Immobilization of Functionalized Ionic Liquid on Sol-Gel Derived Silica for Efficient Removal of H2S
2019-05-10MaYunqianMaoJiamingXiaoCongLiYanZangLihua
Ma Yunqian; Mao Jiaming; Xiao Cong; Li Yan; Zang Lihua
(1. School of Environmental Science and Engineering, Qilu University of Technology, (Shandong Academy of Sciences),Jinan 250353;
2. Jiangsu Key Laboratory of Anaerobic Biotechnology, Jiangnan University, Wuxi 214122;
3. Shandong Province Metallurgical Engineering Co., Ltd., Jinan 250101;
4. Langfang Meihua Biotechnology Development Co. Ltd., Langfang 065001)
Abstract: An innovative approach to H2S capture has been developed using several metal-based ionic liquids ([Bmim]Cl·CuCl2, [Bmim]Cl·FeCl3, [Bmim]Cl·ZnCl2, [Bmim]Br·CuCl2, and [Bmim]Br·FeCl3) immobilized on the sol-gel derived silica, which is superior to purely viscous ionic liquid with a crucial limit of high temperature, low mass transfer rate,and mass loss. The adsorbents were characterized by the Fourier transform infrared spectrometer, transmission electron microscope, N2 adsorption/desorption, X-ray photoelectron spectroscopy, and thermal analysis techniques. The effects of the metal and halogen in IL, the loading amount of IL, and the adsorption temperature were studied by dynamic adsorption experiments at a gas flow rate of 100 mL/min. The H2S adsorption results have showed that the optimal adsorbent and adsorption temperature are 5% [Bmim]Cl·CuCl2/silica gel and 20—50 C, respectively. H2S can be captured and oxidized to elemental sulfur, and [Bmim]Cl·CuCl2/silica gel can be readily regenerated by air. The excellent efficiency of H2S removal may be attributed to the formation of nano-scaled and high-concentration [Bmim]Cl·CuCl2 confined in silica gel, indicating that the immobilization of [Bmim]Cl·CuCl2 on the sol-gel derived silica can be used for H2S removal promisingly.
Key words: functionalized ionic liquid; supported ionic liquid; silica; sol-gel; H2S removal
1 Introduction
H2S removal from gaseous emissions is commonly achieved by absorption, adsorption or biological process.Nevertheless, the gas-liquid absorption process with the aqueous solution of ammonia, alkanolamine or alkaline salts suffers from the drawbacks of foaming,inapplicability for high temperature, and requiring high energy consumption for regeneration[1]. Biological process can efficiently remove H2S at low concentration,but it would cost much time[2]. The gas-solid adsorption by metal oxides, activated carbon, zeolite or silica is regarded as an efficient technology for H2S removal at high temperature, but requires a modification with a high adsorption capacity and selectivity[3-4].
Ionic liquid (IL) has attracted great attention due to its unique properties, such as designability, non-volatility,and high thermal and chemical stability[5]. Compared with conventional water-based system with an upper limit of working temperature which is normally below 50 C, IL shows a much higher stability in a wide range of temperature and higher selectivity thanks to its functionalized groups that can be designed. Hence, pure IL has been used as an absorbent for oxidative removal of H2S. The physical solubility of H2S in many ILs has been investigated under static high pressure conditions[6].In recent years, studies on the dynamic H2S removal using the chemical property of functionalized ionic liquid (FIL) under atmospheric pressure have received much attention[7-8]. However, FILs generally have high viscosity, which would decrease the mass transfer rate to cause mass loss under experimental and engineering conditions, and thus are applied to H2S removal at extremely high temperature to make their viscosity reduced. To overcome the above shortcomings of pure functionalized ionic liquid solvents, the supported ionic liquid (SIL) materials have a promising application in gas adsorption to bring into full play the nature of both ionic liquid and heterogeneous support material.
The preparation of these materials was achieved by three different immobilization approaches. The first approach involves the covalent attachment of ionic liquids to the support surfaces, the second approach aims at physically depositing the ionic liquid phases containing chemically active species on the surface of the support, whereas the third approach embeds the ionic liquid in silica gel by the sol-gel method. Among the three methods, the sol-gel process can achieve a molecular level mixing, which is favorable to the preparation of in situ coated materials. IL can be entrapped in the pores of silica-gel. Being different from the physical deposition method, the immobilization of ILs by the sol-gel process can result in a more intimate biphasic system[9]. The solid matrix forms a porous prison that prevents ILs from leaching, but can allow for H2S gas and products[10]. Recently, the supported ionic liquid materials prepared by the sol-gel method have been used in the desulfurization of fuels[11], and in some catalytic reactions, such as carbonylation[12], hydroformylation[13],and alcohol oxidation[14]. Moreover, previous studies mostly focused on liquid phase systems, while only a small amount of studies were targeted for H2S removal using the supported ionic liquids prepared by physical deposition[15],and no work was referred to the sol-gel method.
The main aim of this work is to test the efficiency for H2S removal by the functionalized ionic liquids immobilized on silica prepared by the sol-gel method. Metal-based(Cu, Fe, and Zn) ILs with different halogens (Cl and Br)were embedded in silica using an ultrasonic technology which could disperse ionic liquid more uniformly than conventional stirring[16]. This is meaningful for introducing the sol-gel method into gas adsorption. The H2S removal capacity and regeneration performance of the supported ionic liquid materials were evaluated, and the product was further determined.
2 Experimental
2.1 Materials
Anhydrous ferric chloride (FeCl3, >98%), zinc chloride(ZnCl2, >98%), and copper chloride (CuCl2, >98%) were purchased from the Shanghai Macklin Biochemical Co.,Ltd.; 1-butyl-3-methylimidazolium chloride ([Bmim]Cl, >99%) was purchased from the Shanghai Chengjie Chemical Co., Ltd.,; tetraethylorthosilicate (TEOS, AR)was purchased from the Tianjin Damao Chemical Co.,Ltd.,; ethanol (99.9%) and hydrochloric acid (37.5%)were purchased from the Tianjin Kermel Chemical Reagent Co., Ltd. All the above chemicals were used as received.
2.2 Synthesis of IL and IL supported silica
[Bmim]Cl·FeCl3, [Bmim]Cl·ZnCl2, [Bmim]Cl·CuCl2,[Bmim]Br·FeCl3, [Bmim]Br·CuCl2, [Bmim]Br·FeCl3,and [Bmim]Br·CuCl2were prepared by the procedures previously described[17], and their viscosity increased sharply, as the temperature decreased. The acid-catalyzed sol-gel process was used for preparation of the silica gel supported IL[18], denoted as IL/silica gel. Taking [Bmim]Cl·CuCl2/silica gel as an example, TEOS was selected as the sol-gel precursor. 10 mL of TEOS and 7 mL of ethanol were added into a 250 mL round-bottom flask and mixed with a certain amount of [Bmim]Cl·CuCl2under stirring at 60 C. After the mixture became a clear and uniform liquid, the mixture was acidified with 2 mL of hydrochloride acid (36%—38%) and deionized water. The final mixture gelled during 5 min-10 min without further stirring and was subjected to ageing at 60 C for 12 h.The resultant solid material was washed with ethanol and dried in vacuum at 100 C for 4 h, and then the [Bmim]Cl·CuCl2/silica gel was obtained. The loading amount was confirmed in terms of the mass percent (1%, 3%, 5%, and 7%) of [Bmim]Cl·CuCl2to TEOS.
2.3 Characterization
The Fourier transform infrared (FT-IR) spectra of the samples were recorded on a Vertex 80 infrared spectrometer (Bruker Corp., Germany) at a wavenumber range of 400—4 000 cm-1. The microstructure of the sample was observed by a transmission electron microscope (TEM) (JEM-2100, Japan) operated at 180 kV. The Brunauer-Emmett-Teller (BET) method was used to calculate the specific surface area. The pore size distribution (PSD) was obtained by using the Barrett-Joyner-Halenda (BJH) model. Gold was used as a conductive material for sample coatings. The X-ray photoelectron spectroscopy (XPS) was carried out on an ESCALAB250 X-ray energy spectrometer.The binding energy (BE) was normalized by using the binding energy of carbon (284.6 eV) as the external standard. Thermogravimetric analysis (TGA) and differential thermogravimetric (DTG) analysis of the sorbents were done on a SDT Q600 Universal V4.1D TA instrument under a flow of N2stream from room temperature to 800 C at a temperature increase rate of 10 C/min.
2.4 Measurement details
The H2S adsorption measurement of IL/silica (0.5 g)was done at atmospheric pressure. N2was used instead of methane (CH4), which is the main component of natural gas. The H2S gas mixture (1 000 mg/m3) obtained by blending H2S and N2passed through a fixed bed system at a gas flow rate of 100 mL/min. The residual exhaust H2S-containing gas was absorbed by NaOH solution. The concentration of H2S in the outlet gas was detected by a H2S monitor (TH-990S). The breakthrough concentration of H2S was defined as 1% of the initial concentration (C0), and the breakthrough time of H2S was defined when the outlet H2S concentration (C)came up to the breakthrough concentration value. The breakthrough sulfur sorption capacity was calculated by integration of the areas above the breakthrough curves(with time serving as the x-coordinate and the outlet H2S concentration serving as the y-coordinate), under a given flow rate, an initial concentration of H2S, and mass of the sorbent.
3 Results and Discussion
3.1 Properties of the adsorbents
3.1.1 FT-IR anlyses
The FT-IR spectra of the pure silica gel, [Bmim]Cl,[Bmim]Cl/silica gel, [Bmim]Cl·FeCl3/silica gel, [Bmim]Cl·ZnCl2/silica gel, and 1%—7% [Bmim]Cl·CuCl2/silica gel are shown in Figure 1. The broad band around 3 420 cm-1was the vibrations of O-H in the silica gel and the physically absorbed water. The peaks at 1 645 cm-1and 956 cm-1were the vibrations of Si-OH. The characteristic absorption peak of Si-O-Si was found at 1 085 cm-1and 460 cm-1. The bands around 3 150 cm-1,2 960 cm-1, and 1 450 cm-1were the stretching of C-H and deformation vibrations of the imidazole section and the alkyl chain. The absorption peak around 1 591 cm-1was the C=N stretching vibration in the imidazole ring[18]. With an increasing loading amount, the peaks of the groups C=N (1 591 cm-1)and C-H (2 960 cm-1and 1 450 cm-1) in the imidazole ring of [Bmim]Cl·CuCl2became more distinct and stronger[19-20].
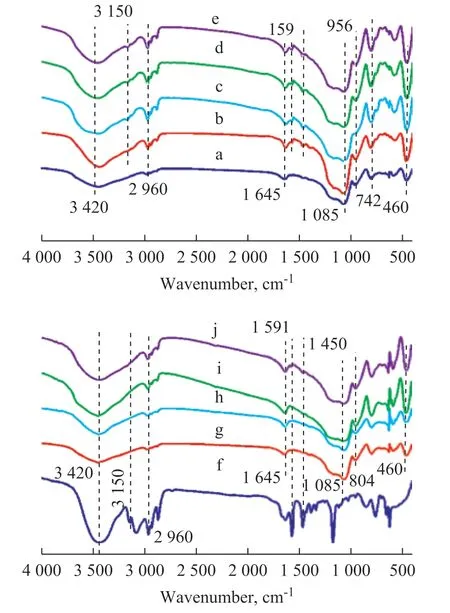
Figure 1 FT-IR spectra of pure silica gel,IL/silica gel and [Bmim]Cl(a) pure silica gel, (b) [Bmim]Cl·CuCl2/silica gel, (c) [Bmim]Cl·FeCl3/silica gel, (d) [Bmim]Cl·ZnCl2/silica gel, (e) [Bmim]Cl/silica gel, and (f) [Bmim]Cl, while (g)-(J) are [Bmim]Cl·CuCl2/silica gel with a loading amount of 1%,3%, 5%, and 7%, respectively
3.1.2 XRD analyses
The pure silica gel, and [Bmim]Cl·CuCl2/silica gel with an IL loading amount of 1%, 3%, 5%, and 7% were analyzed by XRD (in Figure 2). The emergence of a broad peak at the angle 2θ = 21˚—30˚ represents the amorphous phase of the silica gel[21-22]. The diffraction peak for 5% [Bmim]Cl·CuCl2/silica gel shows a right shift,suggesting a reduction of the pore size[23], and then a left shift of diffraction peak for 7% to 5% means an increase of the pore size, indicating the structural collapse of the 7% sample.
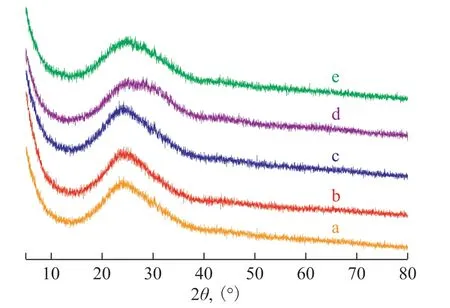
Figure 2 X-Ray Diffraction patterns of silica gel samplesa—pure silica gel; b—1% [Bmim]Cl·CuCl2/silica gel; c—3%[Bmim]Cl·CuCl2/silica gel; d—5% [Bmim]Cl·CuCl2/silica gel;e—7% [Bmim]Cl·CuCl2/silica gel
3.1.3 TEM analyses
The TEM images of pure silica gel and [Bmim]Cl·CuCl2/silica gel with a loading amount of 5% are shown in Figure 3 (a and b), respectively. It can be seen from Figure 3 (a) that the TEM images of pure silica gel showed that the silica gel matrix was non-uniformly mesoporous.Ionic liquid with small molecular size such as [Bmim]Cl, could be washed out from the silica-gel matrix under vigorous reflux conditions, but [Bmim]Cl·CuCl2with larger molecular size could be physically confined into the silica-gel nanopores relatively firmly. It can be seen from Figure 3 (b) that there were some aggregations of the encapsulated ILs inside of the silica gel, indicating that [Bmim]Cl·CuCl2was aggregated and encapsulated into silica gel, and the pore size of [Bmim]Cl·CuCl2aggregation ranged from 1 nm to 30 nm, and was confined in the silica gel matrix of nanoscale dimension. It is evident that the morphology of [Bmim]Cl·CuCl2/silica gel was composed of aggregated and nanoscaled ionic liquid particles, which are well distributed in silica gel.
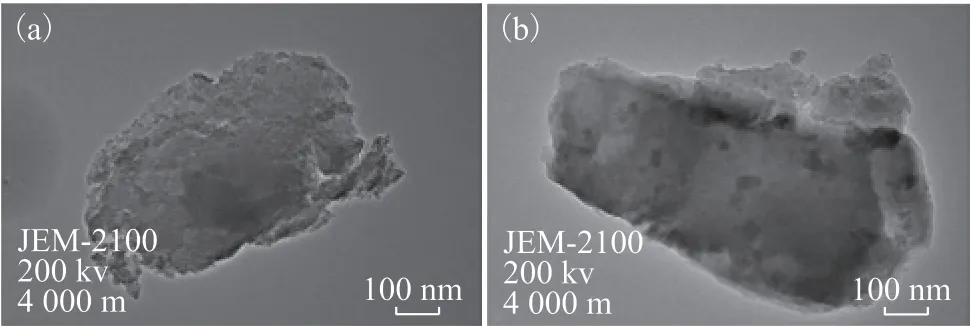
Figure 3 TEM images of the pure silica gel (a) and [Bmim]Cl·CuCl2/silica gel (b)
3.1.4 Textural properties
The surface area, pore volume, and pore diameter of the sorbents are listed in Table 1. As for samples with a loading amount of 3%, the surface area of the sorbents decreased in the following order: Zn > Fe > Cu >sample without metal loading, while the pore volume of sorbents with a loading amount of 3% decreased in the following order: sample without metal loading> Zn> Fe > Cu, respectively. As for [Bmim]Cl·CuCl2/silica gel, the surface areas and pore volumes decreased but the pore diameter increased, when the loading amount increased from 1% to 5%. [Bmim]Cl·CuCl2could be encapsulated into the silica gel, leading to a decreasing surface area and pore volume. The pore size of [Bmim]Cl·CuCl2/silica gel ranged from 1 nm to 30 nm, which showed that the synthesized IL/silica gel adsorbents by the sol-gel method had both the mesoporous and microporous structures. When the loading amount was increased to 7%, the pore diameter sharply increased,but the surface area and pore volume were extremely small, which was caused by the structural collapse of the material.
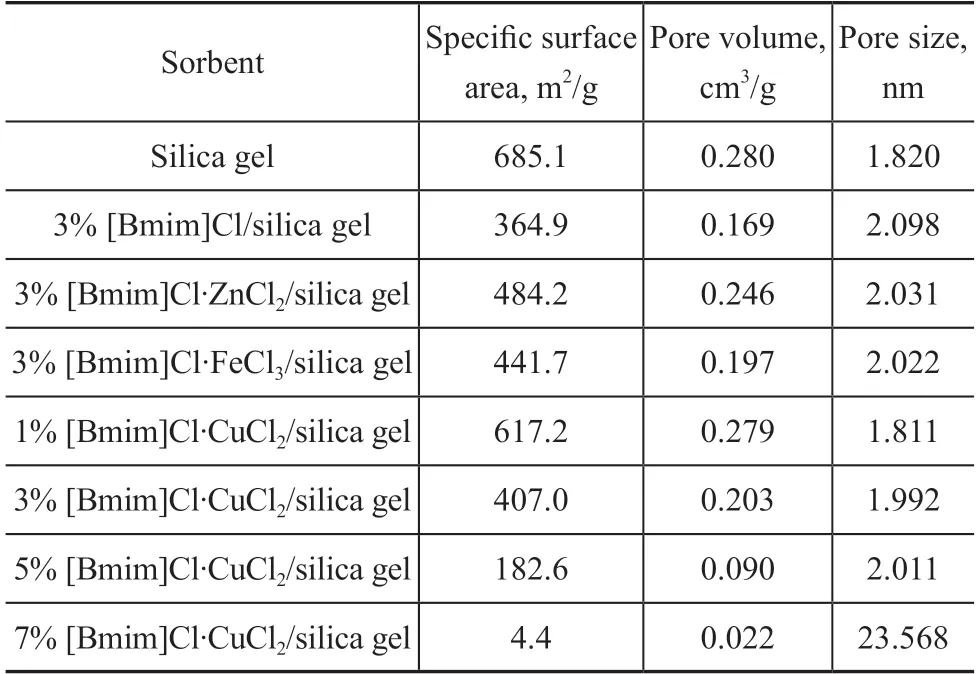
Table 1 Textural properties of IL/silica gel
3.2 H2S adsorption analyses
3.2.1 Effect of metal and halogen on H2S adsorption
Ionic liquids with high-selectivity to H2S adsorption were investigated on their metal (Cu, Fe, and Zn) and halogen (Cl and Br) contents, with the breakthrough curves of IL/silica gel presented in Figure 4. It can be seen from Figure 4(a) that IL/silica gel with Cu loading had a higher desulfurization capacity than those loaded with Fe, and Zn, or those without metal loading, and the excellent desulfurization capacity of Cu-based ionic liquid/silica gel was in agreement with previous results[24].[Bmim]Cl·ZnCl2/silica gel, [Bmim]Cl/silica gel, and the original silica gel all showed low H2S removal capacity with a breakthrough time of 4 min, 0.6 min, and 0 min,respectively. However, it took more time for C coming up to C0with [Bmim]Cl·ZnCl2/silica gel than [Bmim]Cl/silica gel and the original silica gel due to the formation of stable ZnS which was previously mentioned in the literature[15]. The breakthrough time of the sorbents with Fe and Cu was 32 minutes and 72 minutes, respectively,which indicated that they could both remove H2S with an efficiency of 100%. Therefore, the following investigation of halogen content was conducted on Cu- and Fe-based ionic liquid/silica gel, as shown in Figure 4(b).The results indicated that [Bmim]Cl·CuCl2/silica gel had a higher desulfurization capacity than [Bmim]Br·CuCl2/silica gel, while [Bmim]Cl·FeCl3/silica gel on the contrary had a lower H2S removal capacity than [Bmim]Br·-FeCl3/silica gel. Therefore, [Bmim]Cl·CuCl2/silica gel was chosen for further investigation.
3.2.2 Effect of the loading amount of ionic liquid on H2S adsorption
The loading amount of the used ionic liquid was selected and studied in the range from 1% to 7% (Figure 5). For a loading amount of the used ionic liquid in the range of between 1% to 5%, the H2S removal capacity was improved with an increasing loading amount,but it dramatically decreased when a loading amount of [Bmim]Cl·CuCl2increased to more than 5%. The H2S removal capacity was remarkably enhanced with a much lower amount of ionic liquids needed for the silica-supported ionic-liquid that was prepared with simple impregnation, in which the ionic liquid might be deposited as a thin layer on the support. Such unusual enhancement in H2S removal capacity could be attributed to the formation of nanoscaled and high-concentration ionic liquids due to the confinement of the ionic liquids in silica gel, which could result in unusual changes in the symmetry and coordination geometry of the ionic liquids[13]. Moreover, an excessive loading of 7% might lead to collapse of the pores in the sorbent,which could also be confirmed by the results of textural properties analysis in the characterization section.Therefore, [Bmim]Cl·CuCl2/silica gel with a loading amount of 5% had the highest H2S removal capacity and was selected for subsequent studies.
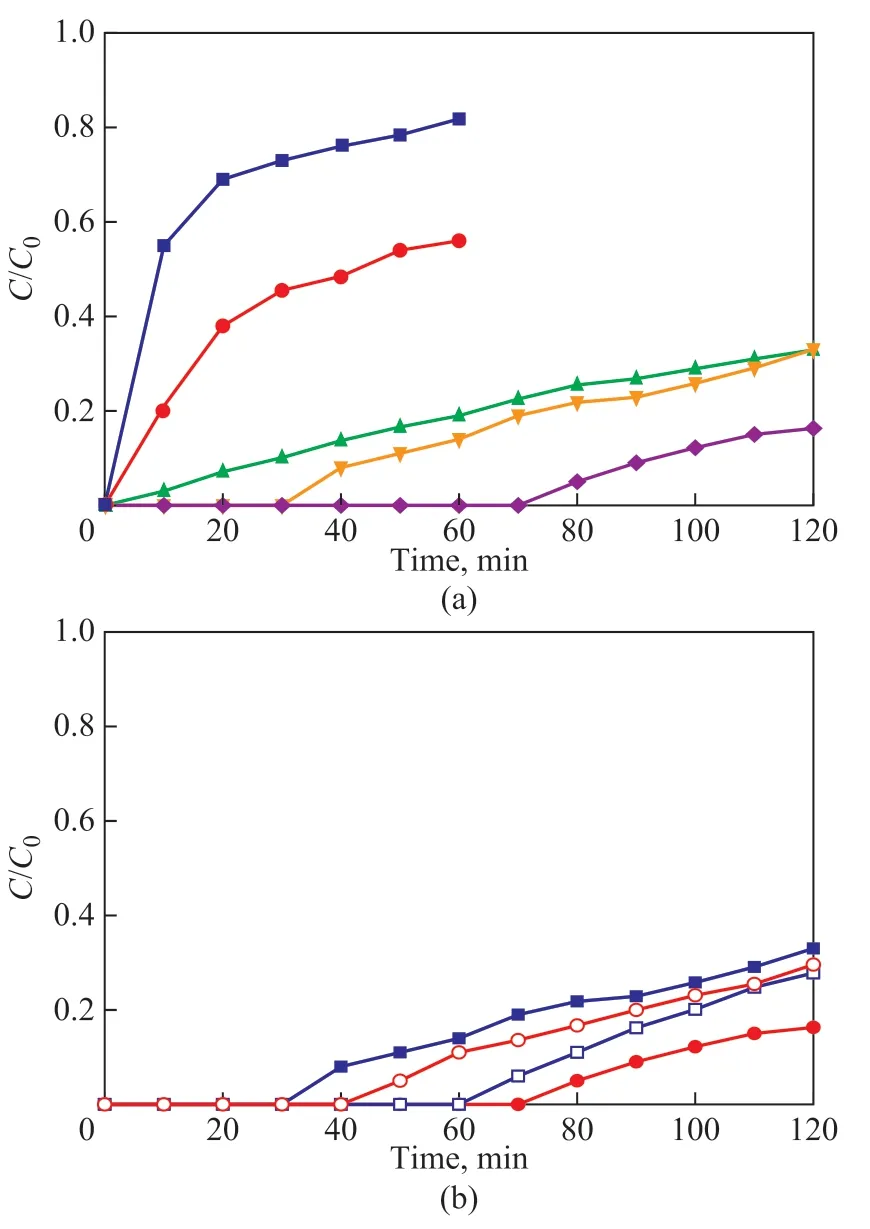
Figure 4 Breakthrough curves of H2S adsorption by the sorbents with different metals (a) and halogens (b) at 20 C with a loading amount of 5%(a) ■—silica gel; ●—[Bmim]Cl/silica gel; ▲—[Bmim]Cl.ZnCl2/silica gel;▼—[Bmim]Cl.FeCl3/silica gel; ◆—[Bmim]Cu.CuCl2/silica gel;(b) ■—[Bmim]Cl.FeCl3/silica gel; ●—[Bmim]Cl.CuCl2/silica gel;□—[Bmim]Br.FeCl3/silica gel; ○—[Bmim]Br.CuCl2/silica gel
3.2.3 Effect of adsorption temperature on H2S adsorption
The breakthrough curves of 5% [Bmim]Cl·CuCl2/silica gel at various mild temperatures (from 10 C to 50 C) are presented in Figure 6. It can be seen that the breakthrough time is almost the same as that obtained at temperatures ranging from 20 C to 50 C. The effect of adsorption temperature on H2S removal depends on some factors[8],since the interaction between H2S and ionic liquid will be stronger as the temperature increases. The chemical reaction rate will be enhanced as the temperature increases,which can improve the mass transfer between H2S and[Bmim]Cl·CuCl2, and will shorten its reaction time.
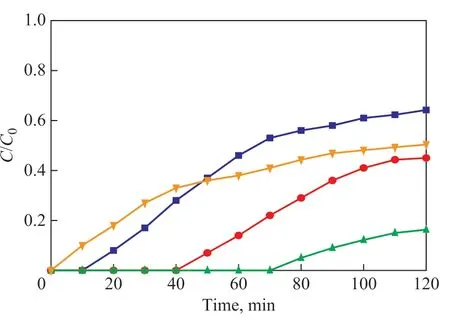
Figure 5 Breakthrough curves of H2S adsorption by [Bmim]Cl·CuCl2/silica gel with different loading amounts at 20 C■—loading amount of 1%; ●—loading amount of 3%;▲—loading amount of 5%; ▼—loading amount of 7%
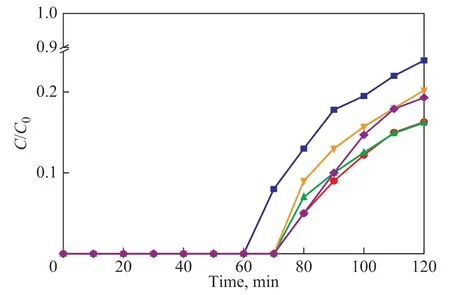
Figure 6 Breakthrough curves of H2S adsorption by 5% [Bmim]Cl·CuCl2/silica gel at different desulfurization temperatures■—10 C; ●—20 C; ▲—30 C; ▼—40 C; ◆—50 C
3.3 Regeneration of [Bmim]Cl·CuCl2/silica gel
After thorough adsorption, the possibility of recycling[Bmim]Cl·CuCl2/silica gel was investigated. Sulfur in the adsorbent was extracted by CS2, and the sulfurremoving adsorbent was dried in vacuum at 50 C.The regeneration of the sorbent was conducted at room temperature by the moving air stream at a flow rate of 100 mL/min for 5 h. The duration of regeneration could be determined according to the colour of the sample after adsorption (black brown), which could be fully recovered to the color of bulk original sample (light green), or could be generally turned into a colour of dark green (Figure 7).
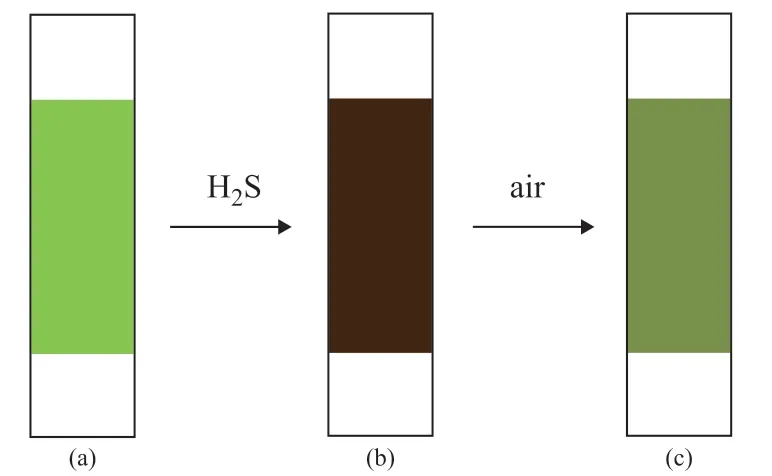
Figure 7 Schematic of the change of H2S in [Bmim]Cl·CuCl2/silica gel (a), [Bmim]Cl·CuCl2/silica gel + H2S (b),and [Bmim]Cl·CuCl2/silica gel (H2S) + air (c)
The regeneration performance of air-sweeping reached the best at 5 h, and a longer time showed no improvement in regeneration. The H2S removal capacity for reabsorption after several cycles is shown in Figure 8. The breakthrough time was reduced from 66 min (first adsorption) to 32 min, 30 min, and 23 min after three regeneration cycles, which indicated that the H2S removal capacity was weakened after the incomplete regeneration of the sorbent inside the silica gel, but the breakthrough time of pristine adsorbent could still reach more than 23 min after the fourth adsorption.
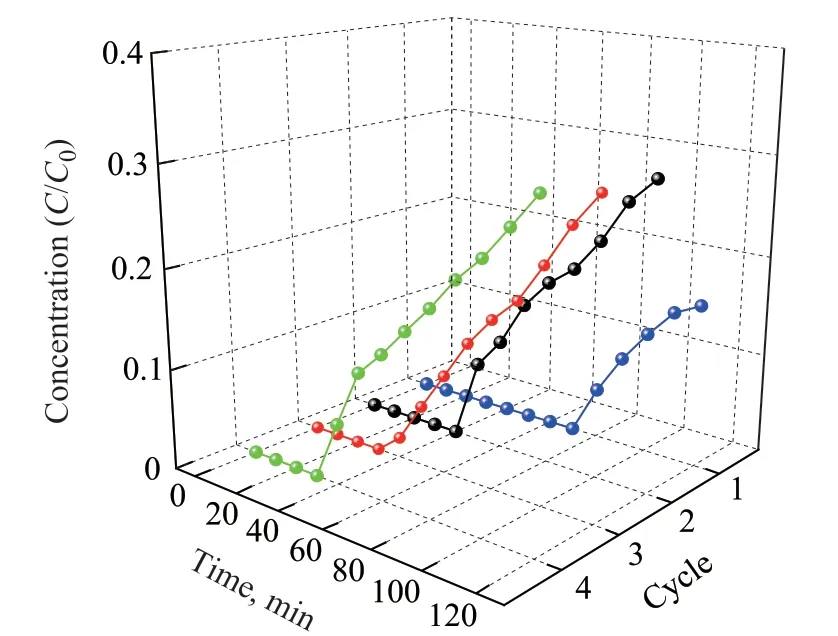
Figure 8 Regeneration performance of [Bmim]Cl·CuCl2/silica gel
3.4 Desulfurization product
To study the chemical reaction between H2S and [Bmim]Cl·CuCl2, the XPS analysis was used to further reveal the sulfur composition in the desulfurized product. Similar to other studies[24], there was no signal observed in XPS spectra of S, because a small amount of sulfur was out of position in the sorbent, so Cu in the [Bmim]Cl·CuCl2/silica gel was studied by XPS as shown in Figure 9. Cu2+was confirmed by the Cu 2p3/2 binding energy within the range of 931 —937 eV and the existence of the shake-up satellite contribution, and Cu+was assigned to the binding energy of the XPS contribution ranging from 931 eV to 933 eV without any shake-up satellite contribution[27]. In the Cu 2p3/2 spectra of [Bmim]Cl·CuCl2/silica gel before adsorption, only one contribution was anticipated and the binding energy was 934.6 eV, which could be assigned to Cu2+[28]. As for [Bmim]Cl·CuCl2/silica gel after adsorption, the binding energy value of the Cu 2p3/2 shifted to a lower value around 932.5 eV that was fitted to two binding energy values of 933.8 eV and 931.0 eV, which were assigned to Cu2+and Cu+, respectively. This shift might be attributed to the transfer of electrons from H2S to Cu2+,which changed the electronic environment of Cu2+, and Cu2+was reduced to Cu+. As regards the regenerated Cu 2p3/2, the binding energy returned to 934.5 eV, or a higher value of 943.6 eV, indicating that Cu+was oxidized to Cu2+by O2from the air[29].
Figure 10 shows the thermogravimetric profiles of 5%[Bmim]Cl·CuCl2/silica gel (before and after adsorption)at temperatures ranging from ambient temperature to 800 C at a temperature increase rate of 10 C/min in nitrogen atmosphere. The physically adsorbed water was removed completely by further heating to about 100 C.Inspection of the TGA results for both samples revealed a small, but noticeable thermal event in the wide temperature region from 100 C to 800 C. This could be attributed to dehydroxylation of the silica surface, in which the silanol groups were condensed to siloxanes,which was related to a process capable of occurring in this thermal region. The modified ionic liquid on SiO2showed two very distinct thermal events, with one occurring in the range of 100—400 C and the other —in the range of 400—600 C[30]. The first of these two regions is located at where the organic moieties on the surface are expected to be thermally decomposed. The second one is associated with the decomposition of the residual methoxy side groups. As for [Bmim]Cl·CuCl2,the temperature from 100—600 C is divided into much more regions. For the sample before adsorption, the regions are identified at 100—200 C, 200—300 C,300—375 C, and 375—600 C. For the sample after adsorption, the regions are found at 100—200 C, 200—300 C, 300—440 C, and 440—600 C. CuCl2evaporates between 350 C and 550 C. CuCl melts and vaporizes at 420 C, and loses most of the weight between 410 C and 560 C[31]. Therefore, the loss of weight at 440 C indicates that there is CuCl in the sample, so that the oxidation of H2S to form S was verified.
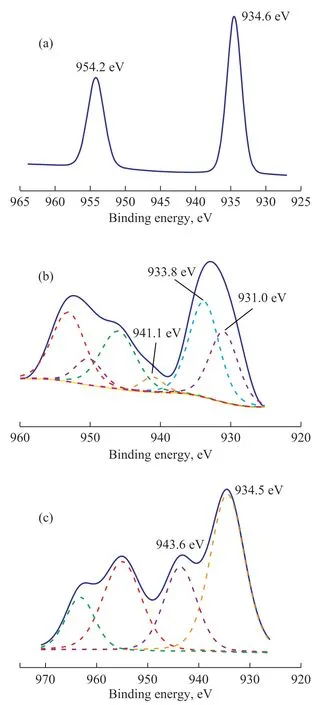
Figure 9 XPS results for Cu 2p1/2 and Cu 2p3/2 over original (a), adsorbed (b), and regenerated (c) [Bmim]Cl·CuCl2/silica gel
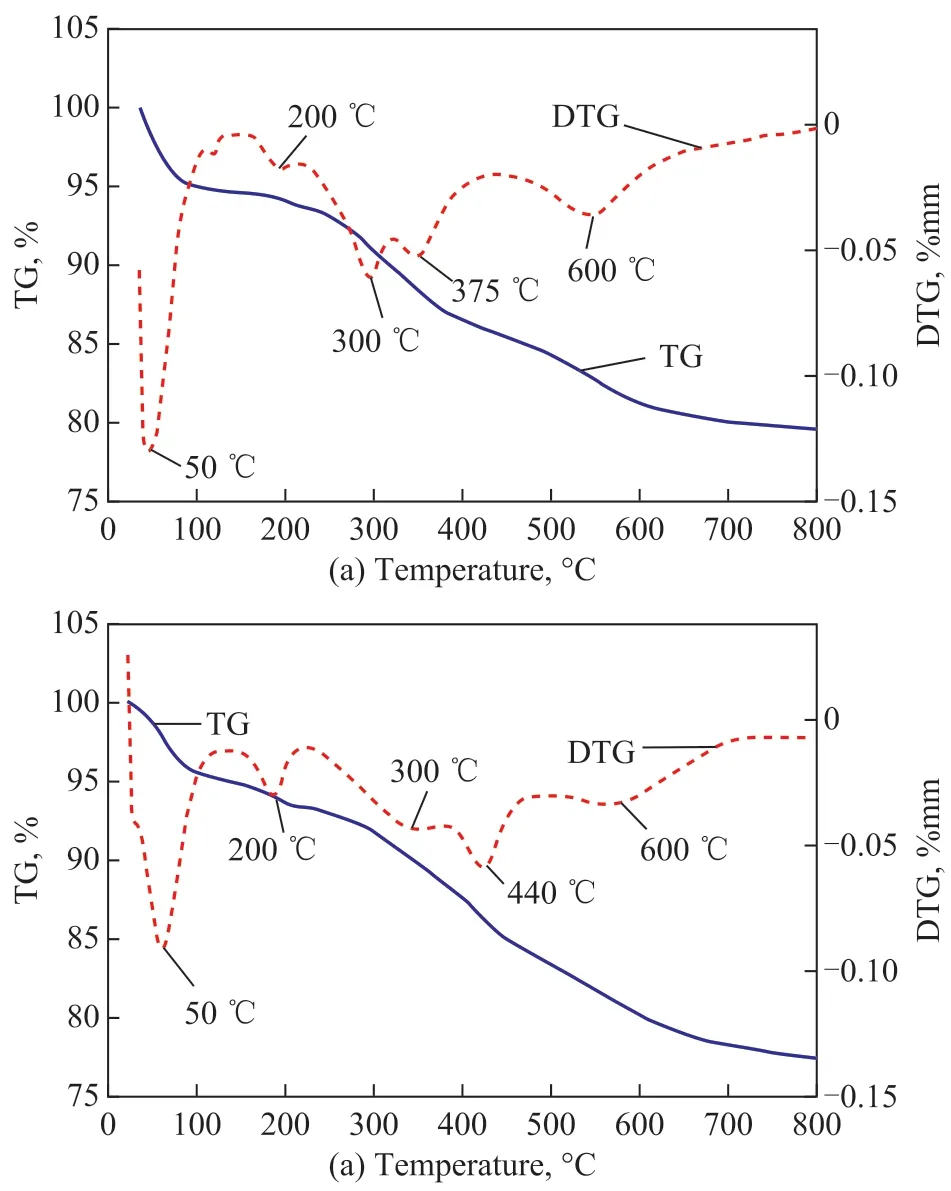
Figure 10 TG-DTG spectra of [Bmim]Cl·CuCl2/silica gel before asorption (a) and after (b) adsorption
4 Conclusions
An innovative approach to H2S capture via the high-viscosity ionic liquids at low temperature had been developed using several metal-based (Cu-, Fe- and Zn-) ionic liquids that were immobilized on silica by the sol-gel method. Being superior to purely viscous ionic liquid system, the ionic liquid solid system [Bmim]Cl·CuCl2/silica gel showed an excellent performence on H2S removal at mild temperature, and possessed good regeneration capacity. The synthesized IL/silica adsorbents were characterized by FTIR, TEM, N2adsorption/desorption, XPS,and TG/DTG techniques. The best desulfurization conditions covered a loading amount of 5% [Bmim]Cl·CuCl2,a mild desulfurization temperature ranging from 20 C to 50 C, and a gas flow rate of 100 mL/min. Compared with H2S removal process by using pure ionic liquid, the excellent adsorption by sol-gel-derived silica described in this study might be attributed to the formation of nanoscaled and high-concentration ionic liquids due to the confinement of [Bmim]Cl·CuCl2in silica gel, which could avoid the disadvantages of pure ionic liquid. H2S could be oxidized to elemental sulfur according to the results of XPS and TGA analyses. By menas of the sweeping air, [Bmim]Cl·CuCl2/silica gel could be recycled at least three times with an acceptable decline of H2S removal capacity. The IL/silica gel system could provide a promising approach for high-viscocity ionic liquid desulfurization of H2S from natural gas at mild temperature. However, the study is still in the laboratory stage and needs further study before practical application.
Acknowledgments:This work was financially supported by the Research and Development Program of the Ministry of Science and Technology of China (Grant No. 2014BAC28B01) and the Jiangsu Key Laboratory of Anaerobic Biotechnology (Jiangnan University) Supported Research Project (No. JKLAB201703).
杂志排行
中国炼油与石油化工的其它文章
- Novel NiMo Catalysts Supported on Sol-Gel Nanosized HY Zeolite-Alumina Composites for Hydrodesulfurization of Diesel
- Kinetic Model of Hydrogenation for Removal of Trace Olefins from Alkylation Mixture Formed during Linear Alkylbenzene Synthesis
- Influence of Cr3+ Concentration on SO2 Removal over TiO2 Based Multi-walled Carbon Nanotubes
- Phosphorous-Modified Carbon Nanotube-Supported Pt Nanoparticles for Propane Dehydrogenation Reaction
- Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
- Polycrystalline Phase WO3/g-C3N4 as a High Efficient Catalyst for Removal of DBT in Model Oil
