Progress in the Application of X Zeolite in Adsorption
2019-05-10WangYubingWangHuiguo
Wang Yubing; Wang Huiguo
(1. SINOPEC Material and Equipment Department, Beijing 100728;2. SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: The application of X zeolite in the areas for producing para-xylene by adsorptive separation, N2/O2 separation,etc., was reviewed, and the framework SiO2/Al2O3 molar ratio, the cations and the water content of X zeolite significantly affected its selectivity and capacity. As the active component of para-xylene adsorbent, the X zeolite with a framework SiO2/Al2O3 molar ratio of 2.2—2.4 coupled with cations of Ba2+ and K+, as well as a water content of 4.0%—5.0% exhibited higher para-xylene selectivity. For N2/O2 separation, higher N2 capacity and N2/O2 separation factor were achieved when the X zeolite had a framework SiO2/Al2O3 molar ratio of 2.0 coupled with Li+ cations. The introduction of another cation in X zeolite could further increase the N2/O2 separation factor, while the adsorption of water, however, could lead to a remarkable decrease of N2 capacity. Besides, the X zeolite could be used in adsorptive separation of highly pure He and selective adsorption of CO2, CO, CH4, N2, Ar, and H2.
Key words: FAU; para-xylene; air; separation; selectivity
1 Introduction
X zeolite is a crystalline material with the FAU topological structure. It belongs to a hexagonal system and its space group is Fd-3m. The tetrahedrons of TO4(Tis Si or Al) in X zeolite crystals are interconnected to form double six-membered rings (D6R) and SOD cage units. The SOD cages are connected by D6R cages and extended to three-dimensional space to gradually form supercages and molecular sieve framework. The supercages in X zeolite have a size of 1.3 nm, and their windows are composed of 12-membered rings with a size of 0.74 nm[1-2].
Usually, X zeolite has a SiO2/Al2O3molar ratio of 2.0—3.0[3]. The X zeolites with a SiO2/Al2O3molar ratio of 2.0—2.2, 2.2—2.4, and 2.4—3.0, respectively, are called low SiO2/Al2O3ratio X zeolite, medium SiO2/Al2O3ratio X zeolite, and high SiO2/Al2O3ratio X zeolite, respectively. With a decreasing SiO2/Al2O3ratio, the number of cations in the cell crystal increases because the gradually increasing negative skeleton charges require more cations to reach a balance. Due to their low SiO2/Al2O3ratio and adjustable types and quantities of cations, X zeolites are widely used in the fields of adsorptive separation of p-xylene[4], N2/O2separation[5], etc.
2 Application of X Molecular Sieve in Adsorptive Separation of p-Xylene
Para-xylene is one of the important basic chemicals, and is usually produced from mixed C8aromatic hydrocarbons. Four C8aromatic hydrocarbon isomers, viz. p-xylene, m-xylene, o-xylene and ethylbenzene, have very small difference in boiling point, especially p-xylene and m-xylene have a difference in the boiling point of only 0.75 C. It is difficult to separate high purity p-xylene from mixed C8aromatic hydrocarbons by conventional distillation method. High purity p-xylene is industrially produced mainly via adsorptive separation by the adsorbent in a moving-bed reactor. The active component of the adsorbent is X zeolite[6].
Early in 1960s, R. N. Fleck, et al.[7]found that X zeolite exhibited a certain adsorption selectivity for C8aromatic hydrocarbon isomers. R. W. Neuzil, et al.[8]used X zeolites with different metal ions as cation sites for adsorptive separation of p-xylene. The test results showed that the metal cations in X zeolites significantly influenced their adsorption selectivity. BaX zeolites had high p-xylene/ethylbenzene separation coefficient and low p-xylene/m-xylene separation coefficient. However, when Ba2+and K+coexisted, X zeolites showed high p-xylene/m-xylene,p-xylene/o-xylene, and p-xylene/ethylbenzene separation coefficients. Zhao Tongfu, et al.[9]used the Rieveld method to refine the parameters in their results based on the diffraction data of BaX zeolite powder and believed that Ba2+cations were mainly distributed at SII in supercages and SI’ in β cages, and a small amount of residual Na+cations remained at SI; Ba2+at SI’ and Na+at SI stabilized the framework structure of the molecular sieve; H2O was mainly located in supercages and at SII’; if an appropriate amount of H2O is coordinated with Ba2+, Ba2+had the same absorptive force field symmetry as p-xylene molecule and therefore the zeolite showed the ability to selectively adsorb p-xylene.
In addition to metal cations, the framework SiO2/Al2O3ratio and water content of X-zeolite affected the adsorption selectivity, which had been systematically studied by the researchers at the SINOPEC Research Institute of Petroleum Processing. The curve of separation coefficient vs. water content of BaX zeolite is shown in Figure 1. With an increasing water content in BaX zeolite ranging from 2.0% to 8.0%, the separation coefficient,β, for p-xylene increased at first and then decreased, as compared with the other three C8aromatic hydrocarbon isomers. The BaX zeolite had the highest adsorption selectivity for p-xylene at a water content of 4.0%—5.0%.The curve of separation coefficient vs. framework SiO2/Al2O3ratio of BaX zeolite is shown in Figure 2. With an increasing SiO2/Al2O3ratio ranging from 2.01 to 2.53,the separation coefficients for p-xylene/m-xylene(βP/M)and p-xylene/o-xylene(βP/O) increased at first and then decreased, i.e. they peaked at a Si-Al ratio of 2.2—2.4, while the separation coefficient for p-xylene/ethylben zene(βP/E) decreased gradually. Therefore, in order to obtain higher adsorption selectivity for p-xylene, X zeolites should have a suitable framework SiO2/Al2O3ratio and water content.
The grain size of X zeolite significantly affected the mass transfer performance. Wang Huiguo, et al.[10]used a directing agent for synthesis of a low SiO2/Al2O3ratio X zeolite with a particle size of 0.2—1.0 μm in a sodium-water system. M. Rasouli, et al.[11]synthesized X zeolite with a particle size of about 100 nm, which was exchanged with Ba ions before use for adsorptive separation of p-xylene from mixed C8aromatic hydrocarbons, leading to excellent separation performance.

Figure 1 Separation coefficient vs. water content of BaX zeolite[10]●—βP/E; ▲—βP/O; ◆—βP/M

Figure 2 Separation coefficient vs. framework SiO2/Al2O3ratio of BaX zeolite[10]●—βP/E; ▲—βP/O; ◆—βP/M
3 Application of X Zeolite in Adsorptive Separation of N2/O2
Pressure swing adsorption (PSA) is industrially used widely for separation of nitrogen and oxygen from air.The active components of the absorbent are mostly composed of X molecular sieves. The quadrupole distance of N2is about 4 times as large as that of O2, resulting in stronger interaction between N2and cations in X zeolite[12]. When N2and O2coexist, the X zeolites containing specific metal cations will preferentially adsorb N2, resulting in separation of N2and O2. Therefore, researchers usually use the following two methods to improve the separation of N2and O2by X zeolites[13], viz.: (1) optimizing the types and quantities of cations in X zeolites; and (2)reducing the framework SiO2/Al2O3ratio of X zeolites to increase the number of cations.
D. W. McKee[5]studied the N2/O2separation performance of X zeolites containing different alkali metal ions and alkaline earth metal ions and found that X zeolites loaded with different cations had their adsorption capacity and separation coefficient decreased in the following order:Li+> Na+> K+, Rb+> Cs+. The LiX zeolites had the highest adsorption capacity and separation coefficient. The research results from M. S. A. Baksh, et al.[13]showed that at a space velocity of 5.65×105L/h, the LiX zeolite had achieved an O2purity and yield of more than 90% and 70%, respectively, but the NaX zeolite had only realized an O2yield of less than 30% along with an O2purity of more than 90%. After being loaded with alkaline earth metal ions, the CaX and SrX zeolites had the highest N2adsorption capacity, while the BaX zeolites had the highest O2adsorption capacity[14]. In order to further improve the N2adsorption capacity of X zeolites, S. Sircar,et al.[15]prepared X zeolites containing both Ca2+and Sr2+ions and studied the effects of Ca2+and Sr2+contents on their N2adsorption capacity. The X zeolites demonstrated higher N2adsorption capacity, when the Ca2+and Sr2+contents reached 17.5% and 82.5%, respectively. As for the NaCaX zeolites, Ca2+cations were mainly located at SI or SI’ sites and could hardly be accessible to N2or O2if about 49% of Na+cations were exchanged; the N2adsorption capacity increased significantly because Ca2+entered the SII' and SII' sites in the supercages, if 49% of Na+cations were exchanged; the O2adsorption capacity increased and an obvious adsorption competition between O2and N2occurred if over 71% of Na+cations were exchanged[16].
In 1987, G. H. Kuhl[17]reported the synthesis of low silicon X zeolite (at a framework SiO2/Al2O3ratio of 2.0). C. C. Chao[18]and F. W. Leavitt[19]studied the effect of framework SiO2/Al2O3ratio and cation type of X zeolites on N2adsorption capacity and N2/O2separation coefficient, and found that when the X zeolite had a framework SiO2/Al2O3ratio of 2.0, the cation Li+demonstrated higher N2adsorption capacity and N2/O2separation coefficient. The adsorption sites of N2or O2in the LiX zeolite were heterogeneous in energy, i.e. the adsorption heat decreased with the increase of N2or O2adsorption capacity, but the N2adsorption sites were much more heterogeneous in energy than the O2adsorption sites[20]. The introduction of the second metal cation,such as Ca2+or Sr2+to the LiX zeolite could maintain a high N2/O2separation coefficient and increase the N2adsorption capacity[21]. N. D. Hutson, et al.[22-23]introduced different amounts of Ag+cations into the LiX zeolite,which was dehydrated under appropriate conditions to form Ag clusters. These results showed that the X zeolite with one Ag atom in the crystal cell could achieve about 10% higher productivity than the LiX zeolite at considerable O2purity and yield. The Rietveld method was used to refine the neutron diffraction data of LiAgX zeolite as shown in Figure 3. After vacuum dehydration at 450 C, Ag+cations at SII sites migrated to SII* sites,while Ag+cations at SII* sites had so strong adsorption capacity that the prepared LiAgX zeolite showed better air separation performance[24]. M. Byulow, et al.[25]prepared the low-silicon X zeolites containing Li+and La3+cations and found that LiLaX zeolites had by about 4 kJ/mol higher in N2adsorption heat than LiX zeolites perhaps because N2interacted with both La3+cations at SII sites and Li+cations at SIII/SIII’ sites in the supercages.
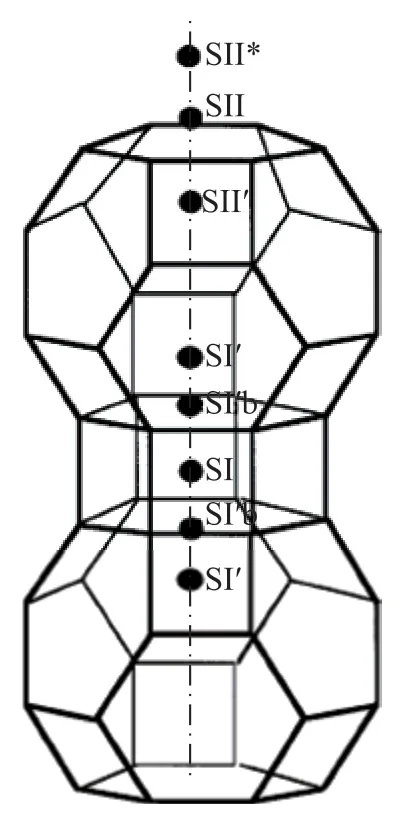
Figure 3 Location of cations outside the framework nearSOD cage in LiAgX zeolite[25]
In addition to metal ions, the water content of LiX zeolite could affect its adsorption capacity. N. D. Hutson, et al.[26]dewatered LiX zeolite with a SiO2/Al2O3ratio of 2.0 at different temperatures to obtain the LiX zeolites with different water contents, and found that when the number of water molecules in the crystal cell of the LiX zeolite increased from 0 to about 32, the amount of adsorbed N2decreased significantly from about 17.4 to less than 2, and only a water content of 12% could decrease the adsorption capacity by about 90%. Li+cations at SIII sites in the LiX zeolite were in a state of high energy with low coordination number. When H2O existed, Li+cations at SIII sites could easily adsorb HO and therefore would reduce the amount of adsorbed
4 Application of X Molecular Sieves in Adsorption of Other Gases
X zeolites with exchanged metal cations can also be used for adsorption of other gases because of their high adsorption capacity and selectivity. N. Kr. Das, et al.[27]found that the LiX zeolite exhibited N2/He and O2/He selectivity. Therefore, the research team used LiX zeolite as an adsorbent to separate the mixed gases containing 30% of He, 60% of N2and 10% of O2(by volume) at a flow rate of 2 L/min, a pressure of 500 kPa, and a feeding duration of 180 s in a single-column pressure swing adsorber, with the He purity and yield equating to 98.3%and 65%, respectively.
R. S. Pillai, et al.[28-29]systematically studied the alkali metal ion-exchanged X zeolites in terms of the performance for adsorption of CO2, CO, CH4, and N2and found that with an increasing radius of alkali metal ions in X zeolite, the CO and N2adsorption capacity gradually decreased and the CO2and CH4adsorption capacity gradually increased, and the adsorption capacity of gases on the LiX zeolite decreased in the following order: CO2>CO>CH4>N2>Ar>H2[30]. F. E. Epiepang, et al.[31]found that LiX molecular sieves with low SiO2/Al2O3ratio could be used for removal of impurities during air pretreatment because of their higher CO2and CH4adsorption capacity at lower pressure than that of conventional 13X molecular sieves. Although the low-silicon LiX zeolites had a H2adsorption capacity of only 0.6% at 298 K and 10 MPa in the domain of H2storage, their H2adsorption capacity at 298 K and 10 MPa was increased to 1.6% by using the hydrogen overflow produced by building the carbon bridges between the Pt-loaded activated carbon and lowsilicon LiX zeolites[32].A. Khelifa, et al.[33-34]studied the CO2adsorption properties of NaX zeolites exchanged by Zn2+, Cu2+, Ni2+,and Cr3+ions, respectively, and found that when less than 50% of Na+ions was exchanged by Zn2+or Ni2+,Zn2+ions, or Ni2+ions were mainly located at SI and SI’sites, so CO2would difficultly interact with Zn2+or Ni2+;when Zn2+or Ni2+exchanged more Na+, Zn2+or Ni2+ions would gradually enter the SII sites which could be easily accessible by CO2, resulting in energy heterogeneity.When the NaX zeolite was exchanged by Cu2+ions and dehydrated at high temperature, these Cu2+ions were inserted into the zeolite to form a Cu-O-Cu structure with low activity, so the Cu2+ions exchanged NaX zeolite showed energy homogeneity. Lower CO2adsorption capacity maybe resulted from the fact that with an increasing Cr3+ion exchange degree in NaX zeolite, the adsorption heat decreased because the electrostatic field in the channel was weakened by the distortion of six O molecules around Cr3+ions.
5 Conclusions
The X zeolite is an active component of the adsorbent for adsorptive separation of C8aromatic hydrocarbon isomers and also an adsorbent for N2/O2separation. In order to obtain higher selectivity for p-xylene adsorption,X zeolites should have an appropriate ratio of SiO2/Al2O,water content, grain size and cation type (such as Ba2+and K+). In the field of pressure swing adsorption of air,the low-silicon LiX zeolites exhibited high adsorption capacity and N2/O2selectivity; their N2/O2adsorption and separation performance could be further improved by introducing the second metal ion, such as Ca2+, Sr2+, Ag+,La3+ions, etc., while their adsorption capacity could be significantly reduced by high water content.
In addition, the X zeolites can be used for adsorption of other gases. The LiX zeolite can be used for separation of He from a mixture consisting of He, N2and O2,with the He purity and yield reaching 98.3% and 65%,respectively. The alkali metal ion exchanged X zeolite also showed selective adsorption capacity for CO2,CO, CH4, N2, etc. Their H2adsorption capacity could be greatly increased by using the hydrogen overflow produced via building the carbon bridges between the Ptloaded activated carbon and the low-silicon LiX zeolites.Their CO2adsorption capacity could be adjusted by Zn2+,Cu2+, Ni2+and Cr3+ions exchanged NaX zeolite.
杂志排行
中国炼油与石油化工的其它文章
- Influence of Cr3+ Concentration on SO2 Removal over TiO2 Based Multi-walled Carbon Nanotubes
- Catalytic Cracking Characteristics of Plant Oil for Producing Light Olefins and Light Aromatics
- Polycrystalline Phase WO3/g-C3N4 as a High Efficient Catalyst for Removal of DBT in Model Oil
- Novel NiMo Catalysts Supported on Sol-Gel Nanosized HY Zeolite-Alumina Composites for Hydrodesulfurization of Diesel
- Design, Optimization and Control of Extractive Distillation for Separation of Ethyl Acetate-Ethanol-Water Mixture Using Ionic Liquids
- Kinetic Model of Hydrogenation for Removal of Trace Olefins from Alkylation Mixture Formed during Linear Alkylbenzene Synthesis
