Catalytic Cracking Characteristics of Plant Oil for Producing Light Olefins and Light Aromatics
2019-05-10ChengXiaojie
Cheng Xiaojie
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Catalyst containing shape selective zeolite is used to investigate the catalytic cracking characteristics of palm oil and three types of hydrocarbon VGOs on a fixed fluidized bed (FFB) unit. The advantage of producing light olefins and light aromatics by catalytic cracking of plant oil is discussed. Results indicate that the hydrocarbyl group of the plant oil molecule is quite readily crackable; the C6—C8 aromatics yield is well above and the light olefins yield is about the same with the hydrocarbon feeds, while the yields of low value products are lower; the hydrocarbyl group of the plant oil molecule has strong tendency of aromatization, and can enter the zeolite pores to selectively form C6—C8 aromatics; during catalytic cracking of plant oil and fatty acids, a portion of the oxygen is removed in the form of water through hydrogen transfer reaction, while olefins are prevented from being saturated, which can ensure proper yields of both low-carbon olefins and light aromatics.
Key words: plant oil; catalytic cracking; hydrogen-transfer; light olefins; light aromatics
1 Introduction
Carbohydrates such as cellulose, starch, and plant oil are composed of carbon, hydrogen and oxygen, all of which are produced by plants through photosynthesis. They are renewable, clean, and widely distributed, featuring low-carbon emission, abundance of resources, and huge energy availability. They not only can serve as food for human being, but also are being increasingly used in the chemical industry in recent years[1]. By now, people can produce bio-gasoline, biodiesel, bio-jet fuel and a variety of biological chemicals from biomass, and more new applications are continuously being explored.
Researches on biomass started early in Europe, the United States, and other developed countries. Biomass refining has long been industrialized, and is still a hotspot for study[2]. However, the research on biomass utilization abroad mainly focuses on the production of biomass-based fuel[3-7]. China also attaches great importance to the research and development of biomass utilization. Benefiting from the trend of global plant oil market oversupply in recent years and the policy support from the government for clean and renewable energy, the application of biomass as raw material for manufacturing fuel and chemical products has become more extensive[8-10]. On the other hand, the disposal of waste oil rich in animal or plant oils, such as waste cooking oil, has forced people to speed up the research in related fields. Utilization of animal and plant oils to produce fuel and chemical products is a convenient way to the era of “carbohydrate”, which includes not only the trans-esterification between the plant oil and methanol to produce bio-fuel, but also the refining process similar to the way, by which petroleum is refined to produce fuel or chemical products.
The main components of plant oil are triglycerides formed by esterification of glycerol and fatty acids. The plant oil molecules contain a large amount of long-chain fatty acids, the hydrocarbyl group of which is similar to the paraffinic VGO. It is well-known that paraffinic VGO is the ideal feedstock to produce light olefins through catalytic cracking process. Therefore, it is worth studying the feasibility of plant oil on taking the place of petroleum to produce chemical products. On the basis of the existing studies[11-13], by using palm oil as the feedstock and combining with the experimental study of model compounds, this study aims to compare the product distribution and yields of target products from plant oil with those from conventional petroleum hydrocarbon feedstocks so that the catalytic cracking characteristics of plant oil can be explored.
2 Experimental
2.1 Feedstocks and catalyst
In this study, palm oil (with a melting point of 24 C), #1 VGO, #2 VGO, and HTVGO are selected as feedstocks.They are respectively the representatives of plant oils,different types of vacuum gas oils and hydrotreated vacuum gas oil. The main properties of each feedstock are shown in Table 1.
It can be seen from Table 1 that the properties of palm oil have much in common with those of the petroleum feedstocks. Main difference lies in the content of oxygen,which in palm oil is much higher. The density of palm oil is higher than HTVGO and #1 VGO, and is similar to #2 VGO. The Conradson carbon residue of palm oil is higher than HTVGO and #1 VGO, but is lower than #2 VGO. In terms of elementary composition, the oxygen content of palm oil is 11.70%, and the sulfur and nitrogen content shows a trace amount. The nH/nCratio of the four feedstocks decreases in the following order: HTVGO >#1 VGO > palm oil > #2 VGO.
Fatty acid composition of the palm oil (with a melting point of 24oC) that is obtained by transesterification is shown in Table 2. It can be seen that the palm oil contains C14—C20fatty acids, and the unsaturation degree of the carbon chains is between 0—2. Oleic acid and palmitic acid (n-hexadecane acid) make up more than 80% of the total mass, with their mass fraction equating to 42.73% and 40.37%, respectively.Moreover, the mass fraction of linoleic acid and stearic acid accounts for more than 1%, too.
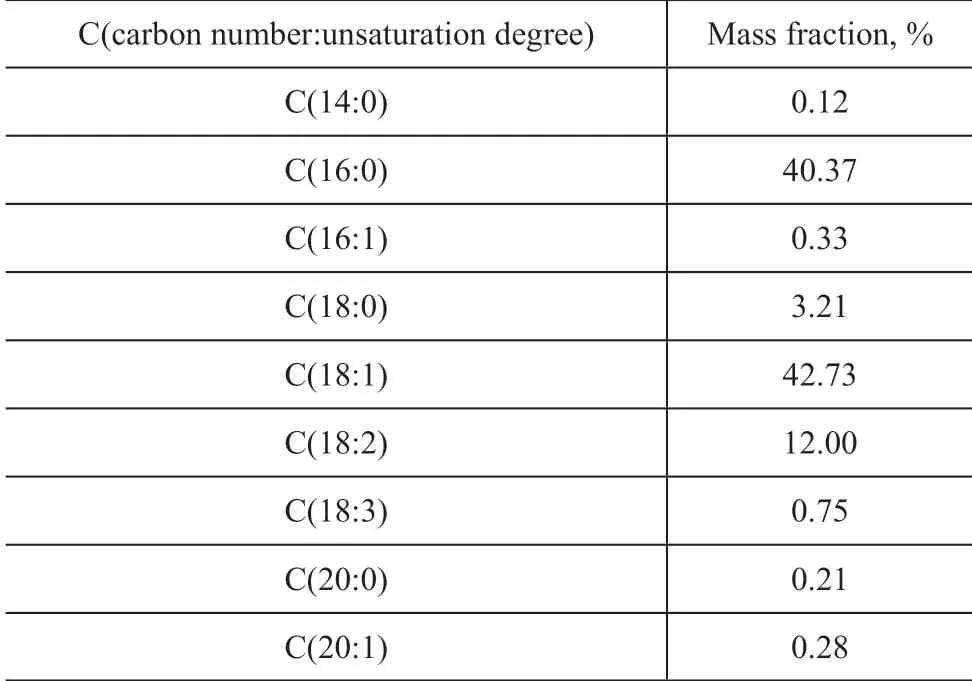
Table 2 Fatty acid composition of the 24 oC palm oil
According to the fatty acid composition of palm oil,model compounds are selected, which are n-hexadecane,octadecene, oleic acid, palmitic acid and stearic acid, with their purity being higher than 97%.
The catalyst used is a lab-made model catalyst containing a single zeolite, which is labeled as ZSP-2.
2.2 Data processing method
Light olefins refer to C2—C4olefins, and light aromatic hydrocarbons refer to C6—C8aromatics in this study.
During the catalytic cracking process of plant oil, almost all oxygen atoms are removed in the form of CO, CO2and H2O, and the deoxidation degree increases with the conversion rate. Since deep catalytic cracking occurs at a high reaction temperature and has a relatively high conversion level, it is assumed in this study that all oxygen atoms are converted into CO, CO2, and H2O. The yield of H2O in this study was obtained by analyzing the CO and CO2contents in the cracking gas and by calculating the mass balance of oxygen element in the feedstock and products.
Due to the particularity of oxygen-containing compounds,the hydrocarbyl group of palm oil was taken as the main research object. The experimental results were discussedbased on the same hydrocarbyl mass. The so-called“hydrocarbyl mass” is defined as follows: Since almost all oxygen atoms in palm oil molecules are removed in the form of inorganic compounds, they are not recorded as the feedstock. For example, when the mass of palm oil is 100 g, its hydrocarbyl mass is 88.3 g. For the convenience in discussing the reaction pathway, the amount of hydrocarbon feedstock is specified as 88.3 g.
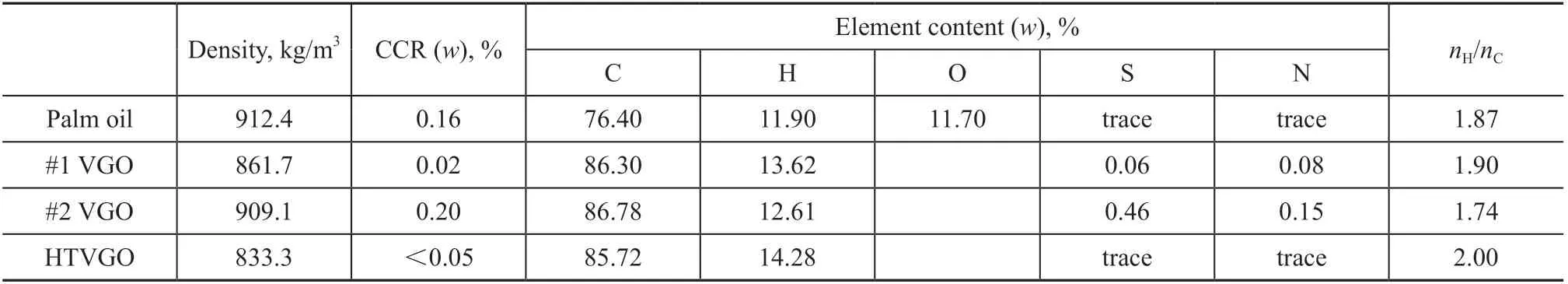
Table 1 Main properties of the feedstocks
3 Results and Discussion
3.1 Distribution of catalytic cracking products
On a bench scale fixed fluidized bed (FFB) unit, catalytic cracking reactions of palm oil, HTVGO, #1 VGO and #2 VGO were carried out at a reaction temperature of 550oC.The distribution of products obtained from catalytic cracking of palm oil was compared with that emanated from hydrocarbon feedstocks, with the results listed in Table 3.
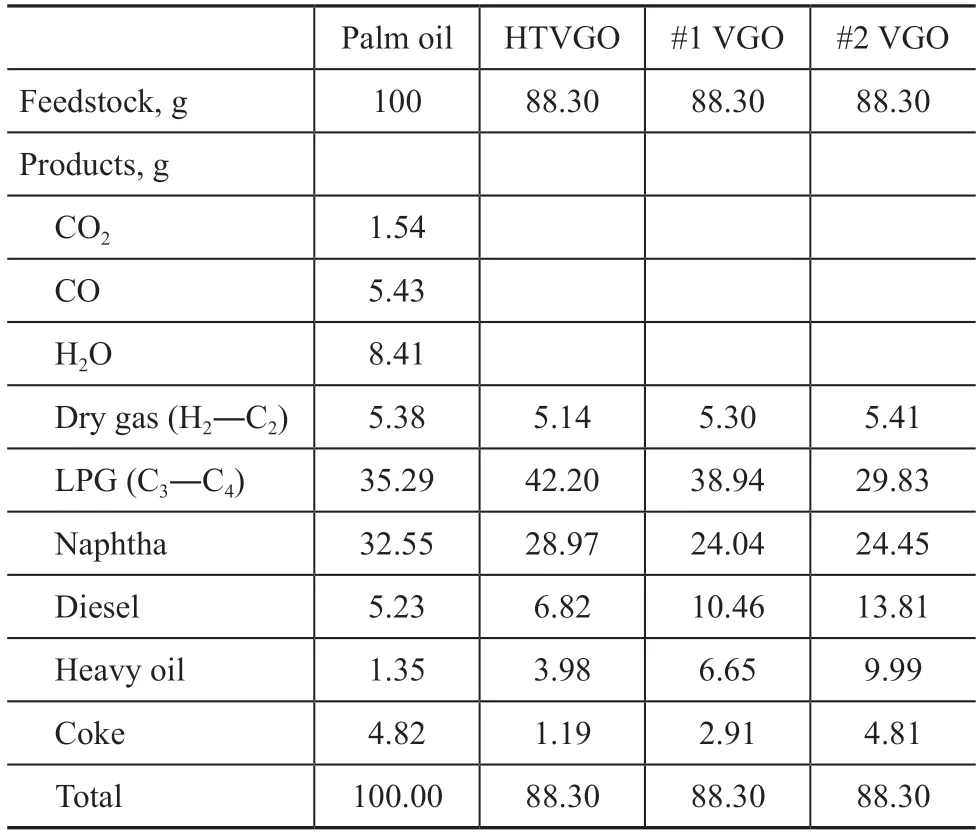
Table 3 Distribution of catalytic cracking products
The production of water was the highest among the three deoxygenating products. By calculation, the oxygen atoms removed in the form of water accounted for 63.88% of the total oxygen in the palm oil. At the same hydrocarbyl mass, there was little difference in the yield of dry gas components obtained from the catalytic cracking of four feedstocks. The LPG yield from different feedstocks decreased in the following order: HTVGO > #1 VGO >palm oil > #2 VGO. The yield of naphtha from palm oil was significantly higher than that from three hydrocarbon feedstocks, while HTVGO demonstrated slightly higher naphtha yield than the two straight-run VGO feedstocks.The yield of diesel and heavy oil distillates from different feedstocks decreased in the following order: #2 VGO>#1 VGO > HTVGO > palm oil. The coke yield from palm oil was higher than HTVGO and #1 VGO, and was close to that from #2 VGO. It can be seen that compared with hydrocarbon feedstocks, palm oil can produce more gas products and light oil products, while the yield of other low-value products, such as diesel and heavy oil, is lower. Since the oxygen atoms contained in palm oil are removed in the form of CO, CO2and H2O, which also contain carbon and hydrogen atoms, therefore some mass loss is inevitable. The loss is usually 3%-5% under the reaction conditions of this study.
3.2 Catalytic cracking of plant oil to produce light olefins
Figure 1 shows the production of light olefins obtained from the catalytic cracking of the four feedstocks.The total production of light olefins obtained from the catalytic cracking of 100 g of palm oil is 35.84 g, and that from the catalytic cracking of 88.3 g of HTVGO and#1 VGO is 37.77 g and 36.21 g, respectively. Among them, the ethylene and propylene production from different feedstock decreases in the following order:HTVGO>palm oil>#1 VGO>#2 VGO. The results show that the hydrocarbyl group in palm oil is capable of producing large amount of light olefins in the process of catalytic cracking, just like those hydrocarbons with high degree of saturation. Since the hydrocarbyl groups of plant oils are entirely in a normal form, they have the inherent advantage of producing light olefins according to the carbocation mechanism. If the catalytic materials and operating parameters are further optimized, it is expected to obtain higher yield of light olefins from palm oil.
Figure 2 shows the relationship between P(propylene)/P(ethylene) and hydrogen content of the feedstocks.According to the preceding part of the text, the nH/nCratio in the four feedstocks decreases in the following order: HTVGO> #1 VGO> palm oil> #2 VGO. It can be seen from Figure 2 that P(propylene)/P(ethylene) ratio of three non-hydrogenated feedstocks, including palm oil, increases significantly with the increase of the ratio of nH/nCin feedstocks, while P(propylene)/P(ethylene) in the hydrogenated oil is not only affected by the ratio of H/C atoms, but is also related to the types of the crude oil before hydrogenation. Palm oil is also identified on the curve. Although the productions of ethylene and propylene from palm oil are both slightly higher than that from #1 VGO, the lower P(propylene)/P(ethylene) ratio indicates that the trend of palm oil on producing ethylene is slightly stronger than that of the latter.
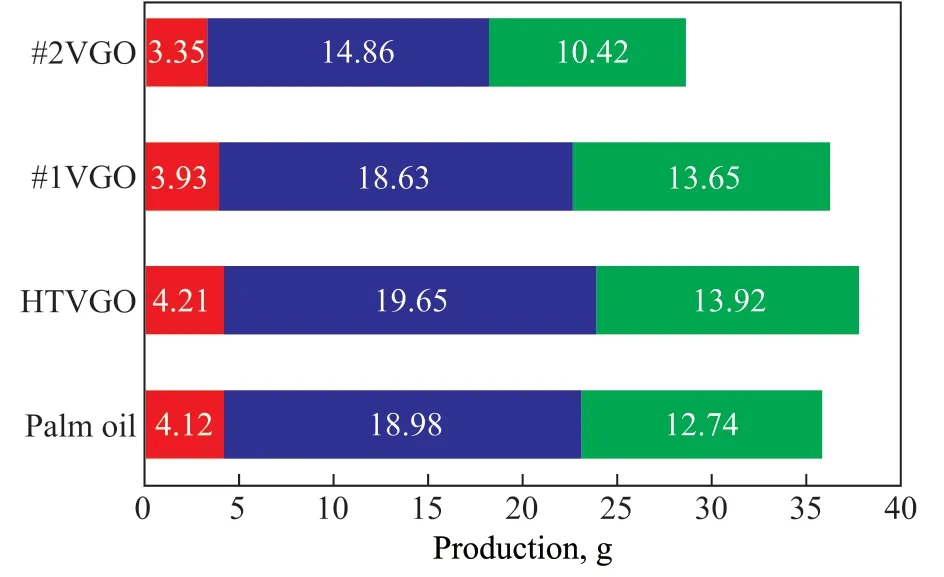
Figure 1 The production of light olefins of different feedstocks■—Ethylene; ■—Propylene; ■—Butenes
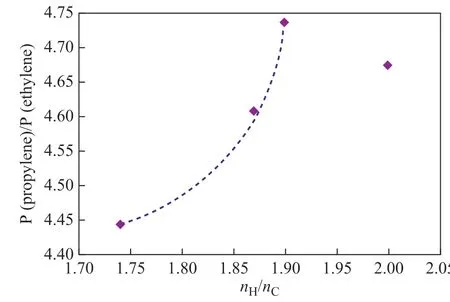
Figure 2 Relationship between P(propylene)/P(ethylene)and hydrogen content of feedstock
3.3 Catalytic cracking of plant oil to produce aromatics
The PIONA compositions of naphtha from different feedstocks were analyzed, with the results shown in Figure 3. The naphtha’s PIONA composition of the three hydrocarbon feedstocks has a notable regularity, which means the hydrocarbon feedstocks with higher hydrogen content (such as HTVGO and #1 VGO) tend to produce more normal and isomeric paraffins, and the feedstocks with lower hydrogen content tend to produce more olefins and aromatics. However, palm oil with a moderate nH/nCratio shows unique characteristics, and the mass fraction of aromatic hydrocarbons is as high as 56.04%, which is far higher than that obtained from other feedstocks, while all mass fractions of n-paraffins, iso-paraffins, olefins, and naphthenes emanated from the palm oil are much lower than those obtained from other three feedstocks.
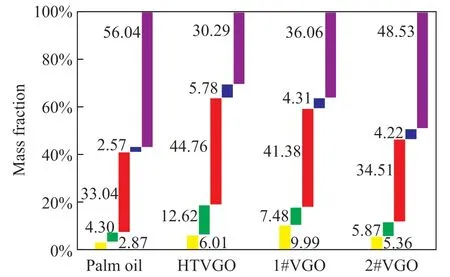
Figure 3 PIONA composition of naphtha of differentfeedstocks■—Paraffin; ■—Iso-paraffin; ■—Olefin; ■—Naphthene; ■—Aromatics
Table 4 shows the carbon number distribution of aromatics in naphtha obtained from different feedstocks.The aromatics content in naphtha decreases in the following order: palm oil> #2 VGO> #1 VGO>HTVGO.The aromatic hydrocarbon composition of naphtha from the three hydrocarbon feedstocks also shows regularity.The mass fractions of C6—C11aromatic hydrocarbons are positively correlated with the hydrogen content of the feedstocks, and the aromatic hydrocarbons in naphtha have similar distribution proportions in different carbon numbers, while palm oil is quite different. The mass fractions of C9—C11heavy aromatic hydrocarbons in the naphtha from the palm oil lie between those of #1 VGO and #2 VGO. The main reason for the increase of total aromatics in palm oil-based cracking naphtha is that the content of C6—C8light aromatic hydrocarbons is significantly higher than those of other three types of naphtha. The benzene content in naphtha from palm oil is 2.63 times as much as that of #2 VGO,the toluene content is 1.82 times as much as the latter,the content of C8aromatic hydrocarbons is 1.38 times as much as the latter, and the total content of C9—C11aromatic hydrocarbons is lower than the latter. It can be seen that the smaller the molecular weight of aromatic hydrocarbons is, the more significant the advantage of the palm oil would be.

Table.4 Carbon number distribution of aromatics in naphthaw, %
Figure 4 further shows the production of light aromatic hydrocarbons from different feedstocks. The total light aromatic production obtained from 100 g of palm oil is 8.85 g, and that obtained from 88.3 g of HTVGO,#1 VGO, and #2 VGO is 2.45 g, 2.29 g, and 3.30 g,respectively. The production of aromatic hydrocarbons from palm oil is much higher than that obtained from hydrocarbon feedstocks. The palm oil especially can produce more benzene and toluene.

Figure 4 Production of light aromatic hydrocarbons from different feedstocks■—Palm oil; ■—HTVGO; ■—#1VGO; ■—#2VGO
The differences in product distribution are usually ascribed to the structural characteristics of feedstocks.Hydrocarbon feedstocks contain some aromatics and naphthenes, which can generate light aromatics through cracking and hydrogen transfer reactions. Being different from the hydrocarbon feedstocks, the hydrocarbyl in palm oil is completely aliphatic. Therefore, the aromatic hydrocarbons in the products can only be generated by cyclization of aliphatic groups.
The highly linearized hydrocarbyls of the palm oil are much easier than isomeric and cyclic hydrocarbons to enter the pores of shape-selective zeolite, so that the shape-selective effect of zeolite channel improves the selectivity of light aromatics, and cracks the long side chains of aromatic hydrocarbons to generate light olefins and light aromatics, while sometimes ethylene is also generated at the same time by the β-scission (e.g., from propyl benzene), which may explain why the tendency of palm oil to produce ethylene is slightly higher than that of hydrocarbon feedstocks. In conclusion, the palm oil has a unique advantage in the production of light aromatic hydrocarbons because of its double-bonded straight chain structure.
3.4 Hydrogen transfer reaction in catalytic cracking of plant oil
When hydrogen transfer reaction occurs between two molecules, usually one molecule loses hydrogen atoms and its unsaturation degree increases, while the other molecule gains hydrogen and its unsaturation degree decreases, which is reflected in the product distribution showing that the olefin content decreases coupled with the increase in the aromatic hydrocarbon and alkane contents.Therefore, when we focus on the yields of light olefins and light aromatics, the discussion of hydrogen transfer cannot be avoided. In the process of producing light olefins we often attempt to reduce the hydrogen transfer reaction, but the generation of aromatic hydrocarbons depends on the hydrogen transfer reaction. The catalytic cracking of plant oil can achieve high yields of light olefins and light aromatics.
The olefincity of LPG reflects the level of hydrogen transfer reaction. Figure 5 shows the relationship between the production and olefincity of LPG obtained during the catalytic cracking of 88.3 g of hydrocarbon feedstocks with different nH/nCratio of feedstocks. It can be seen that both of the production and olefincity have an approximately linear relationship with the nH/nCratio of feedstocks. Along with the increase of nH/nCratio in the feedstocks, the production of LPG increases while the olefincity of LPG decreases.
Then the production and olefinicity of LPG obtained during catalytic cracking of palm oil are also marked in Figure 5. It can be concluded that there is a positive correlation between the LPG production and nH/nCratio of feedstocks, which not only applies to hydrocarbon feedstocks, but also to palm oil.
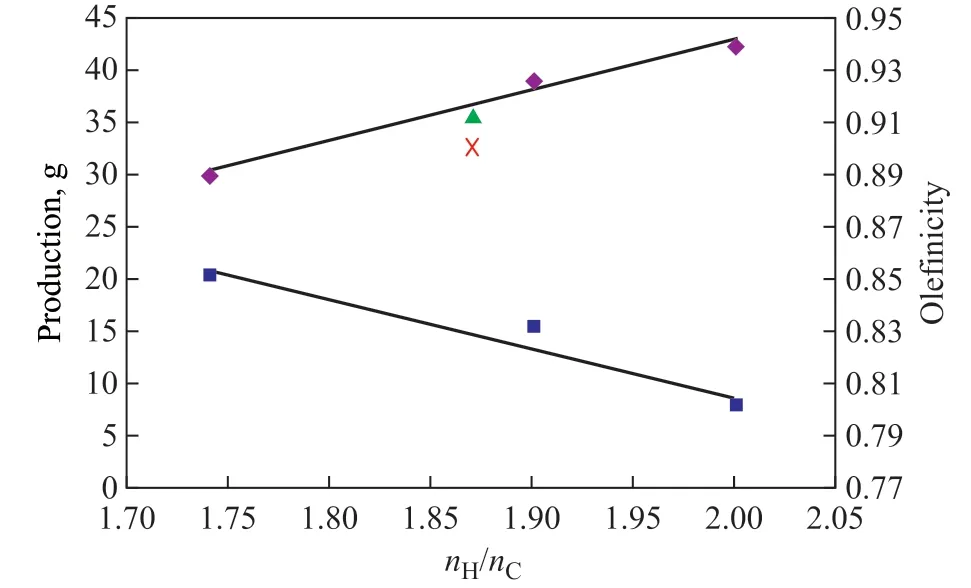
Figure 5 Relationship between production and olefincity of LPG and nH/nC of feedstocks◆—LPG production of hydrocarbon feed; ▲—LPG production of palm oil;■—LPG olefincity of hydrocarbon feed; X—LPG olefincity of palm oil
Subsequently, similar phenomena are also observed in the catalytic cracking of several fatty acid model compounds,as shown in Figure 6. Figure 6 shows that the production ratio of propane to propylene and that of iso-butane to iso-butene emanated from every hydrocarbon feedstock,including the VGOs and model compounds, are greater than 0.1 and 0.4, respectively, while those values of fatty acids and vegetable oil feedstocks are all below 0.1 and 0.4, respectively. The author calls it “the phenomenon of 0.1 & 0.4”.
Upon taking the n-hexadecane and palmitic acid as examples, the production ratio of alkanes to alkenes in LPG is significantly different from the above-mentioned two cases, which can be easily related to the presence of carboxyl groups.
As regards the hydrocarbon feedstocks, the hydrogen transfer reaction is divided into two steps, namely: the olefin accepts a proton to form a carbocation; then the carbocation extracts a hydrogen anion from the hydrogen donor molecule to form an alkane, and the donor finally forms an aromatic hydrocarbon. The charge distribution of palmitic acid and oleic acid is shown in Figure 7, in which the C=C bond of oleic acid acts as a weak negative center, and by contrast, the carboxyl oxygen atom forms a much stronger one. Because of the existence of the stronger negative charge center, protons preferentially combine with carboxyl group to form a carbocation, the carbocation binds to a hydrogen anion and then removes the oxygen in the form of water. It can be seen that, being different from hydrocarbon cracking reaction, hydrogen transfer is involved in the process of deoxidation of vegetable oil, and the hydrogen receptor would change from olefin to carboxyl group, thus generating a large number of aromatic hydrocarbons, while also preserving the olefin generated during cracking reaction.
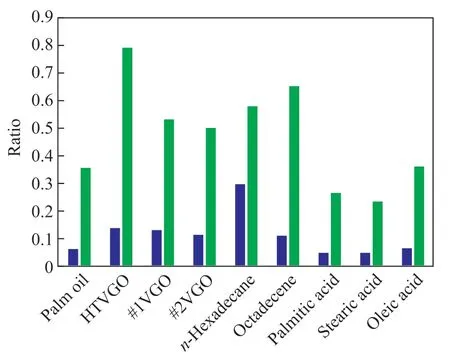
Figure 6 Production ratio of alkanes to olefins in LPG emanated from different feedstocks■—P(propane)/P(propylene); ■—P(iso-Butane)/P(iso-Butene)
4 Conclusions
(1) Compared with hydrocarbon feedstocks, the hydrocarbyl part of palm oil is quite readily crackable and tends to produce more gas product and naphtha product,along with relatively low production of diesel, heavy oil and other low-valued products.
(2) The production of ethylene and propylene obtained from catalytic cracking of the palm oil hydrocarbyl groups is slightly higher than that from the paraffinic VGO, while the production ratio of propylene to ethylene is lower than the latter.
(3) The structure of palm oil molecule is beneficial to being treated over the shape-selective zeolite, since the output of light aromatics is significantly higher than that of hydrocarbon feedstocks, in particular, more benzene and toluene can be formed.
(4) A portion of oxygen in plant oil is removed through hydrogen transfer reaction in the form of water, and at the same time the olefin is prevented from being saturated,which is beneficial to enhancing the yields of light olefins and light aromatics.
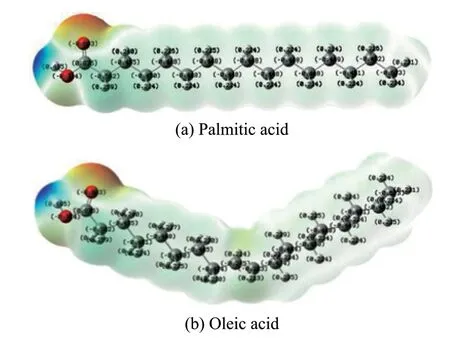
Figure 7 The charge distribution of fatty acids
Acknowledgement:This work was financially supported by the SINOPEC Research and Development Project (Contact No.115010).
杂志排行
中国炼油与石油化工的其它文章
- Novel NiMo Catalysts Supported on Sol-Gel Nanosized HY Zeolite-Alumina Composites for Hydrodesulfurization of Diesel
- Kinetic Model of Hydrogenation for Removal of Trace Olefins from Alkylation Mixture Formed during Linear Alkylbenzene Synthesis
- Influence of Cr3+ Concentration on SO2 Removal over TiO2 Based Multi-walled Carbon Nanotubes
- Phosphorous-Modified Carbon Nanotube-Supported Pt Nanoparticles for Propane Dehydrogenation Reaction
- Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
- Polycrystalline Phase WO3/g-C3N4 as a High Efficient Catalyst for Removal of DBT in Model Oil
