Targeted Mutagenesis of NAC Transcription Factor Gene, OsNAC041, Leading to Salt Sensitivity in Rice
2019-02-19WangBoZhongZhaohuiZhangHuanhuanWangXiaLiuBinglinYangLijiaHanXiangyanYuDeshuiZhengXuelianWangChunguoSongWenqinChenChengbinZhangYong
Wang BoZhong ZhaohuiZhang Huanhuan,Wang XiaLiu BinglinYang LijiaHan Xiangyan,Yu DeshuiZheng XuelianWang Chunguo,Song WenqinChen ChengbinZhang Yong
Targeted Mutagenesis of NAC Transcription Factor Gene,, Leading to Salt Sensitivity in Rice
Wang Bo1, 2,#, Zhong Zhaohui2,#, Zhang Huanhuan1,Wang Xia1, Liu Binglin2, Yang Lijia2, Han Xiangyan1,Yu Deshui1, Zheng Xuelian2, Wang Chunguo1,Song Wenqin1, Chen Chengbin1, Zhang Yong2
(College of Life Sciences, Nankai University, Tianjin 300071, China; Department of Biotechnology, School of Life Sciences and Technology, Center for Informational Biology, University of Electronic Science and Technology of China, Chengdu 610054, China; These authors contribute equally to this study)
Salinity is a major abiotic stress factor that seriously affects plant growth. Many genes are involved in the response to salt stress with various metabolism pathways. A number of plant transcription factor family genes have been found to be involved in the salt stress response, and NAM, ATAF and CUC (NAC) transcription factors are thought to act as active regulators during abiotic stress, especially salt stress. In this study, we detected a rice NAC transcription factor coding gene,, and confirmed that it influenced the germination of seeds under salt stress and salt tolerance of plants.wasprimarily expressed in the leaves and located in the nucleus. Furthermore, the CRISPR/Cas9 method was used to obtain a targetedmutant, of which the plant height was higher than that of the wild-type, showing increased salt sensitivity. Moreover, RNA-seq analysisrevealed a number of differentially expressed genes (DEGs) involved in several important signaling pathways in themutant. Subsequently, Kyoto Encyclopedia of Genesand Genomes annotation also revealed differential expression of DEGs associated with mitogen-activated protein kinase signaling, peroxisome, eukaryotic- type ABC transporters, photosynthesis and plant hormones, which are involved in stress-related signaling pathways. Overall, our study suggested thatwas involved inthe salt stress response in rice. These findings not only provide empirical evidence offunction, but also provide new insight into its potential application in rice resistance breeding.
rice; NAC transcription factor; CRISPR; Cas9; RNA-seq; salinity
Salt stress is one of the most important factors affecting plant cultivation and crop productivity(Hayashi et al, 1998; Kou et al, 2018). High salt concentrations can cause plant wilting and slow growth, and often result indeath. In rice, salinity induces pollen sterility, threatening survival and causing large losses in yield (Dolferus, 2014). Salt stress prevents plants from reaching their full potential via ion toxicity, disruption of protein synthesis and interference with normal enzyme activity. Importantly, salt stress can also cause a reduction in the rate and efficiency of photosynthesis, resulting in wilting and programmed cell death (Ma et al, 2017).
Various factors, for example, kinases, phosphatases, plant hormonesand transcription factors, play a pivotal role in salt stress responses such asthe salt overly sensitive (SOS) pathway(Lata and Prasad, 2011). The plant transcription factor family,including myeloblastosis (MYB), basic leucine zipper (bZIP), WRKY, dehydration responsive element binding (DREB) and NAC (NAM, ATAF1/2 and CUC2), are now considered important components of stress tolerance studies (Zhu, 2002; Duan et al, 2017; Tian et al, 2017). For example, over-expression ofincreases drought resistance, while over-expression and knockout ofwas participated in the response to water stress (Shen et al, 2012). Moreover, combinedover-expression of DREB and phytochrome interacting factor transcription factors improves drought stress tolerance and cell elongation in transgenic plants (Kudo et al, 2017). Transcription factors have also beenshown to have multiple functions during plant growth and development (Petricka et al, 2012), from organogenesis and hormonal signal responses (Nuruzzaman et al, 2012) to abiotic stress responses (Zheng et al, 2009).
The NAC family is plant-specific transcription factor that plays important roles in a number of biological metabolic pathways. The first reported NAC gene is isolated in petunias and plays a role in shoot apical meristem development (Souer et al, 1996). NAC genes found in(ATAF1/2, CUC1) reveal a conserved N-terminal region (Aida et al, 1997). Protein motif is also highly conserved, while the N-terminal end contains five subdomains and the C-terminal end is highly variable (Ernst et al, 2004). Accordingly, the unique structure is related to specific biotic functions during biotic and abiotic stress responses.Meanwhile,is thought to form a synergistic relationship with a subset of abscisic acid (ABA)-responsive-elementbinding factors (ABFs),triggering transcriptional activation of ABA-inducible genes in response to dehydration and osmotic stress (Xu et al, 2013).
A total of 158 NAC transcription factors have been identified in rice and subsequently listed in the Plant Transcription Factor Database (PlantTFDB v4.0). Studies on their functions in rice have since been carried out. The rice transcription factoris involved innumerous molecular mechanisms related to drought tolerance such as root structural adaptions and nicotianamine biosynthesis (Lee et al, 2017). Salinity and drought both can inducetranscription, suggestinginduction of an abiotic stress response via the ABA pathway (Chen et al, 2014). Meanwhile,overexpression lines possess increased resistance to high salt and drought conditions, while RNAi induces anincrease in tolerance at both the vegetative and reproductive stages (Shen et al, 2017).
In the study, we detected a rice NAC transcription factor coding gene,(LOC_Os03g013300). Initial salt treatment of wild-type plants confirmed thatis induced by salt stress. Mutants were then created based on the CRISPR/Cas9 method (Tang et al, 2016; Zhou et al, 2017; Zhong et al, 2018)to further determinethe specific function ofunder salt treatment. The global transcriptome profile ofknockout plants was also determined to explore the major molecular function ofunder salt stress conditions.
Materials and Methods
Plant materials and culture conditions
Wild-type (Nipponbare,L.) andmutant plants were grown in a light incubatorat 28ºC under a 16 h light (3000 Lux)/8 h dark cycle. To induce salt stress, seedlings at the 4-week stage were irrigated with solution consisting of 200 mmol/L NaCl. To examine growth performance, they were thengrown on 1/2 strength Murashige and Skoog (MS) medium, which was supplemented with 150 mmol/L NaCl.
OsNAC041 expression profile analysis
Root, stem and leaf samples from 18-day-old rice seedlings were used to isolate total RNA using the modified Trizol Reagent (Invitrogen, USA) according to the manufacturer’s instructions. Quantitative real- time PCR (qRT-PCR) was then performed to determineexpression in wild-type plants using the primers listed in Supplemental Table 1, with angene as an internal control.
Targeted mutagenesis of OsNAC041
To create targetedmutants, the sgRNA oligonucleotide pair was annealed (Supplemental Table 1), then cloned into the CRISPR-Cas9 backbone vector, pZHY988 (Tang et al, 2016; Zhou et al, 2017; Zhong et al, 2018).strain EHA105 was used to carry the vector and infected rice calli (Toki et al, 2006). Single-strand conformation polymorphism (SSCP) and Sanger sequencing were carried out for mutant identification (Zheng et al, 2016; Zhou et al, 2017).
Subcellular localization confirmation
NLS_Mapper (http://nls-mapper.iab.keio.ac.jp/cgi-bin/ NLS_Mapper_form.cgi) (Kosugi et al, 2009) was used to predict the possible location of, and thepZmUbi::OsNAC041-eGFP::HspT vector was constructedto confirm itslocation. Transformation of rice protoplasts was conducted by Tang et al (2017).
Physiological measurements
Four-week-old seedlings were irrigated with 200 mmol/L NaCl solution. Detached leaf samples were then incubated overnight in 0.5 mg/mL nitro-blue tetrazolium (NBT) or 5 mg/mLdiaminobenzidine (DAB) (pH 3.8) in the dark. Production of O2–and the H2O2content were then quantified according to Rai et al (2013).Malondialdehyde (MDA) concentration was also determined according to the thiobarbituric acid method (Wu et al, 2014). Superoxide dismutase (SOD),peroxidase(POD)and catalase (CAT) activities were determined based on the methods of Wu et al (2014), Munoz-Munoz et al (2009) and Sousa et al (2015), respectively. Each experiment was carried out in triplicate.
RNA-seq and data analysis
RNA-seq analysis was carried out using wild-type and(A/A) mutant plants grown for 18 d under normal conditionsorfor 15 d under normal conditions and 3 d under salt treatment. Three(A/A)T1seedlings were selected and combined to form a mixed sample. Total RNA was then isolated from the mixed sample using the modified Trizol method. RNA-seq was carried out by Beijing Genomics Institute (Shenzhen, China). To confirm the accuracy of the RNA-seq expression profiles, 10 genes with significantly different expression profiles were randomly selected for qRT-PCR. Expression levels were then quantified by normalization against. All assays were carried out in triplicate under identical conditions.
Results
Expression profile of OsNAC041
One NAC transcription factor coding gene in rice was clonedand named asaccording to database serial number (PlantTFDB v4.0). qRT-PCR revealed that the highest expression level was observed in the rice leaves (Fig. 1-A). Salt treatment was then applied to determine whetherexpression was induced by salt stress. Accordingly, after salt treatment (Na+) for 3 d,expression level increasedby two- fold (Fig. 1-B). Using nuclear localization signal(NLS) Mapper, candidate nuclear localization signal elements ofwere revealed (Supplemental Table 1). The pZmUbi::OsNAC041-eGFP::HspT expression vectorwas subsequently constructed to confirmlocalization in rice protoplast. As a result,the- eGFP fusion protein was concentrated in the nucleus, similar to NLS-eGFP (Fig. 1-C).
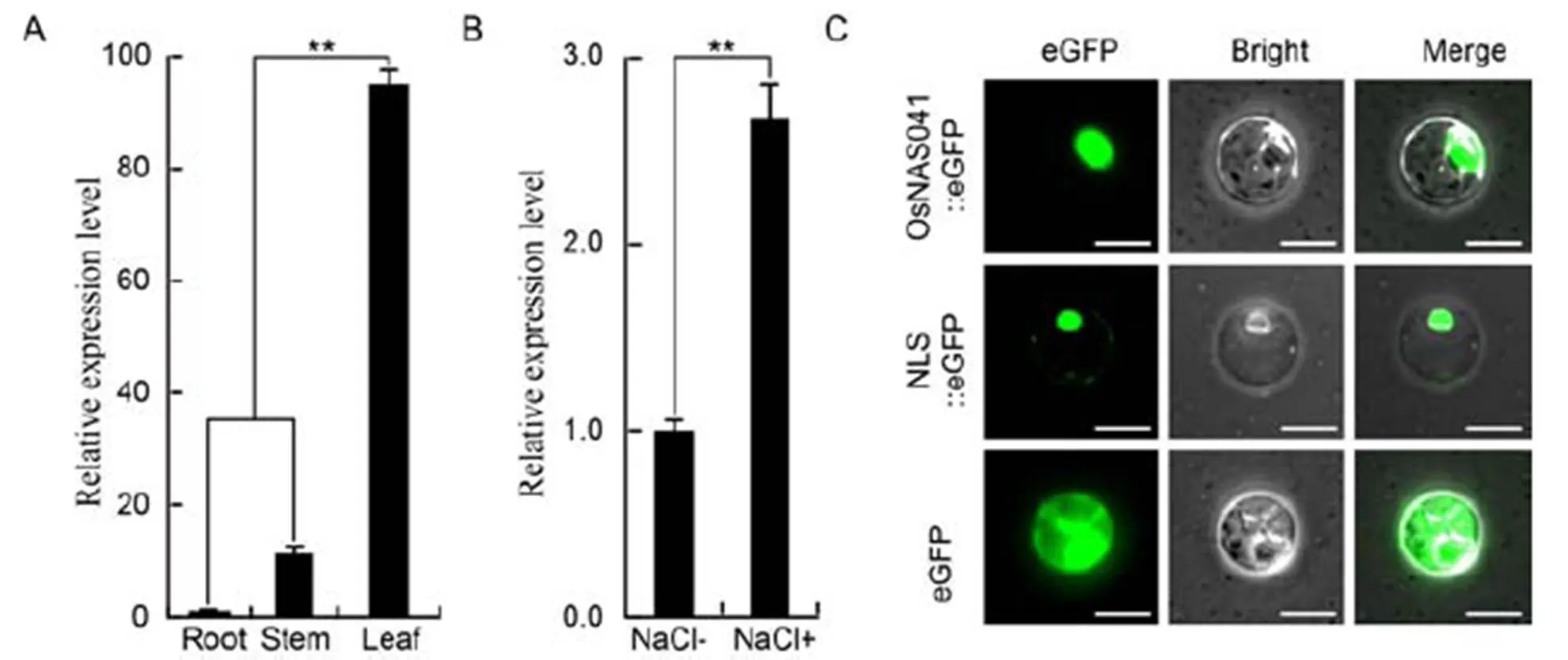
Fig. 1. Expression profile and subcellular location analysis of.
A, Quantitative real-time PCR (qRT-PCR) analysis ofexpression in samples of root, stem and leaffrom two-week-oldwild-type (WT) plants. B, qRT-PCR analysis ofexpression patterns in WT plants before (NaCl-) and after (NaCl+) salt stress. C, Nuclear localization of OsNAC041 protein in the rice protoplast.NLS, Nuclear localization signal. Scale bar = 20 µm.
Data represent Mean ± SE (= 3). **, Significance at the 0.01 level.
OsNAC041 targeted mutagenesis
The designed sgRNA oligonucleotide pair (Supplemental Table 1) was designed into theexons (Fig. 2-A). A total of 27 stable transgenic T0lines were obtained via-medicated rice calli transformation. SSCP was then used to screen the mutant seedlings for the positive transgene,and Sanger sequencing was used to further confirm the mutation genotype (Fig. 2-B and -C). Of the 27-sgRNA01 T0lines, 26 were confirmed as containing mutations (96.3%), andseven of these were biallelic (26.9%). Moreover, the height of the four-week mutant seedlings in T1generation increased significantly compared with the wild-type (Fig. 2-D).
osnac041 mutants possessa salt-sensitive phenotype
To determine the response ofto stress,mutants were treated with 150 mmol/L NaCl. Compared with the wild-type plants, seven days of seed germination and subsequent growth were inhibited under 150 mmol/L NaCl treatment. Moreover, shoots of the wild-type seedlings were longer than those of the mutants under salt stress (Fig. 3-A). Themutants also showeda salt-sensitive phenotypeat the vegetative growth stage. Moreover, after treatmentwith 150 mmol/L NaCl for 15 d, the wild-type seedlingsremained alive, whereas almost all of the mutant seedlings died (Fig. 3-B and -C).
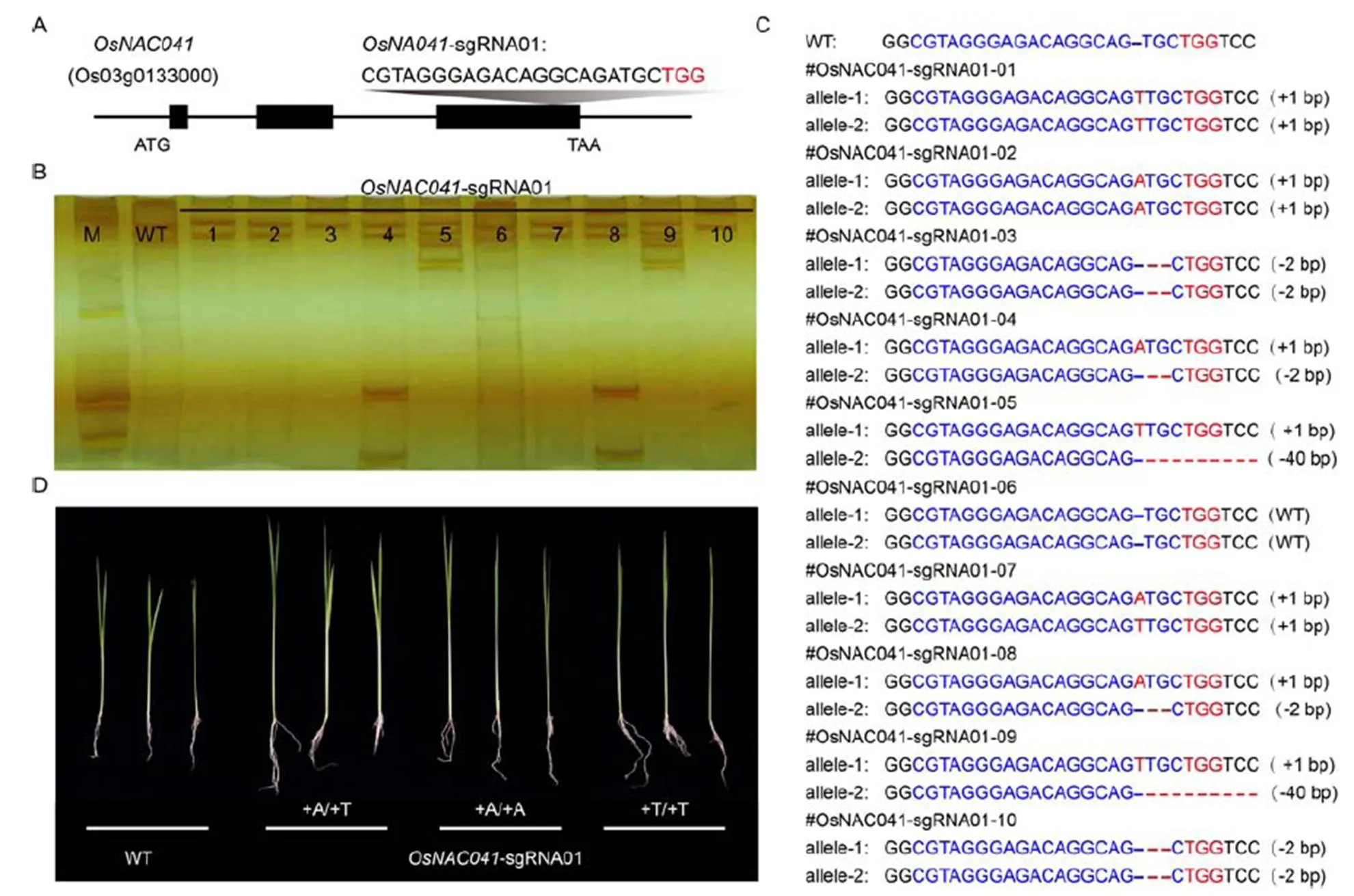
Fig. 2. Targeted mutagenesis of thelocus using CRISPR-Cas9 system.
A, Design of sgRNA sites for theexons. B, Single-strand conformation polymorphism analysis of 10 independent- sgRNA01 T0lines. M, Marker; WT, Wild-type. C, Sanger sequencing of the target site in the-sgRNA01 T0lines. D, Phenotypic analysis ofT1mutant lines under normal condition.
Reactive oxygen species (ROS) accumulation in the mutant plants was subsequently observed under salt stress. O2–and H2O2accumulation were examined using NBT and DAB staining, respectively. As a result, NBT staining was detected in all the three mutant lines,(A/T),(A/A) and(T/T),compared with the wild-type. DAB staining subsequently revealed increased levels of H2O2in the mutantscompared with the wild-type (Fig. 3-F).Quantitative analysis of the O2–and H2O2levels also revealeda significant increase inROS accumulation in the mutants compared with the wild-type (Fig. 3-F and -G), which was consistent with the results of DAB and NBT staining.
MDA content, which reflects lipid peroxidation, was also measured in wild-type andmutantleaves. After 15-day salt treatment, a significant increase in MDA content was observed in themutants compared with the wild-type (Fig. 3-H). In addition, SOD, POD and CAT activities were also determined to examine the response of the membrane protection system, which are involved in scavenging harmful ROS during stress. Overall, activities of these antioxidant enzymes were lower in the wild-type than the mutants (Fig. 3-I to -K), suggesting that knockout ofaffected the membrane protection system, causingan increase in toxic substances, and thereby weakening salt tolerance.
Targeted knockout of OsNAC041 results in global gene expression changes in rice
To understand howresponds to salt and regulates certain pathways, we examined global transcriptome changesfollowingknockout. To do so, RNA-seq assay of wild-type and(A/A) plants was carried out under normal and salt stress conditions (Fig. 4-A). The average mapping ratio ofthe reference genome was 97.22%.Meanwhile, the average mapping ratio at the gene level was 89.06%, with a total of 26 575 genes detected.
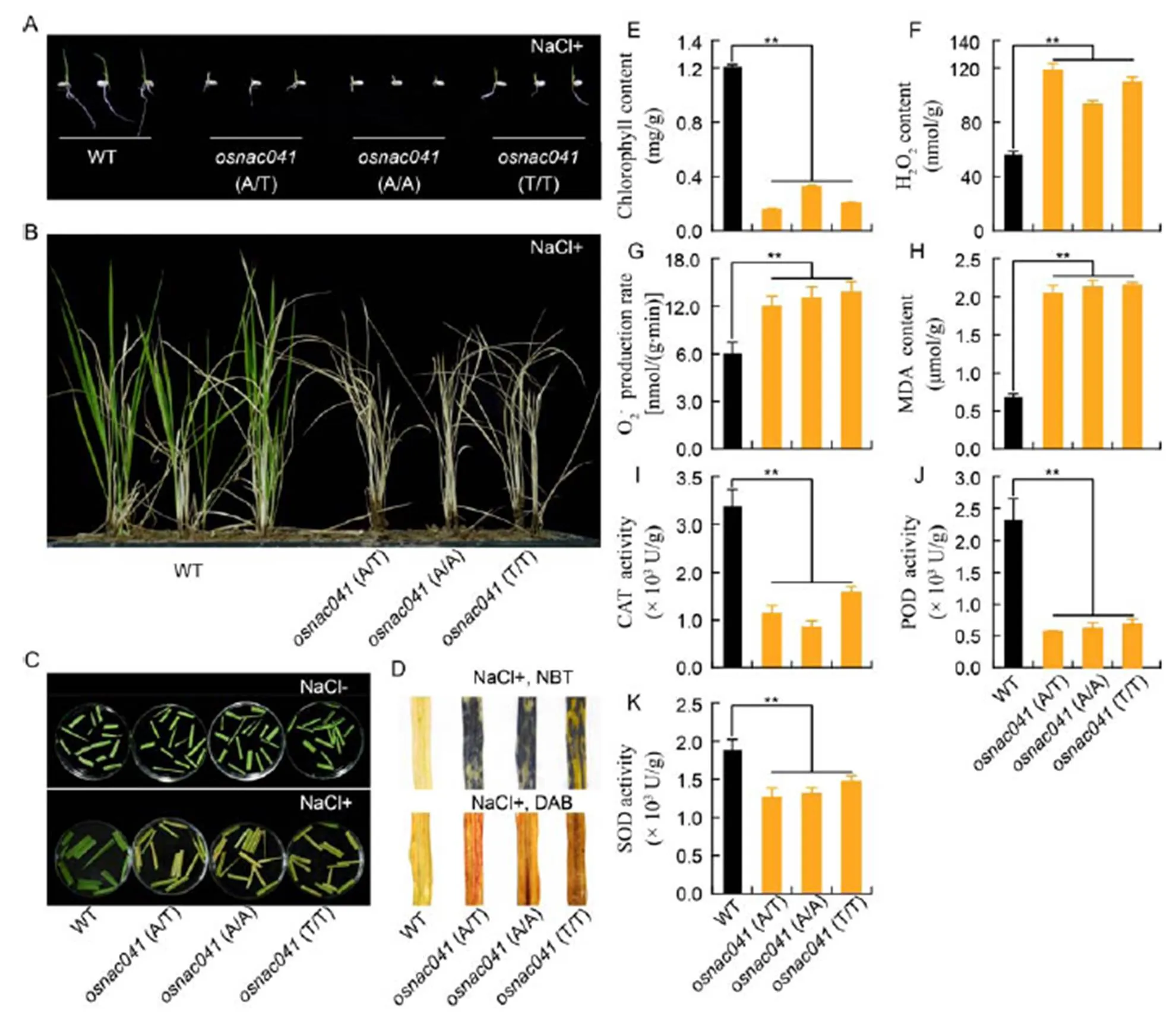
Fig. 3.targeted mutants showed the salt-sensitive phenotype.
A, Germination ofT1mutant and wild-type (WT) seeds grown on 1/2 MS medium supplemented with NaCl (150 mmol/L). B, Phenotypic analysis ofT1mutant lines under salt stress. C, Growth ofT1mutant lines in plugs. D, Levels of O2–and H2O2in WT andT1mutant lines subjected to salt stress. Salt-stressed leaf samples were incubated in nitro-blue tetrazolium (NBT) or diaminobenzidine (DAB) solution. E, Chlorophyll content after 15-day salt stress. F, H2O2content after 15-day salt stress. G, O2–production rate after 15-day salt stress. H, Malondialdehyde (MDA) content after 15-day salt stress. I, Catalase (CAT) activity after 15-day salt stress. J, Peroxidase (POD) activity after 15-day salt stress. K, Superoxide dismutase (SOD) activity after 15-day salt stress.
Data representMean ± SE (= 3). **, Significant at the 0.01 level.
Under normal conditions, a total of 575 genes were up-regulated, while 1 960 were down-regulated in WT compared with mutant without NaCl treatment. Genes with fold change of the expression level ≥ 2.00 with the FDR (false discovery rate)≤0.001 are differentially expressed genes (DEGs).Meanwhile, more than halfof the rice genes (52.78%, 13 494) were differentially expressed under salt stress conditions: 8.35% (2 218) were up-regulated and 44.43% (11 276) were down- regulated. The number of DEGs increased 5.3-fold under salt stress compared to normal conditions (Fig. 4-B). In Venn diagram analysis, 1 447 genes were co-expressedin the WT/::NaCl+ comparison and WT/::NaCl- comparison, while 12 047 genes were expressed only in the WT/:: NaCl+ comparison and 1 088 genes only in the WT/::NaCl- comparison (Supplemental Fig. 1). To validate the results of RNA-seq, qRT-PCR was carried out using 10 genes selected randomly from significantly up-regulated genes in(A/A) compared with wild-type plants following salt treatment (Supplemental Fig. 2). As a result, a high correlation was found between the RNA-seq and qRT-PCR results, confirming the accuracy of the RNA-seq data.
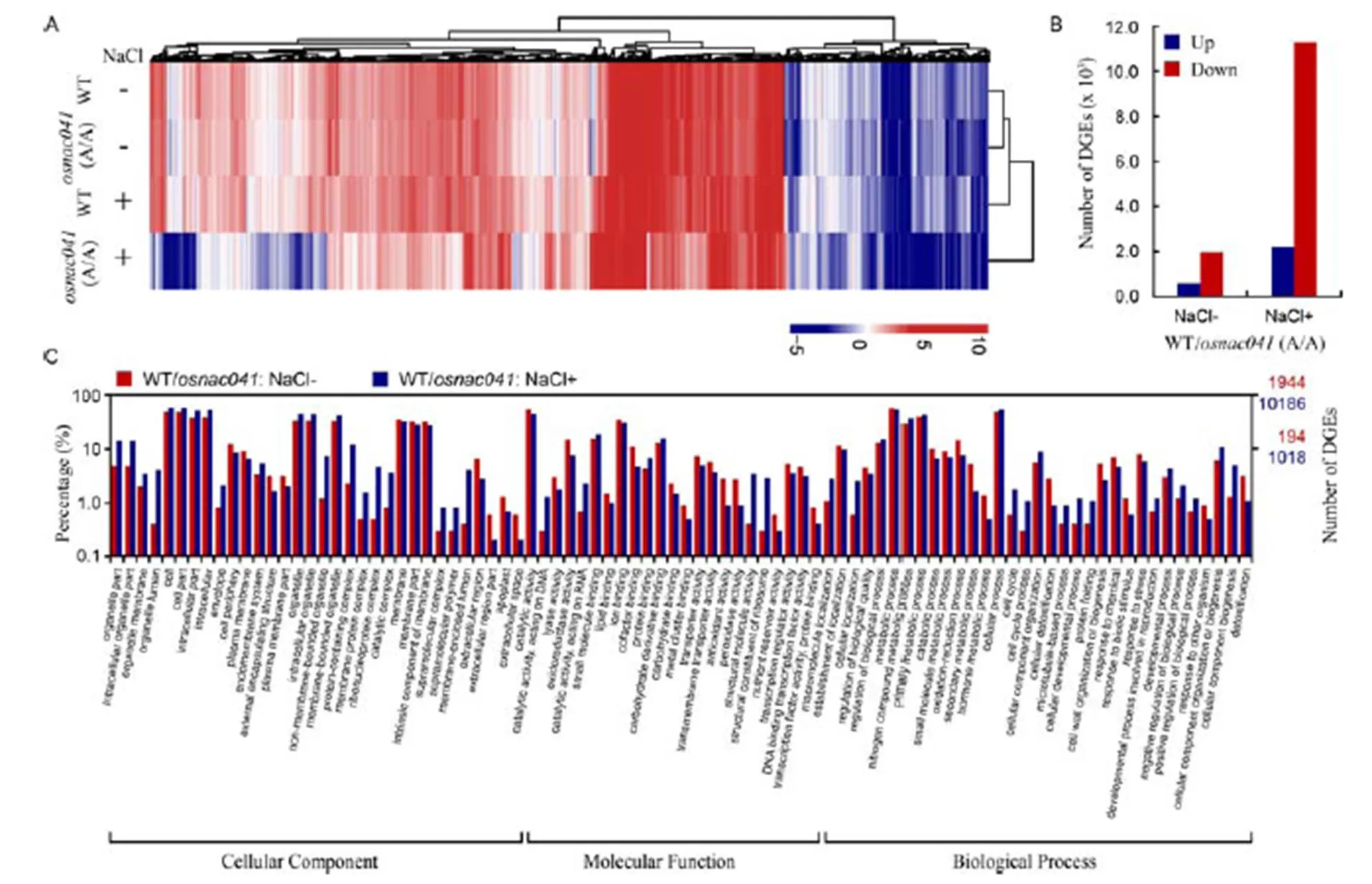
Fig. 4. Targeted knockout ofresulted in global gene expression changes in rice.
A, Number of differentially expressed genes (DEGs) in the wild-type (WT) and(A/A) T1mutant lines, based on expression profiles obtained by RNA-Seq. B, Clustering analysis of DEGs in WT andT1mutant lines. Targeted knockout ofresulted in global changes compared with the WT both with and without salt stress. The color scale corresponds to log2(FPKM) values of the genes. C, Gene ontology classification of DEGs in the following two comparisons: WT andT1mutant lines under normal and salt stress conditions.-axis shows user selected GO terms;-axis shows the percentages of genes (number of a particular gene divided by total gene number).
Gene oncology (GO) classifications further revealed that these DEGs were enriched during ion binding and metabolic processesunder normal and salt stress conditions (Fig. 4-C). A total of 1 697 significant DEGswere related to metabolic processes, while 1 539 DEGs were related to cellular processes, suggesting that salt stress affected biological processes inthe mutants. In the cellular component category, organelle (1 184 DEGs), membrane (996 DEGs) and extracellular region(183 DEGs) GO terms were significantly enriched, suggesting that salt stress also affected the membrane and cell partsin the mutant plants. Moreover, 1 493 DEGs associated with binding, 1 489 DEGs associated with catalytic activity, and 192 DEGs associated with transporter activity were also revealed, suggesting that salt stress also influenced molecular function in the mutants.
Kyoto Encyclopedia of Genesand Genomes (KEGG) analysis wasalso carried out to predict the biochemical pathways associated with the DEGs (Supplemental Fig. 5). Interestingly, a large number of differential genes were concentrated in several signaling pathways associated with salt stress. DEGs identified in the WT/(A/A) comparison were associated with mitogen-activated protein kinase (MAPK) signaling(632, 3.06%), plant hormone signal transduction (804, 3.90%), peroxisome (178, 0.86%), eukaryotic-type ABC transporters (188, 0.91%) and photosynthesis (125, 0.60%).
Differential expression analysis revealed potential transcriptional responses to salt stress
Of the major signaling pathways, photosynthesis is most important, converting light energyinto organic matter and releasing oxygen.In the mutants, most photosynthesis-related genes were down-regulated, consistent with the salt-sensitive phenotype (Fig. 5-A). Similar to photosynthesis, peroxisomes produce important signals during stress responses.,,andare key genes encoding peroxisome membrane proteins, and all are up-regulated during peroxidase synthesis (Supplemental Fig. 6).
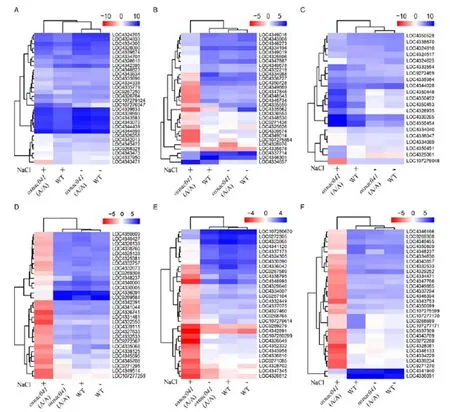
Fig. 5. Transcriptome analysis of genes systemically regulated in the wild-type (WT) andT1mutant lines in response to salt stress.
A, Photosynthesis-related genes. B, Peroxisome-related genes. C, Water deprivation-related genes. D, Mitogen-activated protein kinase signal-related genes. E, Eukaryotic-type ABC transporter-related genes. F, Plant hormone regulatory pathway-related genes.
Log2(Fold change, FC) of differentially expressed genes in WT andT1mutant lines before (NaCl-) and after (NaCl+) salt stress isdefined as [-1.5 > log(FC) > 1.5] with false discovery rate (FDR) < 0.05, and the top 30 most significant differences areshown in the heat-map.
It is well known that when plants are dehydrated, they are more susceptible to injury and death. In this study, GO analysis identified up-regulation of at least 12 DEGs related to water deprivation in mutant(A/A) under salt stress, which may have accelerated dehydration (Fig. 5-C). This finding suggests that the mutantsare more likely to lose water under salt stress. Heat-map analysis revealed that the MAPK pathway expression was down-regulated under salt stress compared to normal conditions, suggesting a decrease in the ability of the mutantsto respond to salt stress (Fig. 5-D). The ABC superfamily is an ancient large family of ATP-driven pumps. As the gatekeeper of cells, ABC transporters preserve nutrients within the cell and expel toxins.in the ABCA subfamily,in the ABCB subfamily, andandin the ABCC subfamily were significantly up-regulated, while more than 20 ABC members were significantly down-regulated in themutant, suggesting that differential expression of ABC family genes also affected the stress response (Fig. 5-E). Moreover, metabolic activities of the auxin, cytokinin, salicylic acid, jasmonic acid, ethylene and brassinosteroid biosynthetic pathways werealso seriously impacted in the mutant plants, suggesting that various pathways associated with plant hormones were also involved in-mediated salt tolerance(Fig. 5-F).
Discussion
It waspreviously revealed that a number of NAC transcription factors are associated with stress. For example, in rice, NAC transcription factors(Hu et al, 2006; Redillas et al, 2012),(Hu et al, 2008),(Jeong et al, 2013),(Liang et al, 2014) and(Kunieda et al, 2008) all affect the abiotic stress pathway. Over-expression ofin rice also enhances drought resistance and salt tolerance (Hu et al, 2006),while over-expression ofimproves salt tolerance (Hu et al, 2008). Meanwhile, over-expression of the homologous gene in(Supplemental Fig. 3),, results in a dwarf phenotype (Kato et al, 2010). In this study, the amino acid sequence ofhas 52% identity withas well as a clear NAC domain (Supplemental Fig. 4). The transgenicover-expression line also shows relatively slow vegetative growth (Kato et al, 2010). These findings suggest that the phenotype of gain-function ofcorrespond with the loss of function observed in this study.
In addition to great changes in photosynthesis (Supplemental Fig. 5), ROS over-accumulation in plants occurs under diverse abiotic stresses including high salinity, and often causes damage (Miller et al, 2010). Many studies have also shown that the plant response to abiotic stresses occurs via regulation of ROS metabolism (Schmidt et al, 2013; Fang et al, 2015). For example, over-expression ofimproves salinity tolerance via regulation of ROS-dependent signaling in rice (Schmidt et al, 2013). Meanwhile,-overexpressing plants show improved drought tolerance as a result efficient ROS scavenging,which reduces membrane lipid peroxidation (Xiong et al, 2018). In this study, theknockout plants weresalt sensitive (Fig. 3-A), and presented with yellow leavesafter salt treatment (Fig. 3-B). Moreover, more H2O2and MDA accumulated in the leaves ofknockout plantscompared with the wild-type (Fig. 3-F and -H), suggesting that the reduction in salt tolerance was the result ofreduced ROS scavenging and increased levels of MDA. Plants have developed a complex antioxidant system, which includes enzymatic antioxidants (Miller et al, 2010). Under salt stress, activities of SOD, POD and CAT, which can scavenge toxic ROS, were all lower inmutant plants compared with the wild-type. These findings suggest thatis associated with the ROS system and membrane protection, thereby regulating salt sensitivity.
Plants rapidly detect environmental changes and, in response, initiate related intercellular and intracellular signal transduction pathways (Hu and Xiong, 2014). In this study,mutation caused global changesin rice transcriptome (Fig. 5). Furthermore, six enriched pathways related to the salt stress phenotype were observed inthemutant plants including MAPK signaling, plant hormone signal transduction, peroxisome, eukaryotic-type ABC transporters and photosynthesis (Fig. 5). Among these, targeted mutagenesis ofhadthe largest impact on the MAPK signalingpathway and ABC transporter pathway (Fig. 5-D and -E).
MAPKs are conserved eukaryote protein kinases that are involved in signaling under environmental stress (Doczi et al, 2012). Plants overexpressing, anC2H2-type zinc finger protein, show improved seed germination under salt stress conditionsvia interaction with phosphorylation by MAPK6 at two sites (Liu et al, 2013). Moreover,, a lipid transfer protein-related hybrid proline-rich protein involved in salt stress, is also phosphorylated by MAPK3(Pitzschke et al, 2014). Up- and down-regulation of core components of the MAPK signaling pathway in theknockout plants therefore suggests flexibility in adapting to salt stress, although the exact mechanisms remain unclear(Supplemental Fig. 7).
ABC transporter proteins contain an ATP binding domain and are grouped into eight subfamilies, ABCA to ABCI. Plants reportedly contain twice as many as ABC transporters as animals (Theodoulou and Kerr, 2015). Since plants are stationary, they have to deal with changing environments (Lefevre et al, 2015). ABC transporters in plants are involved in a number of functions such as secondary metabolite transport (Francisco et al, 2013), heavy metal detoxification (Archer, 2014), antibiotic transport (Mentewab and Stewart, 2005) and phytohormone transport (Hu and Xiong, 2014). The changes to the ABC subfamily observed in theknockout plants therefore suggest thatalso affects ABC transporters,regulating growth and development in rice (Supplemental Fig. 8).
Plant hormones are critical for adaptation to environmental changes. ABA is a stress-responsive hormone, and along with other phytohormones such as brassinosteroids, ethylene, salicylic acid and jasmonic acid,its role in environmental stress responses is becoming increasingly well understood (Peleg and Blumwald, 2011; Cabello et al, 2014; Zhou et al, 2014).Recent studies suggest that plant hormones are involved in multiple signaling pathways, allowing plants to cope with biotic stress (Depuydt and Hardtke, 2011; Shi et al, 2015). Based on our RNA-seq data, various pathways are thought to be involved in- mediated salt sensitive inknockout plants. For example, biosynthesis of auxin, cytokinin, gibberellin, ethylene, brassinosteroid, jasmonic acid and salicylic acid were all affected (Supplemental Fig. 9). These findings suggest that knockout ofalters plant hormone homeostasis, which may be the main cause of the salt sensitive phenotype.
In conclusion, this study presented evidence thatplays important roles in salt resistance inrice. Induction ofby salt stress was confirmedalong with localization in the cell nucleus. Transcriptomeanalysis ofknockout plants showed changes in the expression level of genes involved in MAPK signaling, plant hormone signal transduction, peroxisome, eukaryotic-type ABC transporter and photosynthesispathways. The salt sensitive phenotype of theknockout plants was thought to be the result of changes to ROS-related genes, while differential expression analysis revealed potential transcriptional responses to salt stress. These findings provided a theoretical basis for further molecular studies of salt stress in rice and subsequent salt tolerance breeding programs.
Acknowledgements
This study was supported by the National Science Foundation of China (Grant No. 31771486), the Sichuan Youth Science and Technology Foundation (Grant No. 2017JQ0005), theNational Key Research and Development Program of China (Grant No. 2017YFD01005050102) and the National Transgenic Major Project (Grant No. SQ2018ZD08019-001-003).
SUPPLEMENTAL DATA
The following materials are available in the online versionof this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Table 1. Result of the nuclear localizationsignals-mapper predictions of the location ofexpression.
Supplemental Table 2. Oligonucleotides used in this study.
Supplemental Fig. 1. Validation of the RNA-seq results by quantitative real-time PCR.
Supplemental Fig. 2. Venn diagram analysis of the differentially expressed genes.
Supplemental Fig. 3. Alignment betweenand.
Supplemental Fig. 4. Phylogenetic tree analysis of.
Supplemental Fig. 5. Analysis of photosynthesis-related differentially expressed genes and photosynthesis- antenna proteins.
Supplemental Fig. 6. Analysis of peroxisome-related differentially expressed genes.
Supplemental Fig. 7. Analysis of MAPK signaling-related differentially expressed genes.
Supplemental Fig. 8. Analysis of eukaryotic-type ABC transporter-related differentially expressed genes.
Supplemental Fig. 9. Analysis of plant hormone signal transduction-related differentially expressed genes.
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. 1997. Genes involved in organ separation in: An analysis of the cup-shaped cotyledon mutant., 9(6): 841–857.
Archer E K. 2014. American society of plant biologists: Position statement on the education of young children about plants., 13(4): 575–576.
Cabello J V, Lodeyro A F, Zurbriggen M D. 2014. Novel perspectives for the engineering of abiotic stress tolerance in plants., 26: 62–70.
Chen X, Wang Y F, Lv B, Li J, Luo L Q, Lu S C, Zhang X, Ma H, Ming F. 2014. The NAC family transcription factorconfers abiotic stress response through the ABA pathway., 55(3): 604–619.
Depuydt S, Hardtke C S. 2011. Hormone signalling crosstalk in plant growth regulation., 21(9): 365–373.
Doczi R, Okresz L, Romero A E, Paccanaro A, Bogre L. 2012. Exploring the evolutionary path of plant MAPK networks., 17(9): 518–525.
Dolferus R. 2014. To grow or not to grow: A stressful decision for plants., 229: 247–261.
Duan M, Zhang R X, Zhu F G, Zhang Z Q, Gou L M, Wen J Q, Dong J L, Wang T. 2017. A lipid-anchored NAC transcription factor is translocated into the nucleus and activates glyoxalase I expression during drought stress., 29(7): 1748–1772.
Ernst H A, Olsen A N, Larsen S, lo Leggio L. 2004. Structure of the conserved domain of ANAC, a member of the NAC family of transcription factors.,5(3): 297–303.
Fang Y J, Liao K F, Du H, Xu Y, Song H Z, Li X H, Xiong L Z. 2015. A stress-responsive NAC transcription factorconfers heat and drought tolerance through modulation of reactive oxygen species in rice., 66: 6803–6817.
Francisco R M, Regalado A, Ageorges A, Burla B J, Bassin B, Eisenach C, Zarrouk O, Vialet S, Marlin T, Chaves M M, Martinoia E, Nagya R. 2013. ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3--glucosides., 25(5): 1840–1854.
Hayashi H, Sakamoto A, Alia, Murata N. 1998. Enhancement of stress tolerance by gene-engineering of betaine accumulation in plants.: 2419–2424.
Hu H H, Dai M Q, Yao J L, Xiao B Z, Li X H, Zhang Q F, Xiong L Z. 2006. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice., 103: 12987–12992.
Hu H H, You J, Fang Y J, Zhu X Y, Qi Z Y, Xiong L Z. 2008. Characterization of transcription factor geneconferring cold and salt tolerance in rice., 67: 169–181.
Hu H H, Xiong L Z. 2014. Genetic engineering and breeding of drought-resistant crops., 65(1): 715–741.
Jeong J S, Kim Y S, Redillas M C F R, Jang G, Jung H, Bang S W, Choi Y D, Ha S H, Reuzeau C, Kim J K. 2013.overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field., 11(1): 101–114.
Kato H, Motomura T, Komeda Y, Saito T, Kato A. 2010. Overexpression of the NAC transcription factor family generesults in a dwarf phenotype in., 167(7): 571–577.
Kosugi S, Hasebe M, Tomita M, Yanagawa H. 2009. Systematic identification of yeast cell cycle-dependent nucleocytoplasmic shuttling proteins by prediction of composite motifs., 106: 10171–10176.
Kou L L, Hu H C, Ma L, Ke X N, Liu M Y, Lian W M, Jin K, Xie L J, Liu Q P. 2018. Functional analysis of a copper/zinc SOD encoding gene in response to arsenite stress in rice., 32(5): 437–444. (in Chinese with English abstract)
Kudo M, Kidokoro S, Yoshida T, Mizoi J, Todaka D, Fernie A R, Shinozaki K, Yamaguchi-Shinozaki K. 2017. Double overexpression of DREB and PIF transcription factors improves drought stress tolerance and cell elongation in transgenic plants., 15(4): 458–471.
Kunieda T, Mitsuda N, Ohme-Takagi M, Takeda S, Aida M, Tasaka M, Kondo M, Nishimura M, Hara-Nishimura I. 2008. NAC family proteins NARS1/NAC2 and NARS2/NAM in the outer integument regulate embryogenesis in., 20(10): 2631–2642.
Lata C, Prasad M. 2011. Role of DREBs in regulation of abiotic stress responses in plants., 62(14): 4731–4748.
Lee D K, Chung P J, Jeong J S, Jang G, Bang S W, Jung H, Kim Y S, Ha S H, Choi Y D, Kim J K. 2017. The ricetranscription factor orchestrates multiple molecular mechanisms involving root structural adaptions and nicotianamine biosynthesis for drought tolerance., 15(6): 754–764.
Lefevre F, Baijot A, Boutry M. 2015. Plant ABC transporters: Time for biochemistry?, 43(5): 931–936.
Liang C Z, Wang Y Q, Zhu Y N, Tang J Y, Hu B, Liu L C, Ou S J, Wu H K, Sun X H, Chu J F, Chu C C. 2014.connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice., 111: 10013–10018.
Liu X M, Nguyen X C, Kim K E, Han H J, Yoo J, Lee K, Kim M C, Yun D J, Chung W S. 2013. Phosphorylation of the zinc finger transcriptional regulator ZAT6 by MPK6 regulatesseed germination under salt and osmotic stress., 430(3): 1054–1059.
Ma C Q, Wang Y G, Gu D, Nan J D, Chen S X, Li H Y. 2017. Overexpression of S-adenosyl-L-methionine synthetase 2 from sugar beet M14 increasedtolerance to salt and oxidative stress., 18(4): 847.
Mentewab A, Stewart C N. 2005. Overexpression of anABC transporter confersresistance to transgenic plants., 23(9): 1177–1180.
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. 2010. Reactive oxygen species homeostasis and signalling during drought and salinity stresses., 33(4): 453–467.
Munoz-Munoz J L, Garcia-Molina F, Garcia-Ruiz P A, Arribas E, Tudela J, Garcia-Canovas F, Rodriguez-Lopez J N. 2009. Enzymatic and chemical oxidation of trihydroxylated phenols., 113(2): 435–444.
Nuruzzaman M, Sharoni A M, Satoh K, Moumeni A, Venuprasad R, Serraj R, Kumar A, Leung H, Attia K, Kikuchi S. 2012. Comprehensive gene expression analysis of the NAC gene family under normal growth conditions, hormone treatment, and drought stress conditions in rice using near-isogenic lines (NILs) generated from crossing Aday selection (drought tolerant) and IR64., 287(5): 389–410.
Peleg Z, Blumwald E. 2011. Hormone balance and abiotic stress tolerance in crop plants., 14(3): 290–295.
Petricka J J, Winter C M, Benfey P N. 2012. Control ofroot development., 63: 563–590.
Pitzschke A, Datta S, Persak H. 2014. Salt stress in: Lipid transfer protein AZI1 and its control by mitogen-activated protein kinase MPK3., 7(4): 722–738.
Rai G K, Rai N P, Rathaur S, Kumar S, Singh M. 2013. Expression ofin tomato alleviates drought induced oxidative stress by regulating key enzymatic and non- enzymatic antioxidants., 69: 90–100.
Redillas M C F R, Jeong J S, Kim Y S, Jung H, Bang S W, Choi Y D, Ha S H, Reuzeau C, Kim J K. 2012. The overexpression ofalters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions., 10(7): 792–805.
Schmidt R, Mieulet D, Hubberten H M, Obata T, Hoefgen R, Fernie A R, Fisahn J, Segundo B S, Guiderdoni E, Schippers J H M, Mueller-Roeber B. 2013. SALT-RESPONSIVE ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice., 25(6): 2115–2131.
Shen H S, Liu C T, Zhang Y, Meng X P, Zhou X, Chu C C, Wang X P. 2012.is activated by MAP kinases to confer drought tolerance in rice., 80(3): 241–253.
Shen J B, Lv B, Luo L Q, He J M, Mao C J, Xi D D, Ming F. 2017. The NAC-type transcription factorregulates ABA-dependent genes and abiotic stress tolerance in rice., 7: 40641.
Shi Y T, Ding Y L, Yang S H. 2015. Cold signal transduction and its interplay with phytohormones during cold acclimation., 56(1): 7–15.
Souer E, van Houwelingen A, Kloos D, Mol J, Koes R. 1996. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries., 85(2): 159–170.
Sousa R H V, Carvalho F E L, Ribeiro C W, Passaia G, Cunha J R, Lima-Melo Y, Margis-Pinheiro M, Silveira J A G. 2015. Peroxisomal APX knockdown triggers antioxidant mechanisms favourable for coping with high photorespiratory H2O2induced by CAT deficiency in rice., 38: 499–513.
Tang X, Zheng X L, Qi Y P, Zhang D W, Cheng Y, Tang A T, Voytas D F, Zhang Y. 2016. A single transcript CRISPR-Cas9 system for efficient genome editing in plants., 9(7): 1088–1091.
Tang X, Lowder L G, Zhang T, Malzahn A A, Zheng X L, Voytas D F, Zhong Z H, Chen Y Y, Ren Q R, Li Q, Kirkland E R, Zhang Y, Qi Y P. 2017. A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants., 3: 17108.
Theodoulou F L, Kerr I D. 2015. ABC transporter research: Going strong 40 years on.,43(5): 1033–1040.
Tian X J, Li X F, Zhou W J, Ren Y K, Wang Z Y, Liu Z Q, Tang J Q, Tong H N, Fang J, Bu Q Y. 2017. Transcription factorpositively regulates brassinosteroid signaling and plant architecture., 175(3): 1337–1349.
Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S. 2006. Early infection of scutellum tissue withallows high- speed transformation of rice., 47: 969–976.
Wu Y C, Liu C L, Kuang J, Ge Q, Zhang Y, Wang Z Z. 2014. Overexpression ofenhances salt and drought tolerance inand., 251: 1191–1199.
Xiong H Y, Yu J P, Miao J L, Li J J, Zhang H L, Wang X, Liu P L, Zhao Y, Jiang C H, Yin Z G, Li Y, Guo Y, Fu B Y, Wang W S, Li Z K, Ali J, Li Z C. 2018. Natural variation inincreases drought tolerance in rice by inducing ROS scavenging., 178(1): 451–467.
Xu Z Y, Kim S Y, Hyeon D Y, Kim D H, Dong T, Park Y, Jin J B, Joo S H, Kim S K, Hong J C, Hwang D, Hwang I. 2013. TheNAC transcription factorcooperates with bZIP-type transcription factors in dehydration and osmotic stress responses.,25(11): 4708–4724.
Zheng X N, Chen B, Lu G J, Han B. 2009. Overexpression of a NAC transcription factor enhances rice drought and salt tolerance., 379(4): 985–989.
Zheng X L, Yang S X, Zhang D W, Zhong Z H, Tang X, Deng K J, Zhou J P, Qi Y P, Zhang Y. 2016. Effective screen of CRISPR/ Cas9-induced mutants in rice by single-strand conformation polymorphism., 35(7): 1545–1554.
Zhong Z H, Zhang Y X, You Q, Tang X, Ren Q R, Liu S S, Yang L J, Wang Y, Liu X P, Liu B L, Zhang T, Zheng X L, Le Y, Zhang Y, Qi Y P. 2018. Plant genome editing using FnCpf1 and LbCpf1 nucleases at redefined and altered PAM sites., 11(7): 999–1002.
Zhou J P, Deng K J, Cheng Y, Zhong Z H, Tian L, Tang X, Tang A T, Zheng X L, Zhang T, Qi Y P, Zhang Y. 2017. CRISPR-Cas9 based genome editing reveals new insights into microRNA function and regulation in rice.,8: 1598.
Zhou M Q, Xu M, Wu L H, Shen C, Ma H, Lin J. 2014.fromenhances cold tolerance and restrains growth inby antagonizing with gibberellin and affecting cell cycle signaling., 85(3): 259–275.
Zhu J K. 2002. Salt and drought stress signal transduction in plants., 53: 247–273.
(Managing Editor: Wang Caihong)
Copyright © 2019, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2018.12.005
23 October 2018;
27 December 2018
Chen Chengbin (chencb@nankai.edu.cn); Zhang Yong (zhangyong916@uestc.edu.cn)
杂志排行
Rice Science的其它文章
- Improvements of TKC Technology Accelerate Isolation of Transgene-Free CRISPR/Cas9-Edited Rice Plants
- Production of Two Elite Glutinous Rice Varieties by Editing Wx Gene
- Development and Application of CRISPR/Cas System in Rice
- Rapid Creation of New Photoperiod-/Thermo-Sensitive Genic Male-Sterile Rice Materials by CRISPR/Cas9 System
- CRISPR/Cas9-Mediated Adenine Base Editing in Rice Genome
- Characterization and Evaluation of OsLCT1 and OsNramp5 Mutants Generated Through CRISPR/Cas9-Mediated Mutagenesis for Breeding Low Cd Rice
