Development and Application of CRISPR/Cas System in Rice
2019-02-19RenJunHuXixunWangKejianWangChun
Ren Jun, Hu Xixun, Wang Kejian, Wang Chun
Development and Application of CRISPR/Cas System in Rice
Ren Jun#, Hu Xixun#, Wang Kejian, Wang Chun
(,,, China; These authors contributed equally to this work)
In the past several years, the CRISPR (clustered regularly interspaced short palindromic repeats)/Cas (CRISPR-associated protein) system has been harnessed as an efficient and powerful tool for targeted genome editing in different prokaryotic and eukaryotic species. Here, we review the development and application of CRISPR/Cas system in rice, emphasizing different varieties of CRISPR/Cas systems have been applied, strategies for multiplex editing, methods for precise gene insertion and replacement, and efficient systems for base editing and site-specific transcriptional regulation. In addition, the biosecurity of CRISPR/Cas system is also discussed, including transgene-free methods and off-target effects of CRISPR/Cas system. Thus, the development and application of CRISPR/Cas system will have significant impact on functional genomic research and variety improvement in rice.
rice; CRISPR; Cas; genome editing; protospacer adjacent motif
Although traditional mutagenesis methods, including chemical [e.g., ethyl methane sulfonate (EMS)] and physical (e.g., gamma ray) mutagenesis, can induce mutations in the rice genome, they still have some limitations, such as high randomness and low efficiency.In contrast, genome-editing technology using sequence- specific nucleases (SSNs) can edit specific genome loci with high accuracy and efficiency. The most prevalent SSNs are the zinc-finger nucleases (ZFNs) (Bibikova et al, 2003), transcription activator-like effector nucleases (TALENs) (Moscou and Bogdanove, 2009; Bogdanove and Voytas, 2011) and Cas proteins (Jinek et al, 2012). However, technologies using ZFNs and TALENs are difficult and costly, with relatively low efficiency, thus greatly limiting their wide application. The CRISPR (clustered regularly interspaced short palindromic repeats) / Cas (CRISPR-associated) system simplifies the operation of genome editing and provides a convenient and powerful tool for genetic engineering, and it has been widely used in rice for gene function analysis, breeding and so on (Shao et al, 2017; Shen et al, 2017b).
Mechanism of CRISPR/Cas system
As an adaptive defence system in bacteria and archaea, the CRISPR/Cas system protects organisms from invading viral and plasmid DNAs (Terns and Terns, 2011; Wiedenheft et al, 2012). Class 2 effectors are the most used Cas effectors, such as Cas9 (type II) and Cpf1 (type V). The fundamental components of the CRISPR/Cas9 system include the Cas9 protein, the trans-activating CRISPR RNA (tracrRNA) sequences and the CRISPR RNA (crRNA). The tracrRNA and crRNA are designed into single guide RNA (sgRNA), which is commonly used in genome editing (Jinek et al,2012). The Cas9 protein-sgRNA complex can generateDNA double-strand breaks (DSBs) at a specific genome locus via two domains of Cas9 endonucleases, HNH and RuvC-like domains. The HNH domain cleaves the complementary strand of sgRNA, and the RuvC-like domain cleaves the non-complementary strand. DSBs generated in eukaryotic organisms can be repaired via two mechanisms. One is the error-prone non-homologous end joining (NHEJ) pathway, which directly ligates two broken ends of DNA, thus often produces insertions and deletions (InDels) around DSBs (Mladenovand Iliakis, 2011). The other repair pathway is homology- directed repair (HDR), which accurately repairs DSBs by relying on a homologous DNA donor (Puchta and Fauser, 2014). This repair pathway occurs only in the G2 and S phases of cells with mitotic activity, whereas the NHEJ pathway exists in all types of cells at different points in the cell cycle.
Zetsche et al (2015) reported that another Cas protein, Cpf1 (CRISPR fromand1), can also be used as an easily programmable tool for genome editing. The mechanism of CRISPR/Cpf1 is similar to that of CRISPR/Cas9. However, the CRISPR/Cpf1 system has its own features: 1) Cpf1 is a nuclease with DNase and RNase activities, which allow it to process the pre-crRNA into mature crRNA. In contrast, the generation of mature crRNA in the CRISPR/Cas9 system requires RNase III. 2) Cpf1 recognises and cleaves targeted DNA with the help of crRNA, whereas Cas9 needs a longer chimera of crRNA and tracrRNA. 3) Before the pairing between crRNA and DNA template, the Cas effector needs to recognise the protospacer adjacent motif (PAM). The PAM recognised by Cas9 is ‘NGG’, located downstream of the target site, whereas the PAM recognised by Cpf1 is a T-rich sequence located upstream of the target site. 4) Cpf1 and Cas9 cleave DNA at sites distal and proximal to PAM, respectively.
CRISPR/Cas system in rice
Gene knock-out mediated by CRISPR/Cas system
Several research teams successively developed the CRISPR/Cas9 system in rice usingCas9 (SpCas9) following the successful achievement of genome editing in human and mice (Cong et al, 2013; Mali et al, 2013). Shan et al (2013) used codon-optimised SpCas9 to target rice endogenousgenes, in which the mutation frequency is 14.5%–38.0% in protoplasts and 4.0%–9.4% in transgenic lines. Meanwhile, Xie and Yang {Li, 2013 #38}(2013) used the CRISPR/ Cas9 system to editat three target sites to enhance the disease resistance of rice, and the mutation rates of these sites are 3%–8% in rice protoplasts. In addition, they reported that off-target mutations exist at a non-target site, and the editing efficiency is lower than the on-target site. Zhang et al (2014) and Ma et al (2015) indicated that homozygous mutations can be found in T0plants.
Cpf1 also can introduce mutations in the rice genome (Endo et al, 2016; Wang et al, 2017b; Xu et al, 2017). Endo et al (2016) and Xu et al (2017) expressed codon-optimisedCpf1 (FnCpf1) andND2006 Cpf1 (LbCpf1), respectively, and successfully achieved gene editing in rice. Moreover, Xu et al (2017) found that pre- crRNAs with a full-length direct repeat sequence have higher editing efficiencies than mature crRNAs. Meanwhile, Hu et al (2017) and Tang et al (2017) codon-optimised LbCpf1 andsp.Cpf1 (AsCpf1) with matched crRNA expression arrays and targeted multiple rice genome loci, respectively, and showed that LbCpf1 can effectively edit the rice genome, whereas AsCpf1 cannot achieve efficient genome editing. The above reports showed that fragment deletion is the most common type of mutation caused by Cpf1 in rice.
Establishment of multiplex gene-editing systems
Many researchers have successfully achieved single gene editing using the CRISPR/Cas9 system in rice. However, simultaneously targeting multiple genes is required in many cases. Based on Golden Gate ligationor Gibson Assembly, multiplex genome-editingsystems have been developed (Xing et al, 2014; Ma et al, 2015). Multiple (2–4) gRNA expression cassettes are assembled into a single binary vector in as little as one cloning step to simultaneously target multiplex genes.
Wang et al (2015) also established a system that can simultaneously integrate multiple gRNAs into a single vector based on the isocaudomer technique including two kinds of vectors in this system. To generate the final binary vector, these gRNAs generated by intermediate vectors will be integrated into the pC1300-Cas9 vector for multiplex genome editing. In this system, the number of targeting genes is not limited in theory. Shen et al (2017a) even successfully obtained an eight- mutant rice line using this system.
In addition, several researchers have designed methods that co-expressed Cas9 protein and gRNAs from a single PolII promoter. Tang et al (2016) designed a single transcriptional unit (STU) CRISPR/Cas9 system. The polyA sequences were used as a linker to co-express the Cas9 protein and gRNAs cassettes from a PolII promoter. A self-cleaving hammerhead ribozyme (RZ) was used to release multiple gRNAs. Moreover, another method was developed based on the endogenous mRNA splicing and tRNA-processing mechanism for simultaneously targeting multiplex endogenous genes (Ding et al, 2018). The polycistronic tRNA-gRNA (PTG) was assembled into the intron of Cas9 to generate multiple gRNA expression cassettes.
Expanding the scope of CRISPR/Cas system
Although the CRISPR/Cas system has been widely used in rice genome editing, the range of genome editing is severely limited by PAMs. Kaya et al (2016) successfully introduced targeted gene mutations with a Cas9 homologous protein,Cas9(SaCas9), which recognises NNGRRT PAMs, and the editing efficiency of SaCas9 is similar to that of SpCas9. The mutations generated by SaCas9 mainly are small deletions and small insertions, whereas large deletions (> 25 bp) are rarely detected. In addition, the T at the 6th position of PAM is proved to be necessary for SaCas9 in this study.
Cas effector variant (Kleinstiver et al, 2015; Gao et al,2017; Yamano et al, 2017) created by artificial evolution is another promising strategy in rice. Hu et al (2016) modified the codon-optimised SpCas9 protein to generate two kinds of Cas9 variants, VQR (D1135V/R1335Q/ T1337R) and VRER (D1135V/G1218R/R1335E/T1337R), in rice. The PAM sequences recognised by the VQR and VRER variants are NGA and NGCG, respectively. The development of the VQR and VRER variants doubles the scope of genome editing in rice.
Li et al (2018b) introduced point mutations in rice codon-optimised LbCpf1 to generate two variants, RR (G532R/K595R) and RVR (G532R/K538V/Y542R). The result demonstrated that only the RR variant can efficiently target genes containing non-canonical TYCV PAMs, and the highest mutation efficiency is about 51%. Similarly, Zhong et al (2018) reported that the RR variant of LbCpf1 can successfully recognise the PAM sequences of CCCC and TYCV in the rice genome. In addition, they reported the RVR variant recognised TATG PAM.
Meng et al (2018) reported that the CRISPR/Cas9 system can efficiently edit the gene locus containing NAG PAMs in rice, in which the efficiency is about three-quarters of NGG PAMs. It was accidentally discovered that SpCas9 can effectively recognise NAG PAMs when researchers detected potential off-target events. It is contrary to previous studies reported in animals and microorganisms, in which SpCas9 could rarely recognise NAG PAMs (about one-fifth of NGG) (Hsu et al, 2013; Jiang et al, 2013). This result not only expanded the available genome loci of SpCas9 in the rice genome but also reminded people that NAG PAMs should be considered when detecting off-target effect of the CRISPR/Cas9 system in rice.
Optimisation of CRISPR/Cas system
To easily and efficiently achieve multiplex genome editing, Xie et al (2015) and Hu et al (2017) engineered the endogenous tRNA-processing system as a robust tool to generate multiple sgRNAs and crRNAs for the CRISPR/Cas9 and CRISPR/Cpf1 systems, respectively. This method allows the multiple sgRNA/crRNA cassettes to be expressed under aU3/U6 promoter.
Hu et al (2016) showed that the Cas9-VQR can recognise and cleave sites containing NGA PAM in rice, which greatly expands the range of genome editing. However, the editing efficiency of Cas9-VQR is relatively low, which limits its application. To increase its efficiency, Hu et al (2018) modified sgRNA structures, and the strong endogenous promoters of rice() and() were used in CRISPR/Cas9-VQR system. Compared with the previous system, the average editing efficiency of CRISPR/ Cas9-VQR increases from three to seven times.
In addition, to improve the specificity of the CRISPR/Cas9 system, Zhang et al (2017) used three more specific Cas9 protein variants, eSpCas9 (1.0), eSpCas9 (1.1) and SpCas9-HF1, to achieve genome editing in rice. It has been demonstrated that the perfectly matched 20-nucleotide sgRNA sequences are necessary for high on-target editing efficiency and high fidelity of eSpCas9 and SpCas9-HF1 (Table 1).
Gene insertion and replacement mediated by CRISPR/Cas system
DSBs generated by the CRISPR/Cas system in the organism are mainly repaired via NHEJ pathway, which usually produce InDel mutations of several bases in DSBs. In certain cases, however, DNA fragment insertion and replacement can be achieved via the NHEJ and precise HDR-mediated repair.
Li et al (2016) achieved intron-mediated gene insertion and replacement based on the NHEJ pathway in the ricegene using the CRISPR/Cas9 system at frequencies of 2.0% and 2.2%, respectively. However, there are still some factors that limit the frequency of gene insertion and gene replacement, such as few copies of the donor DNA template and low insertion efficiency. To deal with these problems, Wang et al (2017a) co-transformed CRISPR/Cas9 and geminiviral vectors offering donor DNA templates into rice cells. The wheat dwarf virus was selected to provide abundant donor DNA templates because of its strong replication ability. According to Wang et al (2017b), the efficiency of accurate knock-in is up to 19.4% in transgenic rice plants.
Gene replacement was also achieved in the ricegene via the HDR pathway with CRISPR/Cas9 system (Sun et al, 2016). The designed vectors involves Cas9 expression cassettes, donor DNA template and two sgRNAs cassettes. The two sgRNAs were used to delete the fragment involving two sites of amino acid residues for substitution (W548L/S627I) in the ricegene. The two sites simultaneously target the vector to release the donor DNA, which include a 100-bp left homologous arm and 46-bp right homologous arm. he free 476-bp donor DNA templates and the vector were co-introduced into rice calli for providing enough donor fragments. The same substitution of amino acid in thegene is achieved by the CRISPR/Cpf1 system as well (Li et al, 2018a). It was also shown that precise targeted gene replacement mediated by HDR repair can be achieved with templates lacking the right homologous arm.
Other systems based on CRISPR/Cas system
Base editing
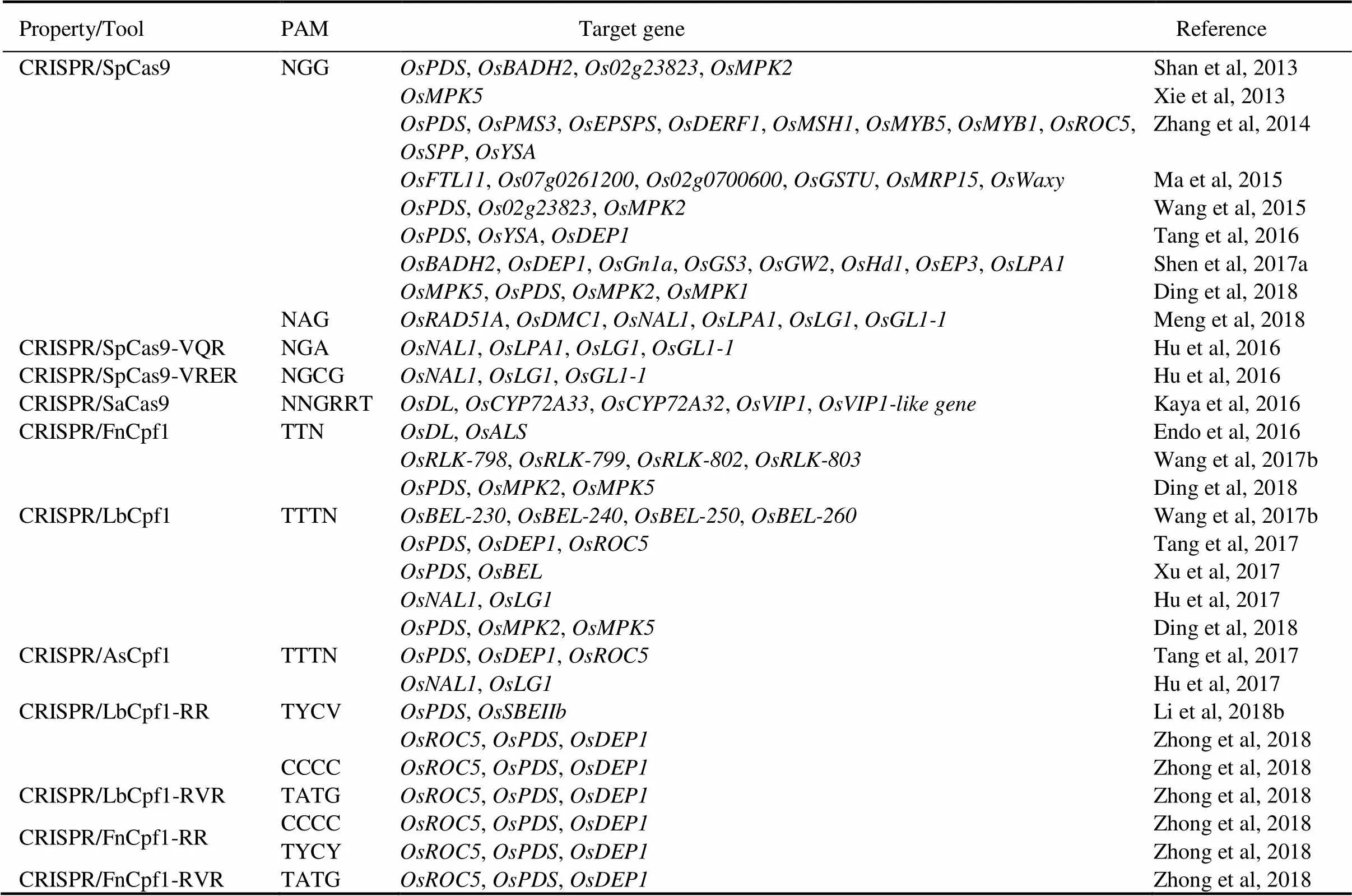
Table 1. CRISPR/Cas system used in rice.
PAM, Protospacer adjacent motif. N represents A/T/G/C base. R represents A/G base. Y represents T/C base. V represents A/C/G base.
The precise modification, such as point mutations and gene replacements generated by CRISPR/Cas system, remains a serious challenge because of its limited frequency.
Base editing (BE) systems combining CRISPR/ Cas9 and cytidine deaminase or adenine deaminase were first successfully applied in animal cells (Komor et al, 2016; Gaudelli et al, 2017), and were then quickly applied in rice. Researchers (Li J Y et al, 2017; Lu and Zhu, 2017; Zong et al, 2017) have reported the successful conversion of cytosine (C)-guanine (G) base pairs to adenine (A)-thymine (T) base pairs in rice using the BE system involving the cytidine deaminase enzyme APOBEC1, nSpCas9 (Cas9-D10A) and the uracil glycosylase inhibitor (UGI). The efficiency of the C to T conversion ranges from 0.39% to 43.48% in,and. Meanwhile, InDel mutations are also found in the target sites.
Subsequently, the conversion of A∙T base pairs to G∙C base pairs is achieved in rice (Li C et al, 2018; Hua et al, 2018a; Yan et al, 2018). The wild-typegene and its mutants are fused together to nSpCas9 for generating several BE systems in rice. The efficiency of A to G conversion is up to 62.26%, when TadA and TadA*7.10 were fused together to the N-terminus of nSpCas9. To expand the scope of the adenine base editor, nSaCas9 (Hua et al, 2018a), SaCas9 and SpCas9 variants (Hua et al, 2018b) were used in rice for BE as well, such as VQR- nSpCas9, VRER-nSpCas9 and SaKKH-nCas9. Moreover, no InDel mutations are found in the target sites.
Transcriptional regulation
Similarly, transcriptional regulatory elements fused with dCas9 can achieve transcriptional inhibition or activation in an organism. Lowder et al (2018) developedCRISPR-Act2.0 (dCas9-VP64 coupled with MS2-VP64via gRNA2.0) in rice, which can perform transcriptionalactivation of multiple genes. The system has a stronger effect on transcriptional activation than the first- generation dCas9-VP64 system previously reported (Lowder et al, 2015), indicating its wider application prospects in plant gene regulation.
Li Z X et al (2017) developed a dCas9-based transcriptional activation system, named dCas9-TV (dCas9-6TAL-VP128), which exhibits relatively strong transcriptional activation effects compared with the dCas9-VP64 activator inand rice. Moreover, dCas9-TV can also work in mammalian cells.
Transgene-free genome editing in rice
mediated callus infection, a prevalent transgenic technique used in rice, causes random insertions of T-DNA into the genome, which may lead to potential security problems. To achieve transgene- free genome editing, a new technology was developed.
Preassembled Cas9 protein-gRNA ribonucleoproteins (RNPs) are directly delivered into rice protoplasts to induce targeted genome modifications, in which mutation frequencies range from 8.4% to 19.0% (Woo et al, 2015). Using this method, the rice genome is edited without integration of exogenous DNA, while it is technically challenging to regenerate the edited rice protoplast into plants. The CYP81A6-hpRNAi expression element was used with the CRISPR/Cas9 system to generate transgene-free rice with expected mutations (Lu et al, 2017). The rice plants with an integrated CRISPR/Cas9 system andRNAi are sensitive to bentazone, showing a lethal symptom. This strategy greatly simplifies the screening process of transgene-free rice. Another strategy for efficient screening and enriching of transgene-free plants was developed using both REG2-BARNASE and 35S-CMS2 expression cassettes (He et al, 2018). It has been demonstrated that the suicide transgenes BARNASE and CMS2 can be used to eliminate transgenic lines in the T0generation without affecting the genome-editing efficiency of CRISPR/Cas9.
Off-target effects of CRISPR/Cas system
Undesired off-target mutations of Cas9 were reported in many studies, which are caused by a few nucleotide mismatches when sgRNAs recognize DNA templates (Jinek et al, 2012; Cong et al, 2013; Fu et al, 2013; Tsai et al, 2015; Kleinstiver et al, 2016). Recently, Tang et al (2018) assessed off-target effects of Cas9 and Cpf1 by a large-scale whole-genome sequencing (WGS) in rice. They found that only one Cas9 sgRNA results in off-target mutations among 12 Cas9 sgRNAs in T0lines, which shows a higher specificity of Cpf1, and the off-target sites could be predicted.
CONCLUSIONs
Compared with the previous two generations of genome-editing technology, the emergence of the CRISPR/Cas9 technology provides a simple, cheap and efficient genome editing platform for researchers. The platform not only provides strong support for basic research, but also accelerates the transformation of research results to application.
With the development and improvement of the CRISPR/Cas system in rice, new Cas9 variant proteins and homologous proteins, such as Cas9-VQR, Cas9-VRER, Cpf1-RR, Cpf1-RVR and SaCas9, have been created and applied in rice genome editing, which has greatly expanded its editing range. With the use of an endogenous promoter, improvement of sgRNA expression elements and other optimisation strategies, the editing efficiency of the rice CRISPR/ Cas9 system has been greatly improved, providing researchers with more powerful genome-editing tools. At the same time, genetic operating systems based on CRISPR/Cas9, such as gene insertion and replacement, single-BE and transcriptional regulation, have continued to emerge, providing new ideas for basic research and crop breeding. In terms of transgenic safety, rice transgene-free technology also achieves a great breakthrough. Mutants without transgenic ingredients can be obtained in their progeny through an instantaneousediting or screening system (e.g., resistanceand suicide genes). In addition, several studies have shown that the off-target effect of the CRISPR/Cas technology is generally controllable, and Cpf1 has a smaller off- target effect on the rice genome than Cas9.
However, there are still some pressing problems for rice genome editing, such as the efficient delivery of the CRISPR/Cas9 system without integrating into the rice genome. The means to accurately knock-in and replace endogenous genes via the HDR repair are relatively limited. Previous studies have revealed significant differences in the efficiency of SpCas9 recognising NAG PAM in rice and human cells (Hsu et al, 2013; Jiang et al, 2013; Meng et al, 2018). Therefore, the investigation of the CRISPR/Cas system in rice still requires a lot of research due to the differences between mammalian and plant cells.
Acknowledgements
This study was supported by the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences and the National Natural Science Foundation of China (Grant No. 31871703).
Bibikova M, Beumer K, Trautman J K, Carroll D. 2003. Enhancing gene targeting with designed zinc finger nucleases., 300: 764.
Bogdanove A J, Voytas D F. 2011. TAL effectors: Customizable proteins for DNA targeting., 333: 1843.
Cong L, Ran F A, Cox D, Lin S L, Barretto R, Habib N, Hsu P D, Wu X B, Jiang W Y, Marraffini L A, Zhang F. 2013. Multiplex genome engineering using CRISPR/Cas systems., 8(11): 819–823.
Ding D, Chen K Y, Chen Y D, Li H, Xie K B. 2018. Engineering introns to express RNA guides for Cas9- and Cpf1-mediated multiplex genome editing., 11(4): 542–552.
Endo A, Masafumi M, Kaya H, Toki S. 2016. Efficient targeted mutagenesis of rice and tobacco genomes using Cpf1 from., 6: 38169.
Fu Y, Foden J A, Khayter C, Maeder M L, Reyon D, Joung J K, Sander J D. 2013. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells., 31(9): 822–826.
Gao L Y, Cox D B T, Yan W X, Manteiga J C, Schneider M W, Yamano T, Nishimasu H, Nureki O, Crosetto N, Zhang F. 2017. Engineered Cpf1 variants with altered PAM specificities., 35(8): 789–792.
Gaudelli N M, Komor A C, Rees H A, Packer M S, Badran A H, Bryson D I, Liu D R. 2017. Programmable base editing of A·T to G·C in genomic DNA without DNA cleavage., 551: 464–471.
He Y B, Zhu M, Wang L H, Wu J H, Wang Q Y, Wang R C, Zhao Y D. 2018. Programmed self-elimination of the CRISPR/Cas9 construct greatly accelerates the isolation of edited and transgene-free rice plants., 11(9): 1210–1213.
Hsu P D, Scott D A, Weinstein J A, Ran F A, Konermann S, Agarwala V, Li Y, Fine E J, Wu X, Shalem O, Cradick T J, Marraffini L A, Bao G, Zhang F. 2013. DNA targeting specificity of RNA-guided Cas9 nucleases., 31(9): 827–832.
Hu X X, Wang C, Fu Y P, Liu Q, Jiao X Z, Wang K J. 2016. Expanding the range of CRISPR/Cas9 genome editing in rice., 9(6): 943–945.
Hu X X, Wang C, Liu Q, Fu Y P, Wang K J. 2017. Targeted mutagenesis in rice using CRISPR-Cpf1 system., 44(1): 71–73.
Hu X X, Meng X B, Liu Q, Li J Y, Wang K J. 2018. Increasing the efficiency of CRISPR-Cas9-VQR precise genome editing in rice., 16(1): 292–297.
Hua K, Tao X P, Yuan F T, Wang D, Zhu J K. 2018a. Precise A·T to G·C base editing in the rice genome., 11(4): 627–630.
Hua K, Tao X P, Zhu J K. 2018b. Expanding the base editing scope in rice by using Cas9 variants., doi: 10.111/pbi.12993.
Jiang W Y, Bikard D, Cox D, Zhang F, Marraffini L A. 2013. RNA-guided editing of bacterial genomes using CRISPR-Cas systems., 31(3): 233–239.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna J A, Charpentier E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity., 337: 816–821.
Kaya H, Mikami M, Endo A, Endo M, Toki S. 2016. Highly specific targeted mutagenesis in plants usingCas9., 6: 26871.
Kleinstiver B P, Prew M S, Tsai S Q, Topkar V V, Nguyen N T, Zheng Z, Gonzales A P, Li Z, Peterson R T, Yeh J R, Aryee M J, Joung J K. 2015. Engineered CRISPR-Cas9 nucleases with altered PAM specificities., 523: 481–485.
Kleinstiver B P, Tsai S Q, Prew M S, Nguyen N T, Welch M M, Lopez J M, McCaw Z R, Aryee M J, Joung J K. 2016. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells., 34(8): 869–874.
Komor A C, Kim Y B, Packer M S, Zuris J A, Liu D R. 2016. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage., 533: 420–424.
Li C, Zong Y, Wang Y P, Jin S, Zhang D B, Song Q N, Zhang R, Gao C X. 2018. Expanded base editing in rice and wheat using a Cas9-adenosine deaminase fusion., 19: 59.
Li J, Meng X B, Zong Y, Chen K L, Zhang H W, Liu J X, Li J Y, Gao C X. 2016. Gene replacements and insertions in rice by intron targeting using CRISPR-Cas9., 2: 16139.
Li J Y, Sun Y W, Du J L, Zhao Y D, Xia L Q. 2017. Generation of targeted point mutations in rice by a modified CRISPR/Cas9 system., 10(3): 526–529.
Li S Y, Li J Y, Zhang J H, Du W M, Fu J D, Sutar S, Zhao Y D, Xia L Q. 2018a. Synthesis-dependent repair of Cpf1-induced double-strand DNA breaks enables targeted gene replacement in rice., 69(20): 4715–4721.
Li S Y, Zhang X, Wang W S, Guo X P, Wu Z C, Du W M, Zhao Y D, Xia L Q. 2018b. Expanding the scope of CRISPR/Cpf1- mediated genome editing in rice., 11(7): 995–998.
Li Z X, Zhang D D, Xiong X Y, Yan B Y, Xie W, Sheen J, Li J F. 2017. A potent Cas9-derived gene activator for plant and mammalian cells., 3(12): 930–936.
Lowder L G, Zhang D W, Baltes N J, Paul J W, Tang X, Zheng X L, Voytas D F, Hsieh T F, Zhang Y, Qi Y P. 2015. A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation., 169(2): 971–985.
Lowder L G, Zhou J P, Zhang Y X, Malzahn A, Zhong Z H, Hsieh T F, Voytas D F, Zhang Y, Qi Y P. 2018. Robust transcriptional activation in plants using multiplexed CRISPR-Act2.0 and mTALE-Act systems., 11(2): 245–256.
Lu H P, Liu S M, Xu S L, Chen W Y, Zhou X, Tan Y Y, Huang J Z, Shu Q Y. 2017. CRISPR-S: An active interference element for a rapid and inexpensive selection of genome-edited, transgene-free rice plants., 15(11): 1371–1373.
Lu Y, Zhu J K. 2017. Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system., 10(3): 523–525.
Ma X L, Zhang Q Y, Zhu Q N, Liu W, Chen Y, Qiu R, Wang B, Yang Z F, Li H Y, Lin Y R, Xie Y Y, Shen R X, Chen S F, Wang Z, Chen Y, Guo J X, Chen L T, Zhao X C, Dong Z C, Liu Y G. 2015. A robust CRISPR/Cas9 system for convenient, high- efficiency multiplex genome editing in monocot and dicot plants., 8(8): 1274–1284.
Mali P, Yang L H, Esvelt K M, Aach J, Guell M, DiCarlo J E, Norville J E, Church G M. 2013. RNA-guided human genome engineering via Cas9., 339: 823–826.
Meng X B, Hu X X, Liu Q, Song X G, Gao C X, Li J Y, Wang K J. 2018. Robust genome editing of CRISPR-Cas9 at NAG PAMs in rice., 61(1): 122–125.
Mladenov E, Iliakis G. 2011. Induction and repair of DNA double strand breaks: The increasing spectrum of non-homologous end joining pathways., 711: 61–72.
Moscou M J, Bogdanove A J. 2009. A simple cipher governs DNA recognition by TAL effectors., 326: 1501.
Puchta H, Fauser F. 2014. Synthetic nucleases for genome engineering in plants: Prospects for a bright future., 78(5): 727–741.
Shan Q W, Wang Y P, Li J, Zhang Y, Chen K L, Liang Z, Zhang K, Liu J X, Xi J J Z, Qiu J L, Gao C X. 2013. Targeted genome modification of crop plants using a CRISPR-Cas system., 31(8): 686–688.
Shao G N, Xie L H, Jiao G A, Wei X J, Sheng Z H, Tang S Q, Hu P S. 2017. CRISPR/CAS9-mediated editing of the fragrant genein rice., 31(2): 216–222. (in Chinese with English abstract)
Shen L, Hua Y F, Fu Y P, Li J, Liu Q, Jiao X Z, Xin G W, Wang J J, Wang X C, Yan C J, Wang K J. 2017a. Rapid generation of genetic diversity by multiplex CRISPR/Cas9 genome editing in rice., 60(5): 506–515.
Shen L, Li J, Fu Y P, Wang J J, Hua Y F, Jiao X Z, Yang C J, Wang K J. 2017b. Orientation improvement of grain length and grain number in rice by using CRISPR/Cas9 system., 31(3): 223–231. (in Chinese with English abstract)
Sun Y W, Zhang X, Wu C Y, He Y B, Ma Y Z, Hou H, Guo X P, Du W M, Zhao Y D, Xia L Q. 2016. Engineering herbicide-resistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase., 9(4): 628–631.
Tang X, Zheng X L, Qi Y P, Zhang D W, Cheng Y, Tang A, Voytas D F, Zhang Y. 2016. A single transcript CRISPR-Cas9 system for efficient genome editing in plants., 9(7): 1088–1091.
Tang X, Lowder L G, Zhang T, Malzahn A A, Zheng X, Voytas D F, Zhong Z H, Chen Y Y, Ren Q R, Li Q, Kirkland E R, Zhang Y, Qi Y P. 2017. A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants., 3: 17018.
Tang X, Liu G Q, Zhou J P, Ren Q R, You Q, Tian L, Xin X H, Zhong Z H, Liu B L, Zheng X L, Zhang D W, Malzahn A, Gong Z Y, Qi Y P, Zhang T, Zhang Y. 2018. A large-scale whole- genome sequencing analysis reveals highly specific genome editing by both Cas9 and Cpf1 (Cas12a) nucleases in rice., 19: 84.
Terns M P, Terns R M. 2011. CRISPR-based adaptive immune systems., 14(3): 321–327.
Tsai S Q, Zheng Z L, Nguyen N T, Liebers M, Topkar V V, Thapar V, Wyvekens N, Khayter C, Iafrate A J, Le L P, Aryee M J, Joung J K. 2015. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases., 33(2): 187–197.
Wang C, Shen L, Fu Y P, Yan C J, Wang K J. 2015. A simple CRISPR/Cas9 system for multiplex genome editing in rice., 42(12): 703–706.
Wang M G, Lu Y M, Botella J R, Mao Y F, Hua K, Zhu J K. 2017a. Gene targeting by homology-directed repair in rice using a geminivirus-based CRISPR/Cas9 system., 10(7): 1007–1010.
Wang M G, Mao Y F, Lu Y M, Tao X P, Zhu J K. 2017b. Multiplex gene editing in rice using the CRISPR-Cpf1 system., 10: 1011–1013.
Wiedenheft B, Sternberg S H, Doudna J A. 2012. RNA-guided genetic silencing systems in bacteria and archaea., 482: 331–338.
Woo J W, Kim J, Kwon S I, Corvalan C, Cho S W, Kim H, Kim S G, Kim S T, Choe S, Kim J S. 2015. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins., 33(11): 1162–1164.
Xie K B, Yang Y N. 2013. RNA-guided genome editing in plants using a CRISPR-Cas system., 6(6): 1975–1983.
Xie K B, Minkenberg B, Yang Y N. 2015. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system., 112(11): 3570–3575.
Xing H L, Dong L, Wang Z P, Zhang H Y, Han C Y, Liu B, Wang X C, Chen Q J. 2014. A CRISPR/Cas9 toolkit for multiplex genome editing in plants., 14: 327.
Xu R F, Qin R Y, Li H, Li D D, Li L, Wei P C, Yang J B. 2017. Generation of targeted mutant rice using a CRISPR-Cpf1 system., 15(6): 713–717.
Yamano T, Zetsche B, Ishitani R, Zhang F, Nishimasu H, Nureki O. 2017. Structural basis for the canonical and non-canonical PAM recognition by CRISPR-Cpf1., 67(4): 633–645.
Yan F, Kuang Y J, Ren B, Wang J W, Zhang D W, Lin H H, Yang B, Zhou X P, Zhou H B. 2018. Highly efficient A·T to G·C base editing by Cas9n-guided tRNA adenosine deaminase in rice., 11(4): 631–634.
Zetsche B, Gootenberg J S, Abudayyeh O O, Slaymaker I M, Makarova K S, Essletzbichler P, Volz S E, Joung J, van der Oost J, Regev A, Koonin E V, Zhang F. 2015. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system., 163(3): 759–771.
Zhang D B, Zhang H W, Li T D, Chen K L, Qiu J L, Gao C X. 2017. Perfectly matched 20-nucleotide guide RNA sequences enable robust genome editing using high-fidelity SpCas9 nucleases., 18(1): 191.
Zhang H, Zhang J S, Wei P L, Zhang B T, Gou F, Feng Z Y, Mao Y F, Yang L, Zhang H, Xu N F, Zhu J K. 2014. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation., 12(6): 797–807.
Zhong Z H, Zhang Y X, You Q, Tang X, Ren Q R, Liu S S, Yang L J, Wang Y, Liu X P, Liu B L, Zhang T, Zheng X L, Le Y, Zhang Y, Qi Y P. 2018. Plant genome editing using FnCpf1 and LbCpf1 nucleases at redefined and sltered PAM sites., 11(7): 999–1002.
Zong Y, Wang Y P, Li C, Zhang R, Chen K L, Ran Y D, Qiu J L, Wang D W, Gao C X. 2017. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion.,35(5): 438–440.
(Managing Editor: Wang Caihong)
Copyright © 2019, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2019.01.001
21 November 2018;
8 January 2019
Wang Chun (wangchun@caas.cn)
杂志排行
Rice Science的其它文章
- Improvements of TKC Technology Accelerate Isolation of Transgene-Free CRISPR/Cas9-Edited Rice Plants
- Production of Two Elite Glutinous Rice Varieties by Editing Wx Gene
- Rapid Creation of New Photoperiod-/Thermo-Sensitive Genic Male-Sterile Rice Materials by CRISPR/Cas9 System
- CRISPR/Cas9-Mediated Adenine Base Editing in Rice Genome
- Targeted Mutagenesis of NAC Transcription Factor Gene, OsNAC041, Leading to Salt Sensitivity in Rice
- Characterization and Evaluation of OsLCT1 and OsNramp5 Mutants Generated Through CRISPR/Cas9-Mediated Mutagenesis for Breeding Low Cd Rice
