al.[16]reported the green process for manufacture of dialdehydes via the selective oxidation of cycloalkene oxides over the mesoporous g-C3N4supported WO3nanorods catalysts using H2O2as the oxidant. Zhu, et al.[17]showed that the g-C3N4supported phosphotungstic acid (H3PW12O40) could effectively catalyze and remove sulfur compounds in fuel and the catalyst could be easily recovered and reused up to fifteen times with only a slight decrease in activity. These results suggested that carbon nitride could be the desirable support for catalyst.
Ionic liquids (ILs) as solvents have been widely researched instead of the traditional organic solvents due to their low melting point, wide liquid range and low vapor pressure, etc. Now, ionic liquids serving as the extraction agents have been widely used in the oxidative desulfurization system, for example, [Bmim]PF6[18],[Bmim]BF4[19], [Omim]BF4[20], etc. It is obvious that the desulfurization efficiency has been greatly improved by the addition of ionic liquids[7,21-22]thanks to the extractant function of ionic liquid for sulphoxide and sulphone.In this paper, upon using [bmim]BF4as the extractant and H2O2as the oxidant in oxidative desulfurization system,the performance of the catalyst, the polycrystalline phase WO3/g-C3N4, was investigated. DBT was selected as the research target because DBT is a sulfur compound contained in the oil. A high desulfurization rate can be realized during the desulfurization reaction which needs fewer doses of extractant and lower apparent activation energy.
2 Experimental
2.1 Materials
Melamine (C3H6N6, CP grade), tungstenic acid, HCl,ethanol, carbamide, tetraethoxysilane, H2O2(AR, 30%),and n-octane were purchased from the Tianjin Damao Chemical Reagent Factory; DBT (98%), BT (97%), and TH (99.8%) were purchased from the Aladdin Reagent Co., Ltd.; [bmim]BF4was purchased from the Shanghai Chengjie Chemical Co., Ltd. The comprehensive microcoulometric analyzer WK-2D (made by the Jiangsu Jiangfen Electroanalytical Instrument Co., Ltd.) was used to analyze the sulfur content.
2.2 Synthesis of g-C3N4
Pure g-C3N4was prepared by direct calcination of melamine in a muffle furnace. A certain amount of melamine was put in an alumina crucible with a cover. The crucible was heated to 550 C at a temperature increase rate of 5 C/min. The crucible was kept at a constant temperature of 550 C for 3 hours and then the yellow product was obtained after natural cooling to room temperature.
2.3 Preparation of WO3/g-C3N4 hybrid materials
The WO3/g-C3N4catalyst was prepared as follows: A certain amount of H2WO4(0.14 g, 0.30 g, 0.47 g, and 0.67 g, respectively,) was completely dissolved into aqueous H2O2(30%) under stirring. After the solution became transparent, g-C3N4(2.69 g) was added into the mixture solution. The mixture was stirred with the condenser pipe submerged in a water bath at 90 C for 5 hours. Then, the WO3/g-C3N4(at a H2WO4mass ratio of 5%, 10%, 15%, and 20%, respectively,) was obtained by drying in an oven at 90 C for 10 hours.
2.4 Characterization of the prepared catalysts
The X-ray diffraction (XRD) patterns of powder samples were performed on an X-ray diffractometer (model D8 Advance; Bruker, Bremen, Germany) operating at 40 mA, 40 kV, and a 10 ()/min speed with Cu-Kα radiation (λ=0.154 06 nm). The results of scanning electron microscopy (SEM) and energy dispersive spectrometry (EDS) were obtained on a FEI Sirion 200 scanning electron microscope, using a scanning voltage of 5.0 kV for morphology. The surface areas (BET) of the as-synthesized samples were tested on a 3H-2000 nitrogen adsorption specific surface area tester at 77.3 K.(Beishide Instruments Corporation, China). The FT-IR spectra were obtained on a WQF-520 Fourier-transform infrared spectrometer (Beifen Ruili Analytical Instrument Company, China).
2.5 Desulfurization experiments
The model oil (with a sulfur content of 500 μg/L) was prepared by dissolving DBT (1.437 g) in n-octane(500 mL). The oxidative desulfurization process was carried out as follows: WO3/g-C3N4, model oil, 30%H2O2, and [bmim]BF4were added into a three-necked flask equipped with a condenser, with the mixture being stirred at a specified temperature. The upper oil phase was sampled every 20 min and then placed until being layered,while the content of sulfur compounds was determined on a WK-2D microcoulometric analyzer. The desulfurization rate was calculated by the following equation[23]:

where Stot(500 μg/L) is the total content of the sulfur compound in the model oil, and Sresis the residual content of the sulfur compound after the ODS process.
3 Results and Discussion
3.1 Characterization
3.1.1 XRD patterns
The phase and crystallinity of H2WO4, g-C3N4, and WO3/g-C3N4with different content of tungstic acid were characterized by XRD, with the results presented in Figure 1. The lattice constants of H2WO4were a=0.523 8 nm, b=1.070 4 nm, and c = 0.512 0 nm, while the XRD peaks of H2WO4were coherent with the standard card of orthogonal crystalline phase (JCPDS 43-0679).

Figure 1 XRD patterns of: (a)H2WO4; (b)WO3/g-C3N4(5%);(c)WO3/g-C3N4(10%); (d)WO3/g-C3N4(15%);(e)WO3/g-C3N4(20%); and (f)g-C3N4◆—Monoclinic WO3; ●—Triclinic WO3;—Hexagonal WO3; ▲—Orthorhombic WO3·H2O
The two diffraction peaks at 12.7 and 27.4 were coincident with the (100) and (002) planes of g-C3N4,respectively. The result was consistent with the literature report of the g-C3N4[24]. The characteristic diffraction peaks of WO3disappeared in WO3/g-C3N4(5%) and WO3/g-C3N4(10%) due to high dispersion of WO3or a too low content of WO3. The characteristic diffraction peaks of g-C3N4and WO3appeared in WO3/g-C3N4(15%) and WO3/g-C3N4(20%), and moreover, these peaks obviously became weaker than those of pure g-C3N4and H2WO4.Many crystallinity phases of WO3appeared in WO3/g-C3N4. The results showed that there was an oxidationreduction reaction between carbon nitride and tungstic acid, because the carbon nitride possessed reducibility and the tungstic acid possessed oxidability. The WO3contained the monoclinic, triclinic, hexagonal and orthorhombic crystal systems[16,25]as shown by Figure 1. Therefore, the polycrystalline phase WO3was dispersed on g-C3N4. The crystalline phase of WO3is tabbed in Figure 1.
3.1.2 SEM and EDS images of samples
The morphology of g-C3N4and WO3/g-C3N4with different content of H2WO4is shown in Figure 2. As shown in Figure 2a, the g-C3N4sample constitutes a sheet structure with lots of pores. The morphology of WO3/g-C3N4is a sheet structure with lots of particles and pores as shown in Figure 2 (b)―(e). The results showed that the particles of WO3, which were dispersed on the sheets of g-C3N4with lot of pores, could exhibit higher catalytic activity for desulfurization. Lots of the particles in WO3could be observed in the inset of Figure 2 (e) and Figure 2 (f). In order to further analyze the constituents of WO3/g-C3N4,the EDS characterization was carried out, with the results shown in Figure 3. The WO3/g-C3N4(20%) contained W,O, C, and N elements and these elements, which were well-dispersed in composites, are shown in Figure 3. The content of W, O, C, and N elements in WO3/g-C3N4(20%)are listed in Table 1. These results showed that WO3were dispersed in the g-C3N4.The results demonstrated that a part of H2WO4was transformed into WO3, or a majority of WO3entered the layers of g-C3N4, because a W content of 0.14% was far less than the added dose of H2WO4(20%).
3.1.3 FT-IR analyses
The FT-IR spectra of pure g-C3N4, H2WO4, and WO3/g-C3N4(x = mass ratio of H2WO4, x=5%, 10%, 15%, and 20%)are shown in Figure 4. It can be seen that four peaks at 668 cm-1, 945 cm-1, 1 617 cm-1, and 3 381 cm-1correspond to ν(O-W-O), ν(W=O), δ(O-H), and ν(O-H) of H2WO4,respectively[26]. The characteristic absorption peaks of g-C3N4appeared at 810 cm-1and 1 200―1 700 cm-1, which are assigned to the triazine units and the stretching vibration of C-N heterocycles. Besides, the broad absorption peak at 3 000 cm-1in g-C3N4is related to the stretching vibration of-NH and =NH[27]. It can be seen that the peaks at 3 381 cm-1for H2WO4and 3 438 cm-1for g-C3N4have changed to 3 187 cm-1and 3 284 cm-1for WO3/g-C3N4. It demonstrates that there is a reaction between W-O of WO3and -NH and=NH of g-C3N4. There is a new peak appearing at 752 cm-1,which is a characteristic peak of WO3. The peak has experienced a red-shift with the increase of H2WO4. These results indicate that a reaction exists between H2WO4and g-C3N4and the FT-IR peaks of WO3and g-C3N4also appear in WO3/g-C3N4.
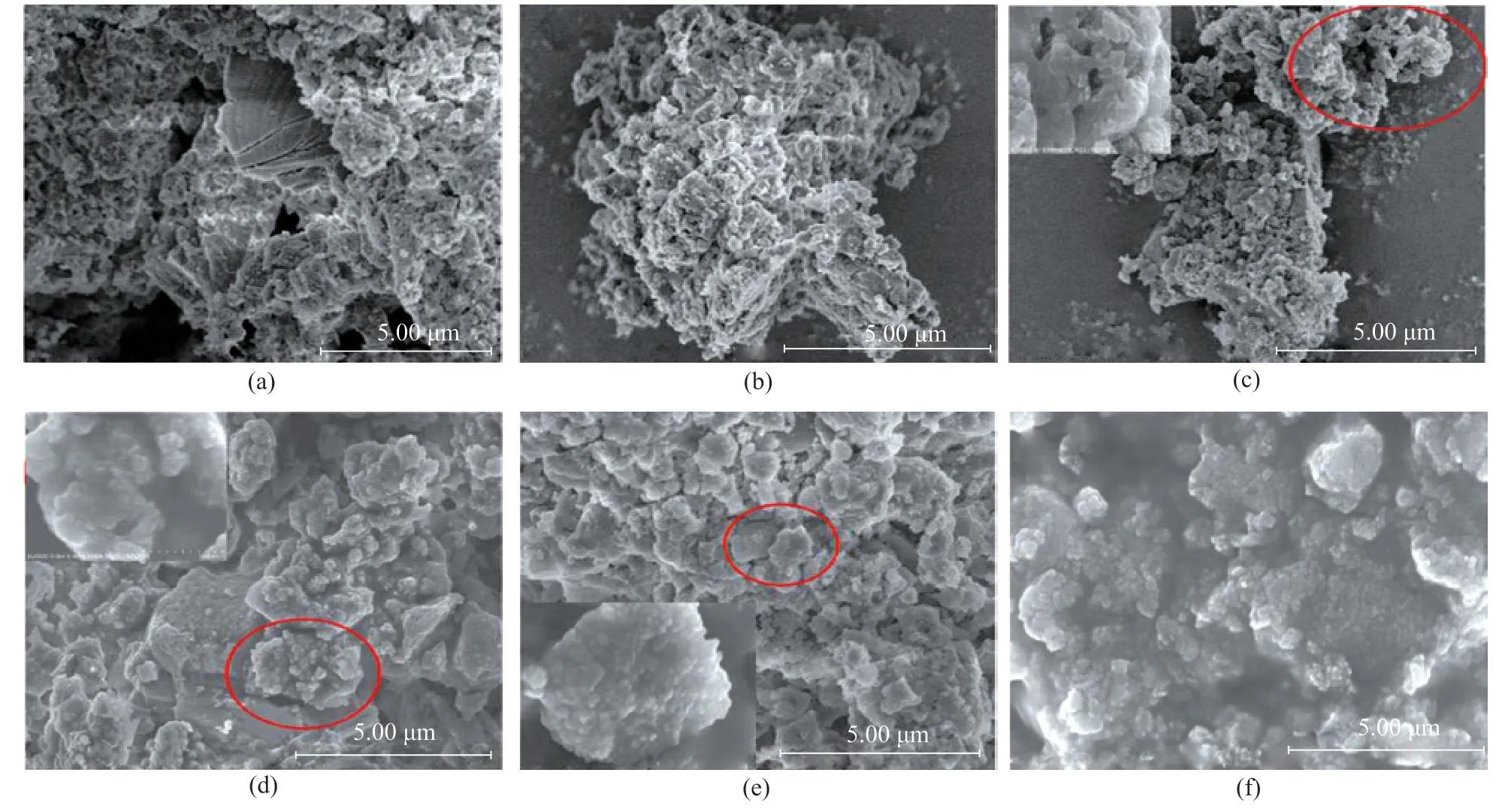
Figure 2 SEM micrographs of: (a)g-C3N4; (b)WO3/g-C3N4(5%); (c)WO3/g-C3N4(10%); (d)WO3/g-C3N4(15%);(e)WO3/g-C3N4(20%); and (f) enlarged WO3/g-C3N4 (20%)
3.1.4 BET analyses of samples
The specific surface areas of different samples are presented in Table. 2. It can be found that the surface area of H2WO4is 11.309 8 m2/g and that of g-C3N4is 8.659 1 m2/g. Specific surface areas of the WO3/g-C3N4increase with an increasing dose of H2WO4. This can occur because more particles are dispersed on g-C3N4and more WO3can enter the layers of g-C3N4, resulting in an enlarged specific surface area. The large surface area can provide more active sites, which are conducive to the adsorption and reaction of the reactants[28]. Therefore,the larger surface area can results in high desulfurization performance of the catalysts.

Table 2 Surface area of samples
3.2 Optimizing reaction conditions for oxidative desulfurization
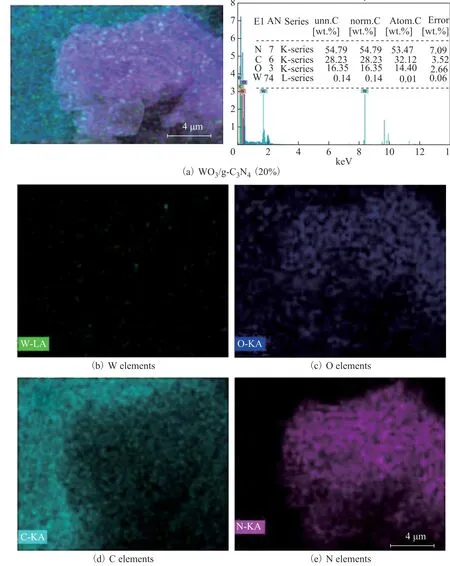
Figure 3 EDS images of WO3/g-C3N4 (20%) and the corresponding EDS mapping of W, O, C and N elements

Figure 4 FT-IR spectra of: (a)H2WO4; (b)WO3/g-C3N4(5%);(c)WO3/g-C3N4(10%); (d)WO3/g-C3N4(15%);(e)WO3/g-C3N4(20%); and (f) g-C3N4
The activity of catalyst depends on the dosage of WO3,because WO3is the active component of catalysts. So the amount of H2WO4has an important influence on the desulfurization activity. The desulfurization rates over catalysts with different content of H2WO4under the same reaction conditions are shown in Figure 5(a). The desulfurization rate of catalyst increased from 17.69%to 95.19% when the content of H2WO4was increased from 0% to 20%. The 1-butyl-3-methylimidazolium tetrafluoroborate serving as a solvent was chosen as the extractant. The influence of IL dose on desulfurization rate was researched, with the results presented in Figure 5(b). The H2O2used as the oxidant has an important effect on the oxidative desulfurization system. The desulfurization rate was only 27.88%in the absence of H2O2as shown in Figure 5(c). The result also demonstrated that WO3was oxidized to H2W[O(O2)2(OH)2][29]under the action of hydrogen peroxide in the desulfurization system. The oxidative desulfurization experiments were implemented at different temperatures. As shown in Figure 5(d), the desulfurization rate obviously increased from 76.15% to 98.46%, when the reaction temperature was raised from 40 C to 70 C. Catalyst played an important role in the oxidative desulfurization system. As shown in Figure 5(e), the desulfurization rate increased from 94.81% to 98.46% when the catalyst dosage was increased from 0.01 g to 0.02 g. In conclusion, the desulfurization rate was optimal under conditions covering a temperature of 70 C, a H2WO4content of 20%, an IL amount of 0.25 mL,a H2O2dose of 0.2 mL, and a catalyst dosage of 0.2 g.
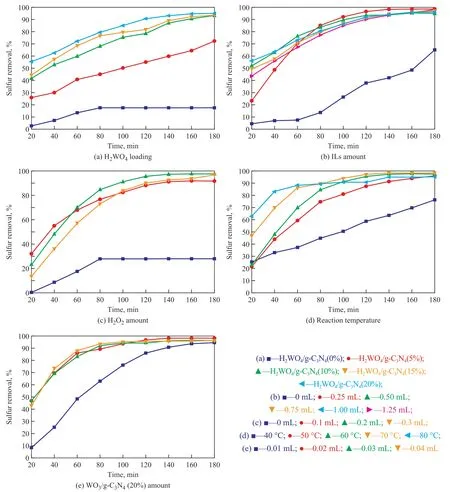
Figure 5 Influence of reaction condition on desulphurization performance
3.2.1 Oxidative desulfurization of different sulfides and reaction dynamics analysis
The ODS system containing WO3/g-C3N4(20%), H2O2,and ILs was used to study the activity of catalyst for treating different sulfides, such as BT, TH, and DBT. As shown in Figure 6(a), the desulfurization rate decreased in the following order: DBT (98.46%)> BT (52.78%)>TH (46.56%). The desulfurization efficiency was related to the electron cloud density of sulfur atoms in the sulfur compounds. The electron density decreased in the following order: DBT (5.758) > BT (5.739) >TH(5.696)[30]. It could be concluded that the desulfurization rate for different sulfur compounds was consistent with the electron density. Some reports have proved that the mechanism of ODS follows the first-order reaction kinetics equation[31].

where C0is the total concentration of sulfur compound,Ctis the remaining concentration of sulfur compound after t, k is the reaction kinetics constants and R2is the correlation coefficient. As shown in Figure 6(b), the reaction kinetics constants (k) of DBT, BT and TH are 0.028 75 min-1, 0.003 633 33 min-1, and 0.001 741 67 min-1, respectively. Meanwhile, the
correlation coefficients (R2) of DBT, BT and TH are 0.990 062 99, 0.951 063 16, and 0.971 122 54,respectively. The half-time of first-order reaction kinetics is t1/2=(ln2/k) = 24.109 min for DBT under the optimal reaction conditions. It can be concluded that the speed of desulfurization is very quick.
3.2.2 Apparent activation energy of chemical reaction
The ODS process follows the first-order reaction kinetics according to the above analysis. The rate of first-order reaction is r = -dC/dt =kC. The ln(C0/Ct) = kt can be written as ln (1-X) = - kt, when Ct=C0(1-X), in which t serves as the abscissa, and ln (1-X) serves as the ordinate.The rate constant can be obtained by linear fitting of the desulfurization rate at different temperatures (333 K,343 K, and 353 K). As shown in Figure 7 (a), the first order reaction rate constant (k) at 333 K, 343 K, and 353 K is 0.018 358 33 min-1, 0.024 541 67 min-1,and 0.028 75 min-1, respectively, when the reaction temperature is 333 K, 343 K, and 353 K, respectively,in which X is the conversion rate, t is the reaction time,C0is the total sulfur content, and r is the rate of reaction.The Arrhenius equation is lnk = lnk0- Ea/(RT), where 1/(RT) serves as the abscissa and lnk serves as the ordinate, k0is the pre-exponential factor, Eais the apparent activation energy, and R is the thermodynamic constant.So, the apparent activation energy Ea=20.7 kJ/mol can be obtained for DBT. In this case the apparent activation energy of chemical reaction was lower than the previous value of research on the desulfurization reaction[31-33].
3.2.3 Recovery-recycles of catalyst

Figure 6 Catalytic oxidation and reaction dynamics analysis of different sulfides■—DBT; ●—BT; ▲—TH
In order to determine the stability of the catalyst, the recovery and recycling use of the ionic liquid phase containing the catalyst were investigated. The WO3/g-C3N4(20%) could be reused in the oxidative desulfurization system containing H2O2and [bmim]BF4. After each oxidative desulfurization cycle, the experiments for recovery of the ionic liquidcontaining catalyst were carried out using CCl4as the extraction agent by means of rotary evaporation. After the fresh H2O2and model oil were added into the recovered ionic liquid phase, the recycling experiments were implemented under the optimal conditions. As shown in Table 3, the desulfurization rate decreased from 98.46%to 96.81% after recycling five times. It may be concluded that the stability of H2WO4/g C3N4(20%) is higher thanks to the heterogeneous catalysis system.
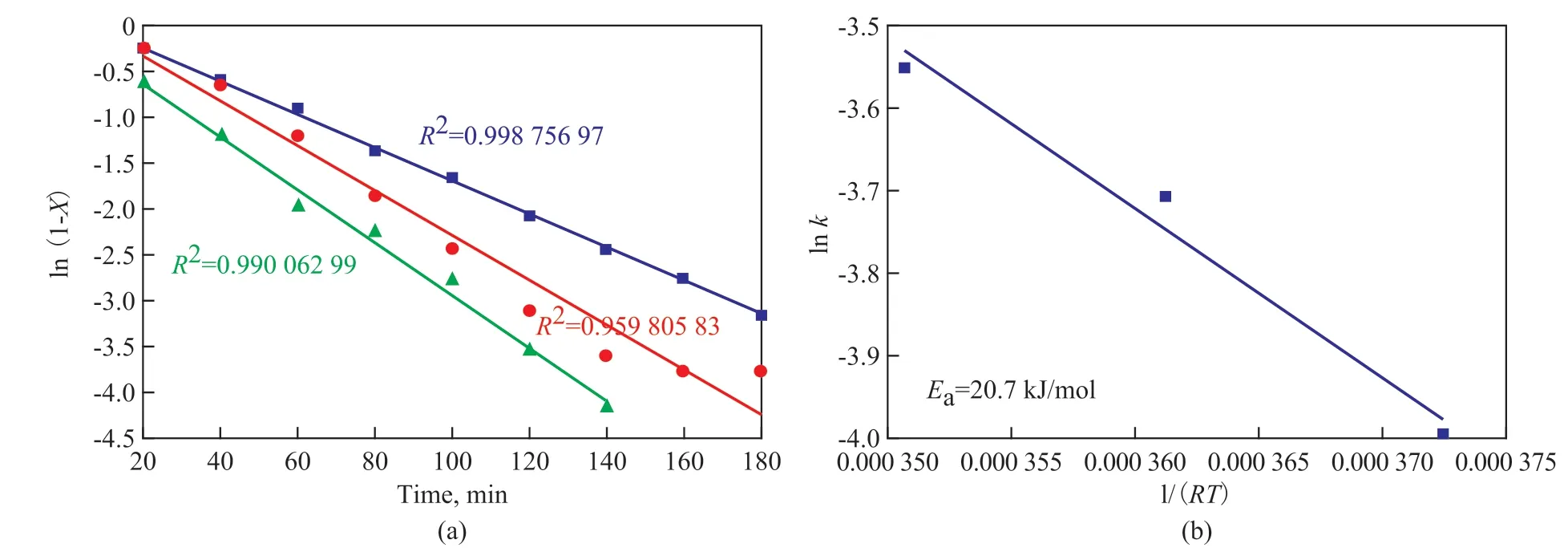
Figure 7 Apparent activation energy of chemical reaction: (a) Fitting of experiment data under different temperature by the pseudo-first-order reaction kinetics equation; and (b) Apparent activation energy of DBT with WO3/g-C3N4(20%) serving as catalyst(a) ■—323 K; ●—333 K; ▲—343 K
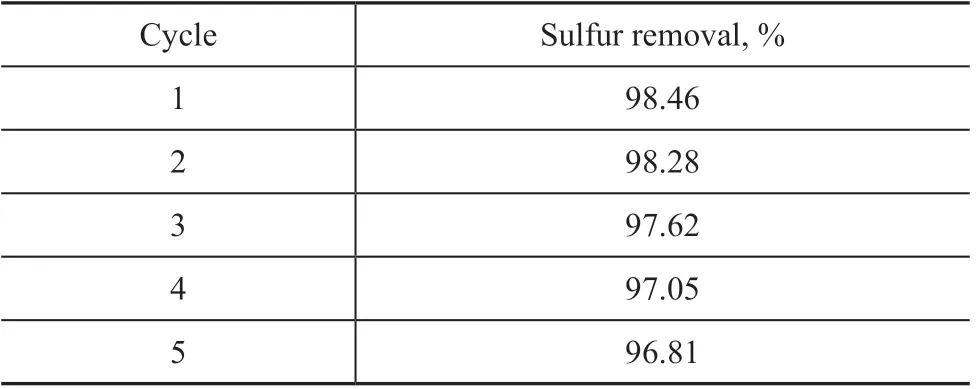
Table 3 Recovery-Regeneration of catalyst
3.3 Mechanism of catalytic oxidative desulfurization
In order to determine the oxidants of desulfurization reaction, the reverse extraction experiments were implemented. The oxidation products of DBT in the reverse extraction experiment were obtained by using carbon tetrachloride (CCl4) as the extraction agent. The infrared spectra of oxidation products of desulfurization are shown in Figure 8, in which three infrared absorption peaks at 1 166 cm-1, 1 047 cm-1, and 1 288 cm-1correspond to three characterization peaks of DBTO2[34-35]. On the basis of the above-mentioned analysis, a mechanism of oxidative desulfurization is proposed. As shown in Figure 9,the oxidative desulfurization system containing the model oil, WO3/g-C3N4(20%), [bmim]BF4, and H2O2forms two phases, one of which is the oil phase (model oil), and the other is the ionic liquid phase (consisting of [bmim]BF4,WO3/g-C3N4(20%) and H2O2). Firstly, WO3/g-C3N4was oxidized to form H2W[O(O2)2(OH)2]/g-C3N4by H2O2[27].A part of DBT in oil phase was extracted into the ionic liquid phase[36]by [bmim]BF4, and then DBT was oxidized to form DBTO2by H2W[O(O2)2(OH)2]/g-C3N4, while H2W[O(O2)2(OH)2]/g-C3N4was reduced to WO3/g-C3N4.The reduced WO3/g-C3N4would continue to be oxidized by H2O2to implement its function in the next cycle. More DBT was extracted into the ILs phase until the end of the desulfurization process or after exhaustion of H2O2.
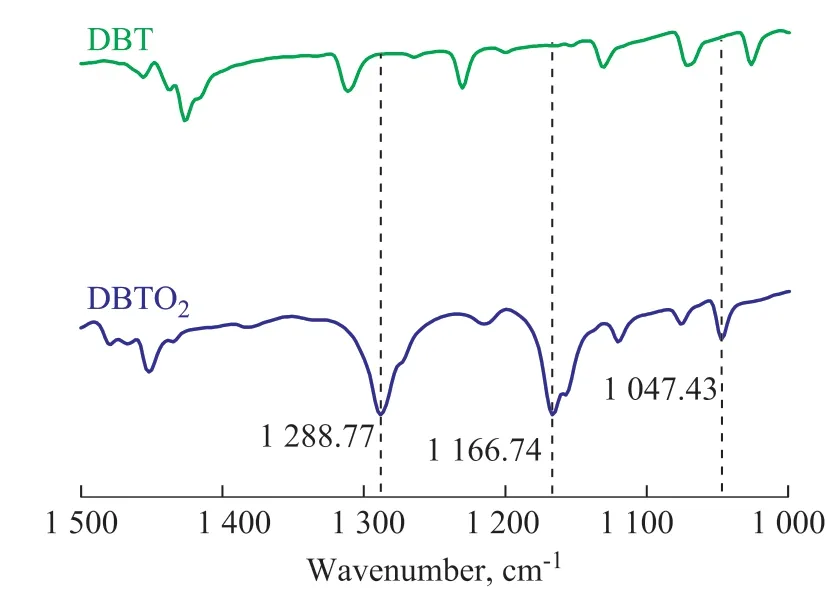
Figure 8 FT-IR spectra of DBT and DBTO2
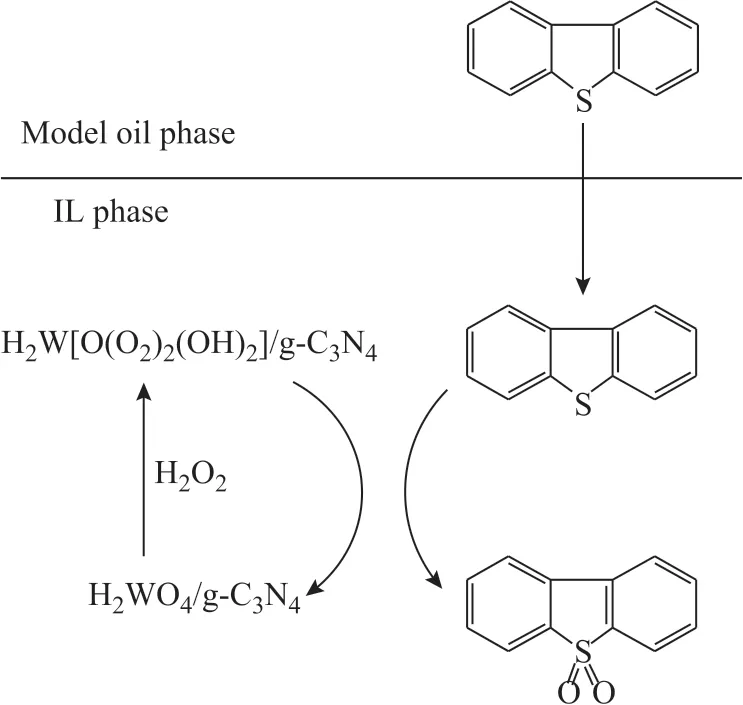
Figure 9 Mechanism of catalytic oxidative desulfurization
4 Conclusions
The WO3/g-C3N4was synthesized by the simple mixture method. The XRD and IR analyses show that there is an interaction between the H2WO4and g-C3N4, and therefore, polycrystalline phase WO3/g-C3N4is obtained.The BET analysis shows that the surface area of catalyst becomes larger. The WO3/g-C3N4(20%) exhibits high catalytic activity in the desulfurization process. The desulfurization rate can reach 98.46% for DBT in the model oil by using a small dosage of catalyst and ionic liquid operated under optimal reaction conditions. The desulfurization reaction is carried out with low apparent activation energy. The desulfurization rate over the catalyst slightly decreases after five times of recycling runs.
Acknowledgements:The authors also acknowledge the financial support of the Doctoral Fund of Liaoning Province(201501105).
杂志排行
中国炼油与石油化工的其它文章
- Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
- Modeling of Continuous Cross-flow Microfiltration Process in an Airlift External-loop Slurry Reactor
- Effects of Nitrogen-Containing Biodegradation Enhancers on Sorption of n-Hexadecane in Soil-Water System
- Novel Approach for Improved Tribological Behavior of Biodiesel Soot in Liquid Paraffin
- Compatibility Evaluation between Direct Coal Liquefaction Residue and Bitumen
- Impacts of Wettability on Immiscible Fluid Flow Pattern-Microfluidic Chip Experiment
