Editing of Rice Isoamylase Gene ISA1 Provides Insights into Its Function in Starch Formation
2019-02-19ChaoShufenCaiYicongFengBaobingJiaoGuiaiShengZhonghuaLuoJuTangShaoqingWangJianlongHuPeisongWeiXiangjin
Chao ShufenCai YicongFeng BaobingJiao GuiaiSheng Zhonghua,Luo JuTang ShaoqingWang JianlongHu PeisongWei Xiangjin
Editing of Rice Isoamylase GeneProvides Insights into Its Function in Starch Formation
Chao Shufen1,#, Cai Yicong1,#, Feng Baobing1, Jiao Guiai1, Sheng Zhonghua1,Luo Ju1, Tang Shaoqing1, 2, Wang Jianlong2, Hu Peisong1, Wei Xiangjin1
()
Isoamylase 1 (ISA1) is an isoamylase-type debranching enzyme which plays a predominant role in amylopectin synthesis. In this study, the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated endonuclease 9 (CRISPR/Cas9) system was used to editgene in rice-mediated transformation. We identified 36 genetic edited lines from 55 T0transgenic events, and classified the mutation forms into 7 types. Of those, two homozygous mutants,(type 1, with an adenine insertion) and(type 3, with a cytosine deletion) were selected for further analysis. Seed sizes of bothandwere affected, and the two mutants also displayed a shrunken endosperm with significantly lower grain weight. Electron microscopy analysis showed that abnormal starch granules and amyloplasts were found inandendosperm cells. The contents of total starch, amylose and amylopectin in the endosperm ofthemutants were significantly reduced, whereas sugar content and starch gel consistency were observably increased compared to the wild-type. The gelatinization temperature and starch chain length distributions of themutants were also altered. Moreover, transcript levels of most starch synthesis-related genes were significantly lower inmutants. In conclusion, the results indicated that gene edition ofaffectedstarch synthesis and endosperm development, and brought potential implications for rice quality breeding.
; rice; starch biosynthesis; starch granule; physicochemical property; CRISPR; Cas9
Rice is a main food crop which provides essential carbohydrates and energy to nearly half of the world’s population. Starch, as the main component (up to 90%) of a milled rice kernel, plays a crucial role in rice quality. With the progress of peopleʼs living standards, the demand for improved rice quality has increased in recent years, and therefore, cultivating high-quality cultivars has received more attention (Rao et al, 2014).
Starch is composed of two different glucose polymers:amylose, formed by linear α-1,4-glucosidic chains,and amylopectin, the major component (approximately 65%–85%) forming cluster structures that consist of α-1,4-linked glucose residues highly branched through α-1,6-linkages (Thompson, 2000). Studies have shown that there are various enzymes involved in amylose and amylopectin biosynthesis, including ADP-glucose pyrophosphorylase (AGPase), granule bound starch synthase (GBSS), soluble starch synthase (SS), starch branching enzyme (BE), starch debranching enzyme (DBE) and plastidial starch phosphorylase (Pho) (Jeon et al, 2010; Pfister and Zeeman, 2016). AGPase catalyzes an adenylyl transfer reaction with adenosine 5-triphosphate and glucose-1-phosphate to produce ADP-Glc and pyrophosphate, which is regarded as the first rate-limiting step in starch synthesis (Haugen et al,1976). Previous studies have shown that mutations in specific AGPase genes lead to serious decline in starch synthesis, resulting in a shrunken endosperm phenotype (Lee et al, 2007). GBSS consists of two isoforms, GBSSI which functions primarily in stored tissues (such as the seed endosperm), and GBSSII which plays a role in non-storage plant tissues (e.g. leaves) to accumulate starch temporarily (Vrinten and Nakamura, 2000). Mutation of the() gene, which encodes GBSSI, causes significant reduction in amylose content (Han et al, 2004). There are at least three SS classes in plants (SSI, SSII and SSIII), which are responsible for the elongation of the glucan chains by creating α-1,4-linked glucose bonds (Smith et al, 1997).A mutant ofdisplays a white-core floury endosperm, alters starch granules morphology, and causes changes in the proportion of the degree of polymerization (DP) values of amylopectin chains (Ryoo et al, 2007). BE has at least three isoforms in rice endosperm (BEI, BEII and BEIII), which forms branch points by introducing α-1,6-linkages with glucose residues into α-1,4-glucosidic chains (Mizuno et al, 1992; Nakamura et al, 1992). Phosphorylase and disproportionating enzymes (DPE) are present in plastidic (Pho1, DPE1) and cytoplasmic (Pho2, DPE2) forms. Both Pho1 and Pho2 catalyze the transfer of glucosyl units to non-reducing ends of α-glucan chains. OsPho1 and OsDpe1 assemble together to form a stable protein complex that participate in the synthesis of oligo-maltose, and play an important role in starch synthesis and degradation (Akdogan et al, 2011; Hwang et al, 2016).Genetic and biochemical analyses have been performed in DBE in(Delatte et al, 2005; Wattebled et al, 2005), rice (Kubo et al,1999), maize (James et al, 1995), barley (Burton et al, 2002), wheat (Sestili et al, 2016) and potato (Ferreira et al, 2017). Two types of DBE have been identified, isoamylase (ISA1, ISA2 and ISA3) and pullulanase (PUL) (Nakamura et al, 1996; Kubo et al, 2005). Mutations in thegene show a dramatic change in the structure of amylopectin (James et al, 1995; Kubo et al,1999; Burton et al, 2002; Sestili et al, 2016; Ferreira et al, 2017). Knocking downRNAi in durum wheat alters the starch composition in endosperm, resulting in decreasing in starch content, increasing in contents of phytoglycogen and phytoglucan, and altering the fine structure of amylopectin (Sestili et al, 2016). Chimeric RNAi of three isoamylase genes in potato displays tissue-specific impairment in starch metabolism, which is translated into significant decrease of starch content and reductionof starch granule size in tuber but without affecting the starch in leaves(Ferreira et al, 2017). ISA1 can exist as a homo-oligomer and can also form hetero-oligomer with ISA2, which is also important in the synthesis of starch (Kawagoe et al, 2005;Utsumi and Nakamura, 2006). Kawagoe et al (2005) used an amyloplast-targeted green fluorescent protein to track amyloplasts and starch granules formation inmutant and found no granules in the sugary endosperm of themutant, indicating that ISA1 is crucial in the early stages of starch granule formation. In addition, ISA1is found to be directly interact with FLO6 (FLOURY ENDOSPERM 6, a CBM-domain protein that binds starch) to affect starch synthesis in developing rice seeds (Peng et al, 2014).
Although there is established information about the effect of up- and down-regulation ofgenes on starch formation in rice and other species (James et al, 1995;Kubo et al,1999; Burton et al, 2002;Delatte et al, 2005; Wattebled et al, 2005; Sestili et al, 2016; Ferreira et al, 2017), detailed effects on amylopectin characteristics controlled byare still incipient. To further understand this, we generated-deficient mutants () using the CRISPR/Cas9 genomic editing system. Such mutants displayed shrunken endosperm, significantly reduced amylose content and increased content of total soluble sugar, altered expression of genes associated to starch synthesis, reduced grain weight and altered starch granule morphology.
MATERIALS AND METHODS
Rice materials and growth conditions
T0and T1generations oftransgenic lines generated using the clustered regularly interspaced short palindromic repeats/CRISPR-associated endo- nuclease 9 (CRISPR/Cas9) systemand wild-type (WT) (subsp.cv. Zhonghua 11, ZH11) were grown under natural conditions in the fields of China National Rice Research Institute, Fuyang, Hangzhou, China in 2016 (T0and WT) and 2017 (T1and WT), respectively. ZH11 was selected as the genetic background due to its efficient transformation rate, moderate growth period and high seed-setting rate. Initial sowing was carried out on 20 May, and seedlings were transplanted into the paddy field on 15 June, with an interplant separation of 13 cm and an inter-row separation of 27 cm. Fertilizer and water managements were used as standard field production. At least five mature plants from wild-type and mutants were used to record the agronomic traits, and the seeds were used to examine the grain shape and weight. All assays consisted of three biological replicates.
Knock out ISA1 gene by CRISPR/Cas9 system
The target site (TGGACGGCGTGAGCACGATC) in the 18th exon of(LOC_Os08g40930) was designed via CRISPR direct website (http://crispr.dbcls.jp) (Naito et al, 2015). The gRNA was controlled by the rice U6 promoter, whereasby thepromoter and theresistance gene () by the CaMV 35S promoter, all in the VK005 vector (Primers Target-F and Target-R, Beijing Viewsolid Biotech Co., Ltd., http://www.v-solid.com/Catalog. No. VK005-01). The Sq-primer was used to sequence the vector. The construct was introduced into wild- type rice using thestrain EHA105 (Hiei et al, 1997). Positive transgenic lines were identified by PCR amplification of afragment (Primers Hyg-F and Hyg-R), and the analysis of the genotypes was performed by sequencing an amplified PCR product of a specific transgenic fragment (500 bp) near the protospacer adjacent motif (PAM) sequence (with the primers Cas9-F and Cas9-R). Primer pairs for PCR amplification and sequencing data are shown in Supplemental Table 1. All the mutation forms were identified by the DSDecode website (http://dsdecode.scgene.com/home/) (Liu et al, 2015; Ma et al, 2015). Prediction of the three-dimensional protein structures was conducted using the website Phyre2 (http://www.sbg.bio.ic.ac.uk/ phyre2), and the comparison of the three-dimensional protein structures between the wild-type and each mutation types was performed using the tool Swiss-PdbViewer 4.1.0 (https://spdbv.vital-it.ch/).
Microscopy analysis
Brown rice grains of wild-type and themutants were cut transversely with a sharp blade, and gold was coated on the surface of the ruptured seed to prepare samples according to Kang et al (2005). The photographs were examined with a scanning electron microscope (S-3400N, Hitachi, Tokyo, Japan). Transverse sections of developing endosperms at 10 d after flowering (DAF) were performed as following: the endosperms were cut into approximately 1 mm thick sections, and then placed overnight in 2.5% glutaraldehyde fixation buffer[0.1 mol/L phosphate buffer (pH 7.2), 2% glutaraldehyde and 2% paraformaldehyde]. After dehydration in a series of progressively concentrated ethanol solution, samples were embeded into LR white resin (London Resin, Berkshire, UK, http://www.2spi.com/), sectionedwith an ultramicrotome (Leica UC7; http://www.leicamicro systems.com) and then imaged with a transmission electron microscope (H-7650, Hitachi, Japan).
Analysis of starch physicochemical properties in mature grains
Total starch content of the brown rice flour was determined using a starch assay kit (Megazyme, Wicklow, Ireland; http://www.megazyme.com) according to the manufacturerʼs instructions. First, 50 mg mature grain flour samples were washed in 5 mL of 80% ethanol to remove sugar. Amylose content was determined using the method described by Liu et al (2009). Total soluble sugar content was quantified using the phenol-sulfuric acid method (Masuko et al, 2005). Briefly, 30 mL ddH2O was added into screw-capped tubes containing about 50 mg brown rice flour. Samples were incubated in a boiling water bath for 20 min, and filtrated, and then the volume was adjusted to 100 mL. One mL each sample and a series of glucose standards were mixed with 5 mL phenol-sulfuric acid, and incubated in boiling water bath for 10 min. Total soluble sugar content was calculated with the absorbance values recorded at 620 nm using a spectrophotometer (DU800, Beckman Coulter, USA). Gel consistency and alkali values were determined and analyzed according to the China Agriculture Industry Standard NY/T147-88 (Agricultural Industry Standard of China, 2002). Thermal characteristics were measured with a Modulated Differential Scanning Calorimeter (MDSC, DSC1 STARe system, METTLER- TOLEDO) as described by Kweon et al (2000) with minor modifications. About 5 mg dry grain flour samples were placed in a sample tray, and 10mL ddH2O was added with gently mix. The tray was then sealed and subjected to heat treatment for 5.5 min from 35 ºC to 90 ºC, increasing 10 ºC per minute. An empty tray was used as reference. Differences in thermal characteristics between the wild-type,andwere shown using MDSC curves. All assays were done with three biological replicates. Determination of the chain length distribution of amylopectin was performed according to Li et al (2017).
RNA extraction and quantitative real-time PCR (qRT-PCR) analysis
To investigate the expression of genes associated to starch synthesis, total RNA was extracted from 10 DAF seeds (due to most of these genes have a relatively stable and high expression levels at this stage) by using the Trizol reagent (Invitrogen, https://www.thermofisher.com). First-strand cDNA was synthesized with 2 μg total RNA using the ReverTra Ace qPCR RT Kit (Toyobo, http://www.bio-toyobo.cn) according to the manufacturerʼs protocol. qRT-PCR was performed using a Light Cycler 480 device (Roche, http://www.roche-applied-science.com) with the SYBR Green Real-time PCR Master Mix (Toyobo, http://www.bio-toyobo.cn) in 20 µL reaction volume. The qRT-PCR conditions were as follows: 95 ºC for 30 s, 40 cycles of 95 ºC for 5 s, 60 ºC for 35 s and 95 ºC for 15 s. Assays included three biological replications. The gene-specific primers related to starch synthesis were those described by She et al (2010). Thegene (Os03g0718150, Supplemental Table 1) was used as an internal control, and the relative expression level was calculated by the 2-ΔΔCTmethod (Schmittgen and Livak, 2008).
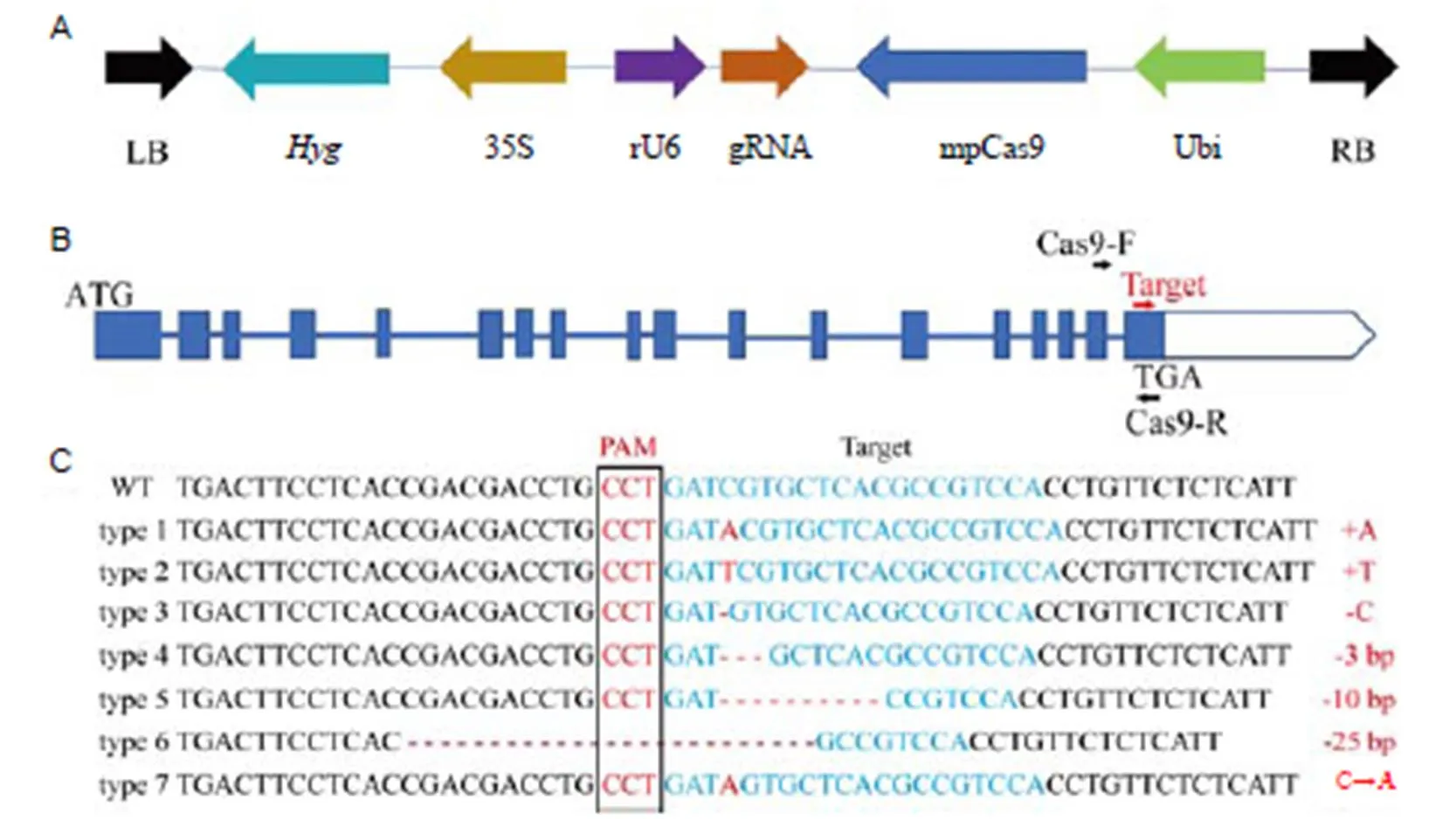
Fig. 1.CRISPR/Cas9 mediated editing of.
A, The structure of the T-DNA region of the Cas9/guide RNV (gRNA) vector. Marker gene() was driven by the CaMV35S(35S) promoter whereas the gRNA was driven by the rice U6 promoter and the mpCas9 was driven by the Ubiquitin (Ubi) promoter. LB, Left border; RB, Right border. B, The structure ofgene. The primer pairs (Cas9-F and Cas9-R) were used to amplify the region that was sequenced in the genotypes. C, Identification of generated mutation forms inby sequencing of the target site (protospacer adjacent motif region) in T0transgenic events. PAM, Protospacer adjacent motif. The mutation forms in all 36 T0transgenic events can be divided into 7 categories. Homozygous mutations included type 1 to type 6, while a heterozygous mutation was classified as type7. Type1 and type3 mutations were selected for further analysis.
RESULTS
Knock out of ISA1 by CRISPR/Cas9 system
To deepen our knowledge on the function of(LOC_Os08g40930), a CRISPR/Cas9 vector containing a gRNA driven by the rice U6 promoter (Fig. 1-A) and carrying one target site for the 18th exon ofwas constructed (Fig. 1-B). The plasmid was then inserted into wild-type (ZH11) calli- mediated transformation. A total of 55 T0transgenic plants were ultimately obtained. Sequencing analysis of thegenomic locus in each T0transgenic plant was performed to determine whether the targeted mutation had occurred. Results showed that 36 independent plants presented an edited sequence near the protospacer adjacent motif (PAM) region. Six types of homozygous and one heterozygous mutations at the target site were found: an adenine (A) insertion, a cytosine (C) deletion, and other types of heterozygous mutations (Fig. 1-C). Alignment of putative amino acid sequences also showed different edited forms of ISA1 (Supplemental Fig. 1). Comparison of the predicted three-dimensional protein structure between wild-type and each type ofedited sequencesSwiss-PdbViewer 4.1.0 indicated that there were differences in the protein between wild-type and mutants (Supplemental Fig. 2). Two transgenic lines,(type 1 mutation form, with an adenine (A) insertion) and(type 3, with a cytosine (C) deletion) with enough grains were selected for further phenotypic analysis (Fig. 1-C and Supplemental Fig. 2).
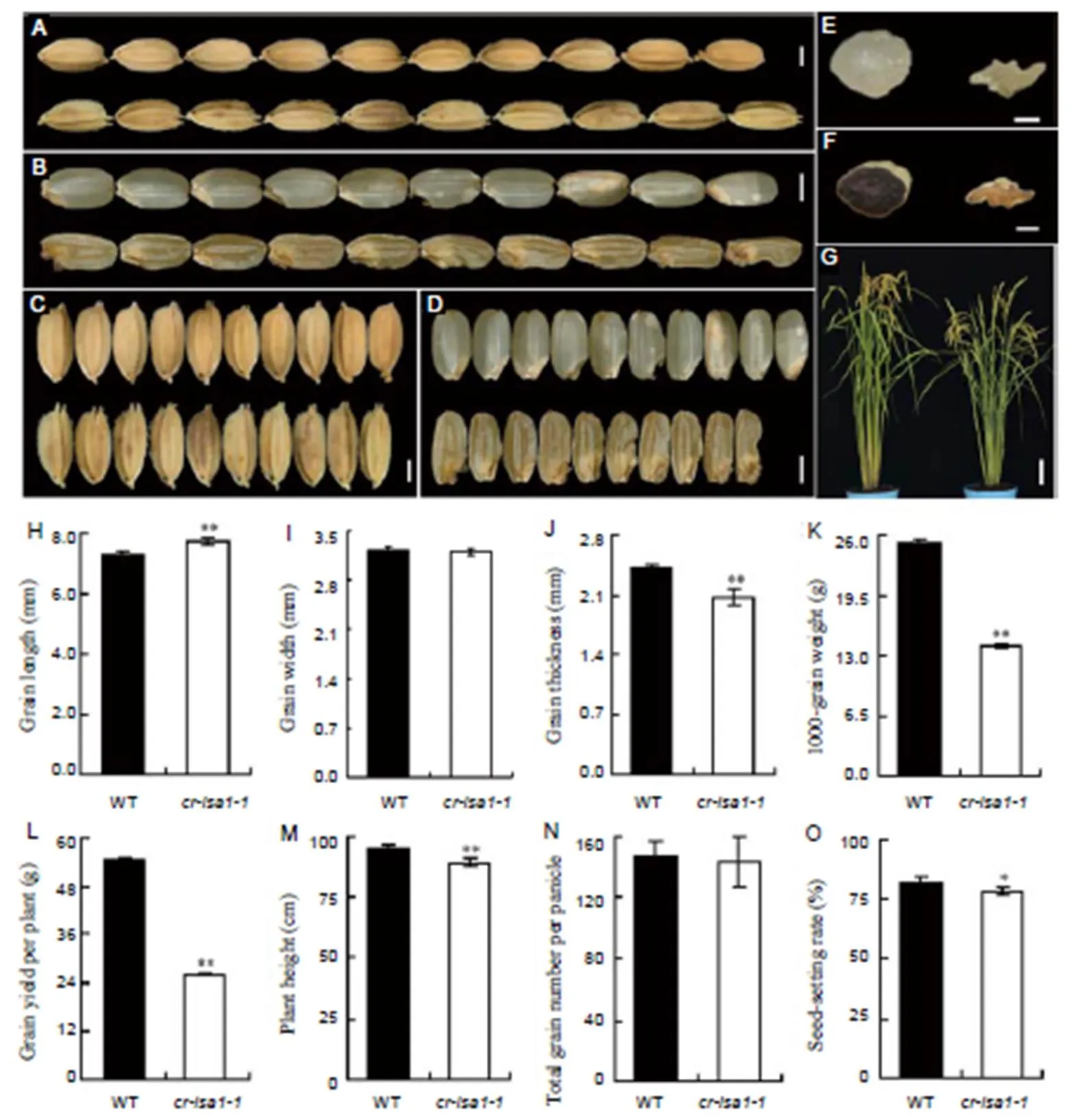
Fig. 2.Phenotypes of the wild-type (WT) andmutant (T1generation).
A,Appearance of mature seeds for WT (above) and(below). B,Appearance of brown rice for WT (above) and(below). C, Grain widths of WT (above) and(below). D,Brown rice widths of WT (above) and thetransgenic line (below). E, Cross sections of WT (left) andtransgenic line (right) brown rice. F, Iodine staining of brown rice in WT (left) andtransgenic line (right). G, Representative plants of WT (left) and(right) after heading. H, Comparison of grain length. I, Comparison of grain width. J, Comparison of grain thickness. K, Comparison of 1000-grain weight. L, Comparison of grain yield per plant. M, Comparison of plant height. N, Comparison of total grain number per panicle. O, Comparison of seed-setting rate. Scale bars are 2mm in A to D, 1 mm in E and F, and 10cm in G. Values in H to O are Mean ± SD from three biological replicates, with no less than 50, 50, 50 and 200 seeds in each replication for H, I, J and K, and no less than 5 plants for L to O, respectively. Asterisks indicate statistical significance by the Student’s-test (*,< 0.05; **,< 0.01).
cr-isa1 mutants have shrunken endosperms and defects in seed development
The seed appearance and yield traits of wild-type plants andmutants were compared. Results showed that the lengths of grain and brown rice in bothandwere increased (Fig. 2-A, -B and -H, Supplemental Fig. 3). Grain width of brown rice rather than the widths of whole seeds of those mutants were significantly reduced when compared to wild-type (Fig. 2-C and -D). The 1000-grain weight and seed thickness were also clearly decreased in the two mutants (Fig. 2-J and -K, Supplemental Fig. 3). In addition, bothandpresented a shrunken endosperm (Fig. 2-E and -F, Supplemental Fig. 3). Cross-sections of brown rice from wild-type and the twomutants were examined by iodine staining(Fig. 2-F, Supplemental Fig. 3). The wild-type stained uniformly with dark blue color, suggesting that the endosperm was filled with starch, whereas the endosperm fractions ofandwere barely stained and only traces of blue staining were visible near the aleurone layer in the mutant endosperms. It suggested that the endosperm of twomutants were mainly filled with the phytoglycogen. Besides, the grain yield per plant, plant height and seed-setting rate ofwere also significantly decreased, but there was no obvious differences in total grain number per panicle compared with the wild-type (Fig. 2-G and -L to -O).
cr-isa1-1 and cr-isa1-2 endosperm cells present abnormal starch granule
Scanning electron microscopy (SEM) of the cross- sections of mature endosperms showed that starch granules in the central region of wild-type endosperm were closely packed with an irregular polyhedron shape (Fig. 3-A and -B, Supplemental Fig. 4). In contrast, no obvious normal starch granules were found in the endosperm ofand(Fig. 3-D and -E, Supplemental Fig. 4). Transmission electron microscopy (TEM) of developing endosperm cells at 10 DAF showed that amyloplasts in the wild-type were filled with polyhedral starch granules to form a typical complex structure (Fig. 3-C and Supplemental Fig. 4). However, no complete compound starch granules or even no individual starch granule were found in the centre region of endosperm cells ofand(Fig. 3-F and Supplemental Fig. 4). These results suggested that ISA1has essential function in the formation of starch grains and amyloplasts in the endosperm cells.
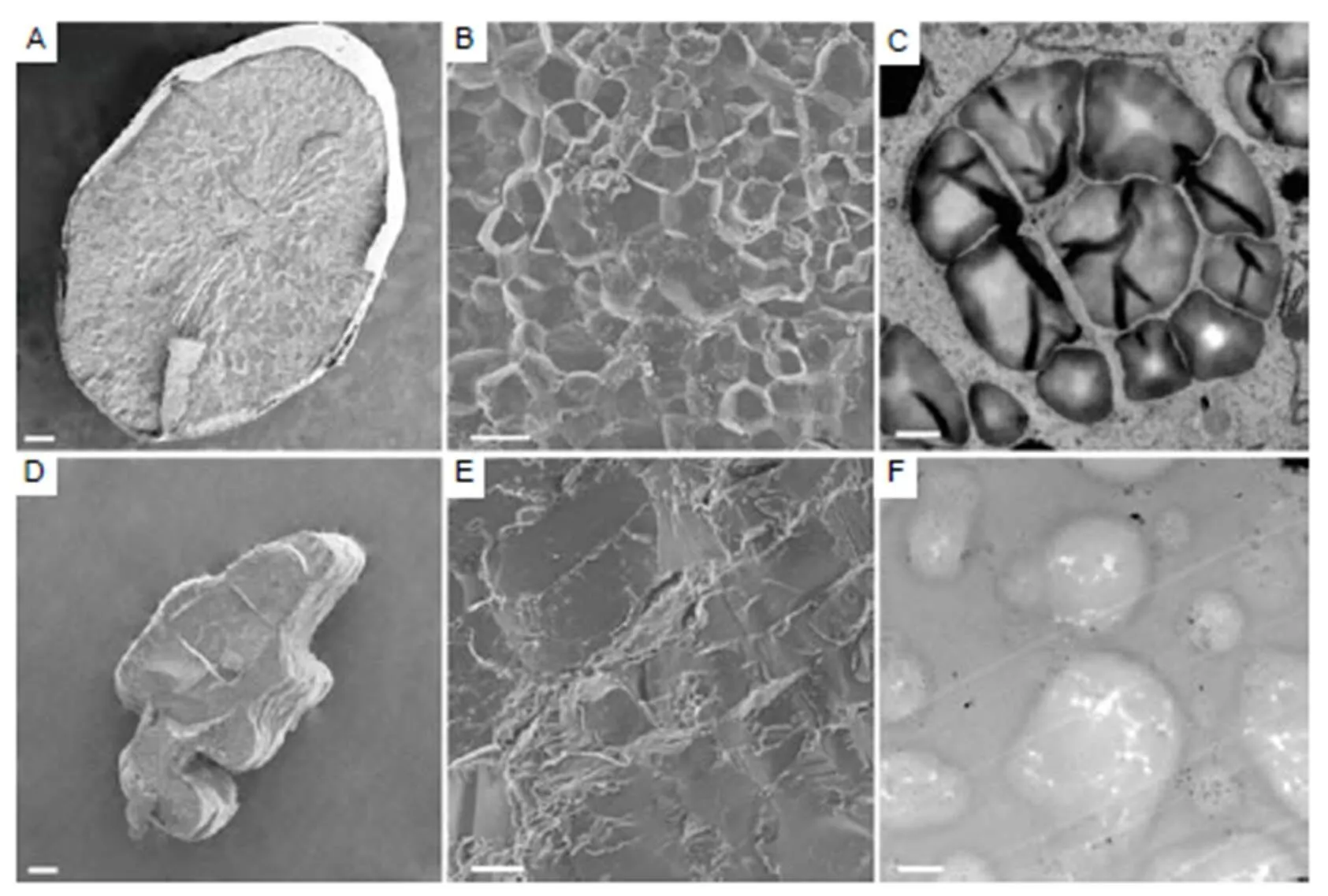
Fig. 3.Electron microscopy images of wild-type and(T1generation).
A,Scanning electron microscopy (SEM) analysis of mature endosperm of wild-type. B, Central region of mature endosperm in the wild-type.C, Amyloplast in endosperm cells of wild-type at 10 d after flowering visualized by transmission electron microscopy analysis. D, SEM analysis of mature endosperm of thetransgenic plant. E, Central region of mature endosperm in. F, Amyloplast in endosperm cells ofat 10 d after flowering visualized by transmission electron microscopy analysis. Scale bars are 0.2mm in A and D, 10mm in B and E, and 1mm in C and F.
cr-isa1 mutants present alteration of starch physicochemical properties
Due to the abnormal starch granules in the endosperm of bothtransgenic lines, the physical and chemical properties of starch were also examined. The data showed that the total starch content in the endosperms ofandwas decreased by 34.4% and 40.0%, respectively, compared to that of wild-type (Fig. 4-A). The amylose content in the wild-type was 14.0%, while there was almost no amylose detected in bothand(Fig. 4-B). These results were consistent with those obtained with iodine staining of endosperm cross-sections (Fig. 2-F, Supplemental Fig. 3). The amylopectin content in the endosperm of bothandwere all significantly lower than that in the wild-type (Fig. 4-C). Otherwise, the total soluble sugar contents inandgrains were 4.3- and 4.5-fold higher than that in the wild-type, respectively (Fig. 4-D). The gelatinization properties of the starch grains were also analyzed. First, the gel consistency of starch grains in the twomutants was significantly higher than that in the wild-type (Fig. 4-E). Second, the starch solubility in KOH solution showed that the endosperm starch ofandwas difficult to gelatinize, whereas the endosperm of wild-type seeds was readily dissolved and dispersed (Fig. 4-F). The thermal gelatinization temperature of the starch from wild-type and the twotransgenic lines were also analyzed by differential scanning calorimetry. Results showed that the onset (To), peak (Tp) and conclusion (Tc) temperatures of gelatinization were undetectable inand, but those behaved normal in the wild-type (Fig. 4-G). To further analyze differences in the fine structure of the polyglucans in endosperm, the chain length distribution was also compared between the genotypes. The short chains with degree of polymerization (DP) values less than 10 glucose units significantly increased in the mutants, whereas the proportion of chains with DP in the range of 10 to 60 was noticeably decreased. Chains with DP in the range of 50–60 were undetectable in bothandwhereas some were presented in the wild-type (Fig. 4-H, Supplemental Fig. 5). In contrast, the proportion of single glucose units (DP1) in themutants was 1.15- to 1.52-fold higher than that in the wild-type (Supplemental Fig. 5). As we know, phytoglycogen was enriched in the chain of DP less than 10 (Fujita, 2015), and therefore, the content of phytoglycogen was possibly increased in the mutants. Together, our data showed that the physicochemical properties and the fine structure of the starch in the twomutantswere significantly altered.
Expression of starch synthesis-related genes is affected in cr-isa1-1
Because the starch content and its physicochemical properties were dramatically changed in, we examined the expression of genes associated with starch synthesis in developing endosperms at 10 DAF (Fig. 5 and Supplemental Fig. 6). Results showed that the expression level ofwas markedly reduced inand. In addition, transcript levels of two AGP genes (and), five starch synthase genes (,,,and), two starch branching enzyme genes (and), two starch debranching enzyme genes (and) andwere significantly lower incompared to the wild-type. Conversely, the expression levels ofandwere significantly higher inand(Fig. 5 and Supplemental Fig. 6). Overall, compared to the wild-type, the expression of most genes associated with starch synthesis was significantly lower inmutants.
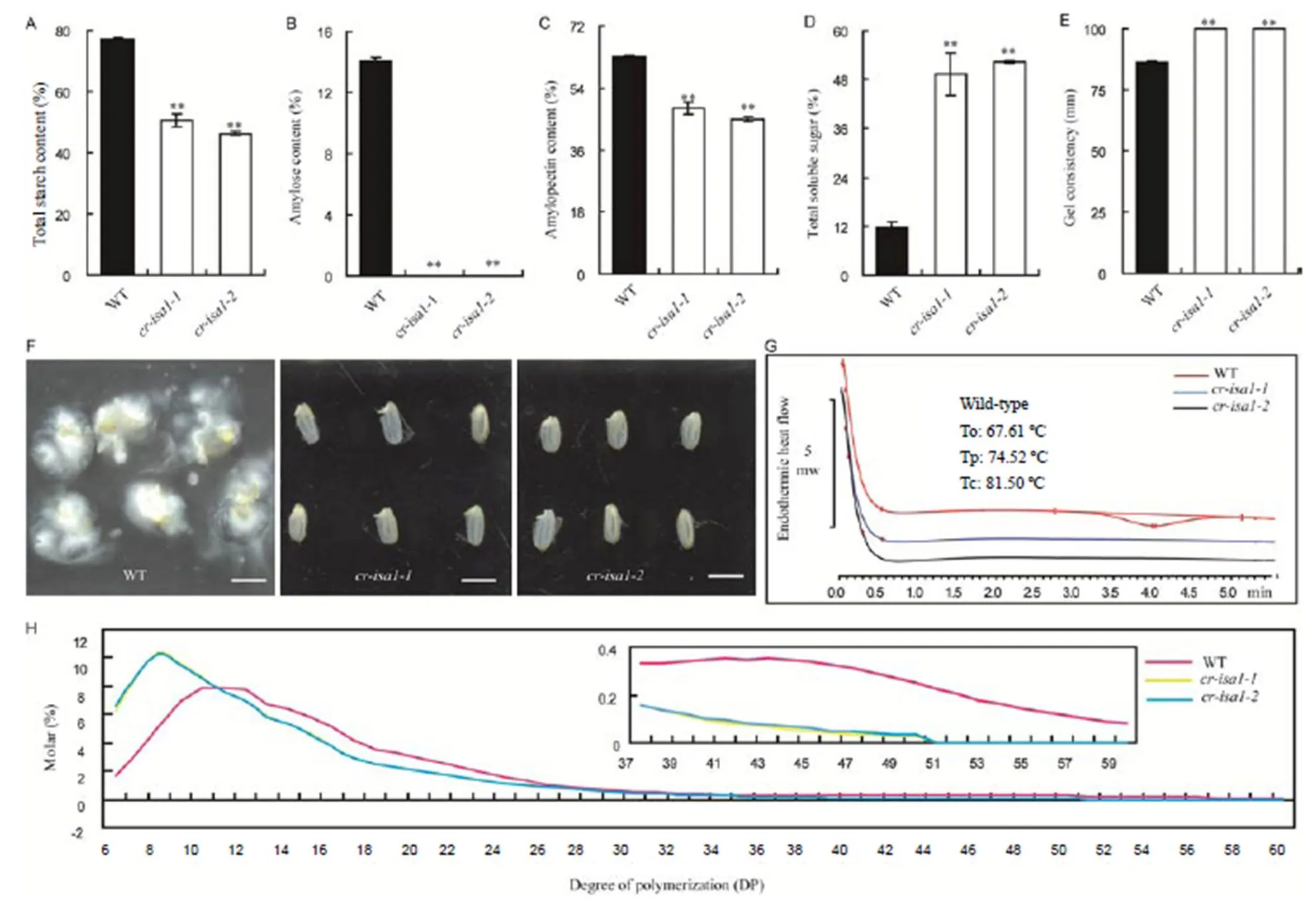
Fig. 4.Starch physicochemical characteristics analysis in T1generation ofmutants.
A, Total starch content. B, Amylose content. C, Amylopectin content. D, Total soluble sugar. E, Gel consistency. F, Starch solubility in KOH solution (Scale bar is 5 mm). G, Thermal characteristics presented by modulated differential scanning calorimetry (MDSC) curves. To, Onset temperature, Tp, Peak temperature; Tc, Conclusion temperature. H, Chain length distributions of amylopectin in wild-type (WT) and. The picture in the upper right corner shows the enlarged region with degree of polymerization ranging from 37 to 60.
Data are shown as Mean ± SD (= 3). Asterisks indicate the statistical significance by the Student’s-test at the 0.01 level.
DISCUSSION
Rice quality is a complex trait that refers to the characteristics of rice grain or rice-related products including milling quality, appearance, as well as cooking, eating and nutritional qualities. Starch makes up to 90% of refined rice grains. The structure and physico- chemical properties of starch in rice endosperm are usually influenced when there are some defects in starch biosynthesis (Fitzgerald et al, 2009). In addition, starch biosynthesis and quality of rice grains can also be impacted by environmental conditions such as temperature, water and fertilizer (Thitisaksakul et al, 2012; Sun et al, 2018).
CRISPR/Cas9 system has emerged as a new technique for precise edition of genomic DNA (Jones, 2015). On one hand, mutants of several genes can be rapidly obtained by this system, which is of great significance to study gene function. On the other hand, valuable genes for breeding programs can be edited to speed up the breeding process and improve crop traits. Furthermore, the system allows to easily obtaining ʻcleanʼ materials without the genetically modified organism (GMO) component in one or two generations. CRISPR/Cas9 system has been applied to edit the() gene, which results in a dramatic reduction in amylose content from 14.6% to 2.6% and a phenotype similar to the natural ricemutants (Ma et al, 2015). High-amylose rice is also created by editing thegenethe CRISPR/Cas9 system, resulting in a 25% increase in amylose content (Sunet al, 2017). Shao et al (2017) succeeded in gettingfragrance mutants without the presence of the CRISPR/Cas9 vector in rice, which provides further genetic information and assistance to fragrance breeding. ISA1 is a type of DBE that plays an essential role in the synthesis of amylopectin (Nakamura et al, 1996; Smith et al, 1997). In this study, the specific target on the 18th exon of thegene was edited through the CRISPR/Cas9 system, and a series of-deficient mutants were obtained with premature termination of the ISA1 protein (Supplemental Fig. 1). Mutation inaccompanied a decrease in expression level ofmRNA (Fig. 5 and Supplemental Fig. 6), and also resulted in frame shift in the translation of protein. The predicted three-dimensional protein structures ofwere different with that of the wild-type (Supplemental Fig. 2). Mutation inmay affect its normal protein function. Compared to the wild-type,mutants presented shrunken endosperm with increased grain length, but reduced grain thickness and weight (Fig. 2-E, -H, -J and -K, Supplemental Fig. 3). Iodine staining of cross-sections of brown rice from wild-type and mutants was extremely different. The endosperm of wild-type was mostly stained with dark blue, while the endosperm of mutants was hardly to stain, and only the aleurone layer can be observed blue(Fig. 2-F and Supplemental Fig. 3), which are in accordance with Kubo et al (1999).

Fig. 5.Relative expression levels of starch metabolism related genes in seeds at 10 d after flowering in wild-type (WT) and(T1).
Total RNA extracted from developing seeds at 10 d after flowering was used for quantitative real-time PCR analysis. Expression level of each gene in the wild-type was set as reference value of 1. Data areMean ± SD(= 3). Asterisks indicate the statistical significance between wild-type and theas determined by the Student’s-test at the 0.01 level.
Mutations inaltered the physicochemical properties of the endosperm starch. The total starch and amylopectin contents were greatly reduced inmutants (Fig. 4-A and -C). Simultaneously, there were almost no integral compound starch granules and any single starch grain present in the central region of endosperm cells ofand(Fig. 3-D to -F and Supplemental Fig. 4). These results are similar to previous observations (Kawagoe et al, 2005; Utsumi et al, 2011). In addition, the amylose could not be detected inmutants, which may due to its extremely low content (Fig. 4-B), and the gelatinization properties of starch were also changed. Particularly, there was a significant difference in the thermal starch gelatinization ofmutants from wild-type, showing no detectable enthalpy curve (Fig. 4-G). It also indicated that there were little starch granules gelatinized in mutants. These observations are new for a mutation of. ISA1 removes the excessive branch points or misplaced branch points in amylopectin introduced by branching enzymes and efficiently promotes the crystallization of neo- amylopectin molecules during starch biosynthesis (Streb et al, 2008). Erlander (1958) proposed that glycogen can be the direct precursor of amylopectin, and amylose can be generated by further debranching amylopectin. Defect inleads to starch to be replaced by soluble phytoglycogen (Erlander, 1958). With a high degree of branching, the structure of phytoglycogen is similar to amylopectin, but contains more short chains (DP£10) and branches at more random positions, which cannot be stained by iodine (Kubo et al, 1999; Streb et al, 2008; Fujita, 2015). Here, the short chains with a DP below 10 were significantly increased and the endosperm could not be iodine stained in(Figs. 2-F, 4-H to -I, and Supplemental Fig. 3), which suggested that the starch may be replaced by soluble phytoglycogen in. Due to the high water solubility and low viscosity of phytoglycogen (Li et al, 2010),can be used as a natural food thickener and stabilizer. Moreover, the total soluble sugar content inmutants was approximately 4-fold higher than that in the wild-type (Fig. 4-D), which is consistent with the sugary endosperm caused by defects in(Kawagoe et al, 2005). In addition, the mutations created inled to lower expression level of, and the transcript levels of main starch synthesis genes were also markedly reduced (Fig. 5 and Supplemental Fig. 6). These transcriptional changes are consistent with the physicochemical properties data obtained from theendosperm. The reduced expression levels of,andmay weaken the ability to extend the short branches, and then generate amylopectin with more short branch and less long chains in mutant.may have feedback physiological regulation on the expression of. However, up-regulation expression ofcannot compensate the effect ofmutation on grain starch synthesis. The considerable changes inmutants were caused by the mutations which likely inhibited ISA1 activity. This is similar to the antisense inhibition ofdescribed by Fujitaet al(2003). Together, our results demonstrated the effect ofin seed development, especially in starch and sugar metabolism, and the structure of starch granules and grain sizes, all of which can contribute to our better understanding and applications of starch synthesis in rice quality improvement.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (Grant Nos. 31471472 and 31521064), the National S&T Major Project of China (Grant No. 2016ZX08001006), and the Central Level, Non-Profit, Scientific Research Institutes Basic R and D Operations Special Fund (Grant Nos. Y2017PT46 and 2017RG002-1).
SUPPlemental DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Table 1.Primers used in this study.
Supplemental Fig. 1.Amino acid sequence alignment of all mutations.
Supplemental Fig. 2.The predicted three-dimensional protein structure of all mutation forms by using the tool Swiss-PdbViewer 4.1.0.
Supplemental Fig. 3.Phenotype of themutant (T1generation).
Supplemental Fig. 4.Electron microscopy images of(T1) endosperm.
Supplemental Fig. 5.Short chain length distributions of amylopectin in.
Supplemental Fig. 6.Expression level of starch metabolism related genes in seeds of wild-type and(T1) at 10 d after flowering.
Akdogan G, Kubota J, Kubo A, Takaha T, Kitamura S. 2011. Expression and characterization of rice disproportionating enzymes.,58(3): 99–105.
Burton RA, Jenner H, Carrangis L, Fahy B, Fincher GB, Hylton C, Laurie DA, Parker M, Waite D,van Wegen S, Verhoeven T, Denyer K. 2002. Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity., 31(1):97–112.
Delatte T, Trevisan M, Parker ML, Zeeman SC. 2005.mutantsandhave identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis.,41(6):815–830.
Erlander SR. 1958.A proposed mechanism for the synthesis of starch from glycogen., 19(5): 273–283.
Ferreira SJ, Senning M, Fischer-Stettler M, Streb S, Ast M, Neuhaus HE, Zeeman SC, Sonnewald S, Sonnewald U. 2017. Simultaneous silencing of isoamylases,andby multi-target RNAi in potato tubers leads to decreased starch content and an early sprouting phenotype.,12(7): e0181444.
Fitzgerald MA, Mccouch SR, Hall RD. 2009. Not just a grain of rice: The quest for quality.,14(3):133–139.
Fujita N, Kubo A, Suh DS, Wong KS, Jane JL, Ozawa K, Takaiwa F, Inaba Y, Nakamura Y. 2003. Antisense inhibition of isoamylase alters the structure of amylopectin and the physicochemical properties of starch in rice endosperm.,44(6):607–618.
Fujita N. 2015. Manipulation of rice starch properties for application.: Nakamura Y. Starch Metabolism and Structure.Tokyo: Springer: 342–343.
Han Y P, Xu M L, Liu X Y, Yan C J, Korban SS, Chen X L, Gu M H. 2004. Genes coding for starch branching enzymes are major contributors to starch viscosity characteristics in waxy rice (L.).,166(2): 357–364.
Haugen T H, Ishaque A, Preiss J. 1976. Biosynthesis of bacterial glycogen: Characterization of the subunit structure ofB glucose-1-phosphate adenylyltransferase (EC 2.7.7.27)., 251(24): 7880–7885.
Hiei Y, Komari T, Kubo T. 1997. Transformation of rice mediated by., 35:205–218.
HwangS K, Koper K, Satoh H, Okita TW. 2016. Rice endosperm starch phosphorylase (Pho1) assembles with disproportionating enzyme (Dpe1) to form a protein complex that enhances synthesis of malto-oligosaccharides.,291:19994–20007.
James M G, Robertson D S, Myers A M. 1995. Characterization of the maize gene, a determinant of starch composition in kernels., 7(4):417–429.
Jeon J S, Ryoo N, Hahn T R, Walla H, Nakamura Y. 2010. Starch biosynthesis in cereal endosperm., 48(6):383–392.
Jones HD. 2015. Regulatory uncertainty over genome editing., 1(1):14011.
Kang HG, Park S, Matsuoka M, An G. 2005. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C-type pyruvate orthophosphate dikinase gene ().,42(6):901–911.
Kawagoe Y, Kubo A, Satoh H, Takaiwa F, Nakamura Y. 2005. Roles of isoamylase and ADP-glucose pyrophosphorylase in starch granule synthesis in rice endosperm.,42(2):164–174.
Kubo A, Fujita N, Harada K, Matsuda T, Satoh H, Nakamura Y. 1999. The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm.,121(2): 399–410.
Kubo A, Rahman S, Utsumi Y, Li Z, Mukai Y, Yamamoto M, Ugaki M, Harada K, Satoh H, Konik-Rose C, Morell M, Nakamura Y. 2005. Complementation ofphenotype in rice endosperm with the wheat isoamylase1 gene supports a direct role for isoamylase1 in amylopectin biosynthesis.,137(1): 43–56.
Kweon M, Haynes L, Slade L, Levine H. 2000. The effect of heat and moisture treatments on enzyme digestibility of AeWx, Aewx and aeWx corn starch., 59:571–586.
Lee SK, Hwang SK, Han M, Eom JS, Kang HG, Han Y, Choi SB, Cho MH, Bhoo SH, An G, Hahn TR, Okita TW, Jeon JS. 2007. Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (L.)., 65(4): 531–546.
Li J, Huang L, Yao Y, Ma Y Q. 2010. The progress in the highly branched carbohydrate polymer phytoglycogen., 4: 35–38. (in Chinese with English abstract)
Li S F, Wei X J, Ren Y L, Qiu J H, Jiao G A, Guo X P, Tang S Q, Wan J M, Hu P S. 2017.encodes an ADP-glucose transporter involved in starch synthesis and compound granule formation in rice endosperm.,7: 40124.
Liu L L, Ma X D, Liu S J, Zhu C L, Jiang L, Wang Y H, Shen Y, Ren Y L, Dong H, Chen L M, Liu X, Zhao Z G, Zhai H Q, Wan J M. 2009. Identification and characterization of a novel waxy allele from a Yunnan rice landrace., 71(6):609–626.
Liu W Z, Xie X R, Ma X L, Li J, Chen J H, Liu YG. 2015. DSDecode: A web-based tool for decoding of sequencing chromatograms for genotyping of targeted mutations., 8(9):1431–1433.
Ma X L, Chen L T, Zhu Q L, Chen Y L, Liu YG. 2015. Rapid decoding of sequence-specific nuclease-induced heterozygous and biallelic mutations by direct sequencing of PCR products.,8(8):1285–1287.
Masuko T, Minami A, Iwasaki N, Majita T, Nishimura SI, LeeYC. 2005. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format.,339(1):69–72.
Mizuno K, Kimura K, Arai Y, Kawasaki T, Shimada H, Baba T. 1992. Starch branching enzymes from immature rice seeds., 112(5):643–651.
Naito Y, Hino K, Bono H, Ui-Tei K. 2015. CRISPRdirect: Software for designing CRISPR/Cas guide RNA with reduced off-target sites.,31(7):1120–1123.
Nakamura Y, Takeichi T, Kawaguchi K, Yamanouchi H. 1992. Purification of two forms of starch branching enzyme (Q-enzyme) from developing rice endosperm., 84(3):329–335.
Nakamura Y, Umemoto T, Ogata N, Kuboki Y, Yano M, Sasaki T. 1996. Starch debranching enzyme (R-enzyme or pullulanase) from developing rice endosperm: Purification, cDNA and chromosomal localisation of the gene., 199(2):209–218.
Peng C, Wang YH, Liu F,Ren Y L, Zhou K N, Lv J, Zheng M, Zhao S L, Zhang L, Wang C M, Jiang L, Zhang X, Guo X P, Bao Y Q, Wan J M. 2014.encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm., 77(6):917–930.
Pfister B, Zeeman S C. 2016. Formation of starch in plant cell., 73(14):2781–2807.
Rao Y C, Li Y Y, Qian Q. 2014. Recent progress on molecular breeding of rice in China., 33(4):551–564.
Ryoo N, Yu C, Park C S, Baik M Y, Park I M, Cho M H, Bhoo S H, Ah G, Hahn T R, Jeon J S. 2007. Knockout of a starch synthase genecauses white-core floury endosperm in rice (L.)., 26(7):1083–1095.
Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method.,3(6):1101–1108.
Sestili F, Sparla F, Botticella E, Janni M, DʼOvidio R, Falini G, Marri L, Cuesta-Seijo JA, Moscatello S, Battistelli A, Trost P, Lafiandra D. 2016. The down-regulation of the genes encoding isoamylase 1 alters thestarch composition of the durum wheat grain.,252: 230–238.
Shao G N, Xie L H, Jiao G A, Wei X J, Sheng Z H, Tang S Q, Hu P S. 2017. CRISPR/CAS9-mediated editing of the fragrant genein rice., 31(2): 216–222. (in Chinese with English abstract)
She KC, Kusano H, Koizumi K, Yamakawa H, Hakata M, Imamura T, Fukuda M, Naito N, Tsurumaki Y, Yaeshima M, Tsuge T, Matsumoto K, Kudoh M, Itoh E, Kikuchi S, Kishimoto N, Yazaki J, Ando T, Yano M, Aoyama T, Sasaki T, Satoh H, Shimada H. 2010. A novel factoris involved in regulation of rice grain size and starch quality., 22(10): 3280–3294.
Smith AM, Denyer K, Martin C. 1997. The synthesis of the starch granule.,48: 67–87.
Streb S, Delatte T, Umhang M, Eicke S, Schorderet M, Reinhardt D, Zeeman SC. 2008. Starch granule biosynthesis inis abolished by removal of all debranching enzymes but restored by the subsequent removal of an endoamylase., 20(12): 3448–3466.
Sun T, Tong L G, Zhao S Y, Wang H W, Han Y F, Zhang Z C, Jin Z X. 2018. Effects of nitrogen fertilizer application on starch quality, activities and gene expression levels of related enzymes in rice endosperm., 32(5): 475–484. (in Chinese with English abstract)
Sun Y W, Jiao G A, Liu Z P, Zhang X, Li J Y, Guo X P, Du W M, Du J L, Francis F, Zhao Y D, Xia L Q. 2017. Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes.,8:298.
Thitisaksakul M, Jimenez RC, Arias MC, Beckles DM. 2012. Effects of environmental factors on cereal starch biosynthesis and composition.,56(1):67–80.
Thompson D B. 2000. On the non-random nature of amylopectin branching., 43(3):223–239.
Utsumi Y, Nakamura Y. 2006. Structural and enzymatic characterization of the isoamylase1 homo-oligomer and the Isoamylase1-isoamylase2 hetro-oligomer from rice endosperm., 225(1):75–87.
Utsumi Y, Utsumi C, Sawada T, Fujita N, Nakamura Y. 2011. Functional diversity of isoamylase oligomers: The ISA1 homo-oligomer is essential for amylopectin biosynthesis in rice endosperm.,156(1): 61–77.
Vrinten PL, Nakamura T. 2000. Wheat granule-bound starch synthase I and II are encoded by separate genes that are expressed in different tissues.,122(1): 255–264.
Wattebled F, Dong Y, Dumez S,Delvallé D, Planchot V, Berbezy P, Vyas D, Colonna P, Chatterjee M, Ball S, DʼHulst C. 2005. Mutants oflacking a chloroplastic isoamylase accumulate phytoglycogen and an abnormal form of amylopectin., 138(1):184–195.
(Managing Editor: Li Guan)
Copyright © 2019, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2018.07.001
1 June 2018;
27 July 2018
Wei Xiangjin (weixiangjin@caas.cn); Hu Peisong (peisonghu@126.com; hupeisong@caas.cn)
杂志排行
Rice Science的其它文章
- Characterization and Evaluation of OsLCT1 and OsNramp5 Mutants Generated Through CRISPR/Cas9-Mediated Mutagenesis for Breeding Low Cd Rice
- Targeted Mutagenesis of NAC Transcription Factor Gene, OsNAC041, Leading to Salt Sensitivity in Rice
- CRISPR/Cas9-Mediated Adenine Base Editing in Rice Genome
- Rapid Creation of New Photoperiod-/Thermo-Sensitive Genic Male-Sterile Rice Materials by CRISPR/Cas9 System
- Development and Application of CRISPR/Cas System in Rice
- Production of Two Elite Glutinous Rice Varieties by Editing Wx Gene
