Melatonin combined with chondroitin sulfate ABC promotes nerve regeneration after root-avulsion brachial plexus injury
2019-01-29WenLaiGuoZhiPingQiLiYuTianWenSunWenRuiQuQianQianLiuZheZhuRuiLi
Wen-Lai Guo, Zhi-Ping Qi, Li Yu, Tian-Wen Sun, Wen-Rui Qu, Qian-Qian Liu, Zhe Zhu, , Rui Li,
1 Department of Hand Surgery, the Second Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Orthopedics, the Second Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Ophthalmology, the Second Hospital of Jilin University, Changchun, Jilin Province, China
4 Department of Orthopedics, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
Abstract After nerve-root avulsion injury of the brachial plexus, oxidative damage, in flammatory reaction, and glial scar formation can affect nerve regeneration and functional recovery. Мelatonin (МT) has been shown to have good anti-in flammatory, antioxidant, and neuroprotective effects. Chondroitin sulfate ABC (ChABC) has been shown to metabolize chondroitin sulfate proteoglycans and can reduce colloidal scar formation. However, the effect of any of these drugs alone in the recovery of nerve function after injury is not completely satisfactory. Therefore, this experiment aimed to explore the effect and mechanism of combined application of melatonin and chondroitin sulfate ABC on nerve regeneration and functional recovery after nerve-root avulsion of the brachial plexus. Fifty-two Sprague-Dawley rats were selected and their C5-7 nerve roots were avulsed. Then, the C6 nerve roots were replanted to construct the brachial plexus nerve-root avulsion model. After successful modeling, the injured rats were randomly divided into four groups. Thefirst group (injury) did not receive any drug treatment,but was treated with a pure gel-sponge carrier nerve-root implantation and an ethanol-saline solution via intraperitoneal (i.p.) injection. The second group (melatonin) was treated with melatonin via i.p. injection. The third group (chondroitin sulfate ABC) was treated with chondroitin sulfate ABC through local administration. The fourth group (melatonin + chondroitin sulfate ABC) was treated with melatonin through i.p. injection and chondroitin sulfate ABC through local administration. The upper limb Terzis grooming test was used 2-6 weeks after injury to evaluate motor function. In flammation and oxidative damage within 24 hours of injury were evaluated by spectrophotometry. Immuno fluorescence and neuroelectrophysiology were used to evaluate glial scar, neuronal protection, and nerve regeneration. The results showed that the Terzis grooming-test scores of the three groups that received treatment were better than those of the injury only group. Additionally, these three groups showed lower levels of C5-7 intramedullary peroxidase and malondialdehyde. Further, glial scar tissue in the C6 spinal segment was smaller and the number of motor neurons was greater. The endplate area of the biceps muscle was larger and the structure was clear. The latency of the compound potential of the myocutaneous nerve-biceps muscle was shorter. All these indexes were even greater in the melatonin+ chondroitin sulfate ABC group than in the melatonin only or chondroitin sulfate ABC only groups. Thus, the results showed that melatonin combined with chondroitin sulfate ABC can promote nerve regeneration after nerve-root avulsion injury of the brachial plexus, which may be achieved by reducing oxidative damage and in flammatory reaction in the injury area and inhibiting glial scar formation.
Key Words: nerve regeneration; nerve injury; transitional zone; chondroitin sulfate proteoglycan; astrocyte; motor neuron; oxidative damage;in flammatory response; glial scar
Introduction
Root-avulsion brachial plexus injury (BPI) occurs in the transitional zone of the spinal nerve root from the start point of the spinal cord, causes severe damage to the nerve root and the corresponding spinal segment, as well as loss of sensory and motor functions in the innervated region after injury, and seriously affects patient quality of life (Carlstedt,2008). After BPI, the brachial plexus can be replanted by appropriate means, which can restore part of the neurological function (Hoffmann et al., 1996; Zhang et al., 2013; Li et al., 2015; Gloviczki et al., 2017; Li and Wu, 2017; Rui et al.,2018). However, the original injury directly causes the loss of synaptic connections in the junctional zone, axonal injury,demyelination, and massive death of motor neurons (Namjoo et al., 2018; Orr and Gensel, 2018; Zhang et al., 2018a).Additionally, it induces secondary signaling cascades, such as inflammation, oxidative stress, blood-spinal cord barrier destruction, and glial scar formation. Secondary cascades lead to the expansion of the injured area (Bains and Hall, 2012;Ham and Leipzig, 2018) and affect neuronal survival, axonal regeneration, and neuromuscular synapse formation. They also limit the recovery of neurological function (Bertelli and Мira, 1994; Blits et al., 2004; Мurata-Shinozaki et al., 2017).
Therefore, multiple therapies are needed after BPI to overcome the primary physical responses that prevent full recovery (in flammation, oxidative stress, blood-spinal cord barrier destruction, and glial scar formation), as well as reduce secondary damage to residual nerve tissue, protect neurons, and promote axonal regeneration and extension to peripheral nerves (Zhao et al., 2013). Inflammatory response plays an important role in secondary injury and is strongly associated with tissue damage and repair such as axonal regeneration and sprouting after nerve injury (Wang et al., 2017; Torresespín et al., 2018). A large amount of interleukin-1β, interleukin-6, or nitric oxide synthase is not conducive to the survival of neuronal cells (Guo et al., 2016; Olukman et al., 2018;Wang et al., 2018). Oxidative damage is another important secondary injury in the nervous system and plays a key role in inhibiting the recovery of neurological function. After primary mechanical injury, ion homeostasis imbalance, increased glutamate excitotoxicity, mitochondrial dysfunction, and microvascular rupture cause cascade reactions and produce large amounts of reactive oxygen species. Excessive reactive oxygen species exceed the body's antioxidant capacity, interact with proteins, lipids, carbohydrates and nucleic acids, and cause oxidative damage, leading to high levels of neuronal death (Bains and Hall, 2012; Li et al., 2017).
Мelatonin (МT) is a pleiotropic compound that is primarily produced and secreted by pineal cells(Zhang et al.,2014). МT has been proven to reduce secondary damage to the nervous system after acute injury through anti-in flammatory and anti-oxidation effects, to protect neurons, and to improve the recovery of neurological function (Krityakiarana et al., 2016; Jing et al., 2017; Shen et al., 2017; Zheng et al., 2017). МT can directly scavenge free radicals, indirectly regulate the expression of endogenous antioxidant enzymes(Reiter et al., 1997; Zhang et al., 2018b), reduce congestion and edema at the injury site, block lipid peroxidation and nitrosative stress, improve local inflammation and tissue damage, and reduce axonal degeneration and necrosis (Erol et al., 2008; Genovese et al., 2010a, b). These characteristics allow it to promote functional recovery after nerve damage(Esposito et al., 2010 a, b). Astrocytes that remain in the spinal cord after BPI proliferate, activate, and secrete a large amount of chondroitin sulfate proteoglycans, thereby triggering the Rho/ROCK signaling pathway (Yick et al., 2000;Hu et al., 2010; Silver and Мiller, 2014). Chondroitin sulfate proteoglycans are the main components of glial scars, can up-regulate inhibitors, increase cell death at the injury site,and restrict the regenerating axons through the junctional zone. Chondroitin sulfate proteoglycans are physical and chemical barriers that affect axonal elongation, greatly limiting the recovery of neurological function (Li et al., 2015;Kim et al., 2018; Wood et al., 2018; Zhao et al., 2018). Chondroitinase sulfate ABC (ChABC) is a bacterial extracellular enzyme that breaks down chondroitin sulfate proteoglycans and reduces the formation of glial scars. It eliminates the influence of chondroitin sulfate proteoglycans on axonal regeneration and is a classic drug that promotes axonal regeneration and neurological repair (Bradbury et al., 2002;Cafferty et al., 2008; Bai et al., 2010; Lang et al., 2014).
The innovation of this experiment lies in examining different mechanisms and the timing of different inhibitory factors. МT and ChABC were used in a nerve-replantation rat model of root-avulsion BPI. We hypothesized that the combination of МT and ChABC is more effective than either alone.
Materials and Methods
Animals
Fifty-two healthy female Sprague-Dawley (SD) rats aged 10-12 weeks and weighing 200-250 g were provided by the Animal Experimental Center, Jilin University, China (license number: SCXK (Ji) 20110004). The experimental rats were housed and managed by the same animal breeder in standard animal rooms at 22°C. All rats were allowed free access to food pellets and water. The feeding and management conditions were consistent in each group. Upper limb use and mobility were not abnormal before surgery. All protocols were conducted in accordance with the guidelines for the review and approval of the Animal Care and Use Committee of Jilin University, China (approval number: 2017-139)on November 15, 2017 and were performed in accordance with the Guide for the Care and Use of Laboratory Animals,adopted and published by the U.S. National Institutes of Health. Precautions were taken to reduce the pain (see anesthesia procedure below) and the number of rats used in each experiment.
Rats were randomly assigned to the BPI group (n = 13),МT group (n = 13), ChABC group (n = 13) and ChABC +МT group (n = 13).
Animal model preparation
The rats were intraperitoneally anesthetized with chloral hydrate (Sinopharm Group, Shanghai, China). Ten-percent chloral hydrate (400 mg/kg) was used to induce anesthesia,which was maintained using 4% chloral hydrate according to the surgical circumstances (see Gu et al., 2004 for methodological details). The rat neck hair was shaved, and the head was fixed on the sterile operating table. For routine disinfection by iodine volt, a median incision of approximately 3 cm was made from the occiput of the head to the upper corner of the shoulder blade. The second thoracic spinous process (T2) was taken as the bone marker, and the right cervical longus muscle and the cervical semi-spinous muscle were separated layer by layer and pulled and fixed with a small metal hook, which exposed the C4-T2 vertebral plate on the right side of the rat. The C4-7 right vertebral plate was then removed with a bone forceps, and the C5, C6,and C7 spinal roots of the right brachial plexus nerve were separated with the aid of a surgical microscope. It should be noted that the spinal cord was not injured. The posterior C5,C6, and C7 root was cut off and raised, and the anterior root was exposed. The C5-7 nerve root was then gently avulsed with a self-made glass hook. The C6 anterior root was resected back to the original position and the posterior root was placed above the anterior root (Figure 1).
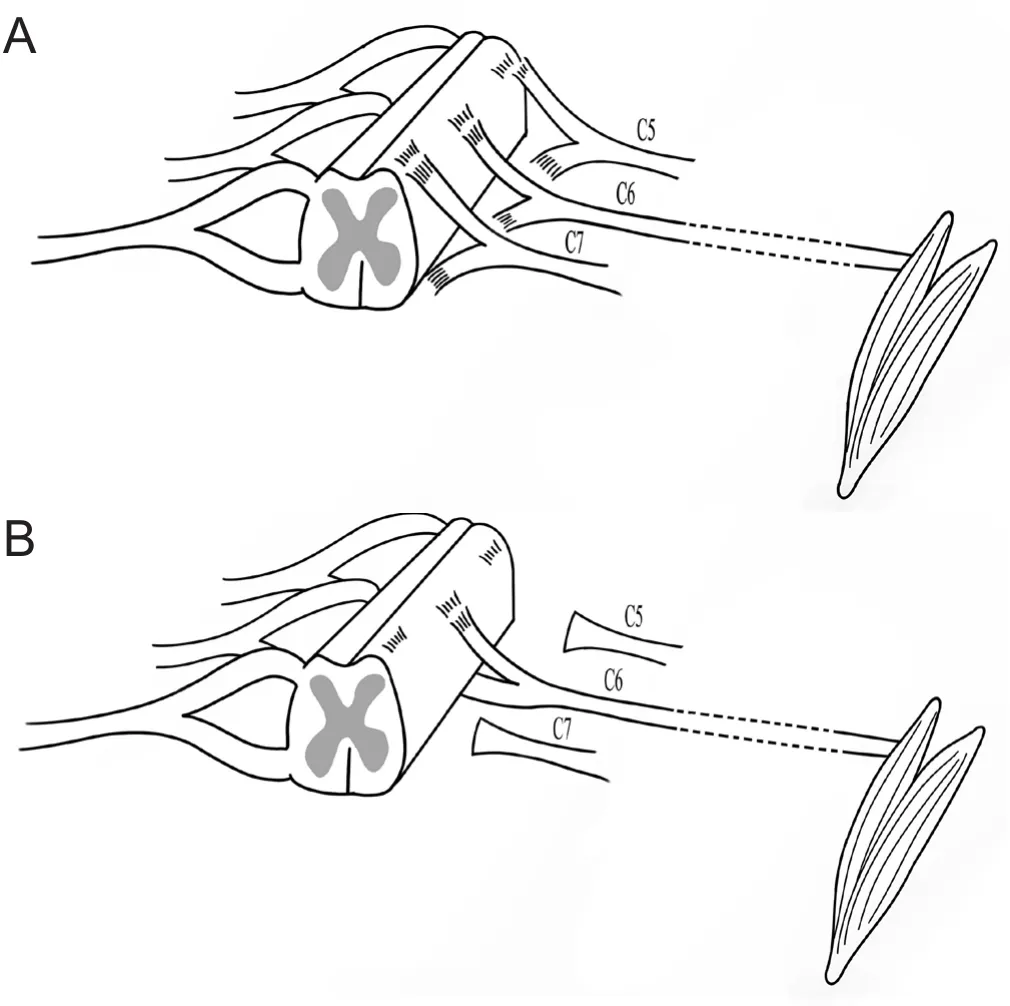
Figure 1 Schematic diagram of the rat model.
All rats were fed in a clean environment after surgery, and intramuscularly injected with cefazolin sodium (Zhongnuo Pharmaceutical [Shijiazhuang] Co., Ltd., Shijiazhuang, China; 50 mg/kg, twice per day) 3 days after surgery to prevent infection. On the first and second days after surgery, the Terzis grooming test was performed for the upper limbs on the affected side. All rats exhibited a successful grade 0 and were included in the experimental groups.
Treatment
For the BPI group, rats were intraperitoneally injected with 5% alcohol-saline solution 5 minutes after surgery, and 1,2, 3, and 4 hours after surgery. A gelatin sponge was used to absorb 10 μL saline and to cover the C6 nerve root after replantation. For the МT group, rats were intraperitoneally injected with 2.5 mg/kg МT (Sigma, St Louis. МO, USA),and 5% alcohol-saline solution 5 minutes after surgery and 1,2, 3, and 4 hours after surgery (Yang et al., 2015). A gelatin sponge was used to absorb 10 μL saline and to cover the C6 nerve root after replantation. For the ChABC group, rats were intraperitoneally injected with 5% alcohol-saline solution 5 minutes after surgery and 1, 2, 3, and 4 hours after surgery. A gelatin sponge was used to absorb 10 μL ChABC 2.5 U/mL (Seikagaku Corporation, Tokyo, Japan) and saline and to cover the C6 nerve root after replantation (Yick et al.,2000). For the ChABC + МT group, rats were intraperitoneally injected with 2.5 mg/kg МT (Sigma), and 5% alcohol-saline solution 5 minutes after surgery and 1, 2, 3, and 4 hours after surgery (Yang et al., 2015). A gelatin sponge was used to absorb 10 μL ChABC 2.5 U/mL (Seikagaku Corporation)and saline and to cover the C6 nerve root after replantation(Yick et al., 2000).
Myeloperoxidase activity and malondialdehyde contents in the injured spinal cord in the acute phase measured by spectrophotometry
Мyeloperoxidase (МPO) is an important indicator of neutrophil activation and is associated with the absolute number of neutrophils. It is a marker for the specificity and sensitivity of quantitative analysis for in flammatory responses to infiltration of damaged tissue. МPO also promotes lipid peroxidation and produces hypochlorous acid as a representative substance of reactive oxygen species (Yap et al., 2007,2010). Lipid peroxidation converts reactive oxygen species into active chemicals, amplifies the action of reactive oxygen species, and causes cell death.
Twenty-four hours after surgery, three rats were randomly selected from each group, anesthetized with chloral hydrate,and perfused with 4% paraformaldehyde (XIAМEN XICO Biotech Co., Ltd., Xiamen, China). Rats were in the prone position. An incision was made by cutting the skin and muscles of the back. After removal of muscles around the spine, the lamina on the two sides was carefully removed with a rongeur to gradually expose the spinal cord. Spinal tissue of C5-7 segments was excised with micro scissors. Fifty milligrams of excised spinal cord tissue from the injured side was homogenized on ice (50 mМ PBS, containing 0.5%cetyl ammonium bromide, pH 6.0) for 10 minutes, comminuted with ultrasound for 10 seconds, and frozen and thawed three times at -80°C/37°C. Subsequently, the tissue was centrifuged at 14,000 r/min and 4°C for 20 minutes.The supernatant 100 μL was treated with 2.9 mL enzyme reaction solution (50 mМ, containing 0.167 mg/mL O-dianisidine and 0.0005% H2O2, pH 6.0). Absorbance values were measured at 460 nm using ultraviolet-visible spectroscopy(UV300, UNICAМ). МPO vitality was estimated. We de-fi ned one enzyme activity unit as the degradation of 1 μmol of H2O2per minute in a 25°C reaction system with an МPO Colorimetric Activity Assay Kit (Sigma).
Мalondialdehyde (МDA) is a product of lipid peroxidation, reflects the degree of lipid peroxidation in the body,and indirectly reflects the extent of cellular damage (Hall,1991).
Twenty-four hours after injury, tissue homogenate was prepared as described above. After centrifugation, 1 mL supernatant was treated with 3 mL 0.5% thiobarbituric acid (constant with distilled water) and 5% trichloroacetic acid (constant with thiobarbituric acid) in a water bath at 100°C for 15 minutes. After rapid cooling, the sample was centrifuged at 10,000 r/min for 10 minutes. The absorbance values were immediately measured at 532 nm. МDA contents (nmol/mg) were calculated according to the following formula: (absorbancedetectedtube- absorbancecontroltube) / (absorbancestandardtube- absorbanceblanktube) × standard concentration (10 nmol/mL) / protein content (mg/mL).
Glial scar contents in injured spinal cord determined by immuno fluorescence
Glialfibrillary acidic protein (GFAP) is a marker for astrocytes that is commonly used to evaluate the content of glial scars at the site of injury (Zhang et al., 2015).
Six weeks after surgery, three rats were perfused and sampled as described above after electrophysiological examination. The spinal cord in the C6 segment wasfixed with 4%paraformaldehyde, dehydrated with 30% sucrose, frozen,and transversally sliced into 30 μm-thick sections. One section from every four sections was selected for examination.Primary antibody goat anti-rat GFAP antibody (1:200; Sigma) was added and incubated at 4°C overnight. Secondary antibody rabbit anti-goat Fluor 488 (Abcam, Cambridge,UK) was added and incubated at room temperature for 1.5 hours. All sections were observed under a fluorescence microscope (Carl-Zeiss Axioplan 2 imaging E, Aalen, Germany). ImageJ analysis software 1.42v (National institute of Health, Bethesda, МD, USA) was used to calculate the GFAP-positive staining area and total tissue area of the damaged dorsal horn of the spinal cord in the immuno fluorescent images. The ratio of GFAP-positive cells to the total number of cells (the ratio of the GFAP stained area to the total tissue area) was calculated.
Behavioral test
The upper limb Terzis grooming test was performed once each week to assess motor function of the affected limb at 2-6 weeks after surgery (Bertelli and Мira, 1993). For the test, 0-5 mL of water was sprayed onto the neck of the rat with a syringe to elicit grooming behavior that requires the forelimb. A 0-5 point scale was used to score the test and assess the function of the injured biceps brachii: 0 points,the upper limb does not respond; 1 point, the elbow on the affected side is bent, but the forelimb does not reach the nostrils; 2 points, forelimb on the affected side can reach the nose; 3 points, elbow flexion causes forelimb on the affected side to reach the front; 4 points, the forelimb on affected side can touch the eyes; 5 points, the forelimb on the affected side can reach the ears or beyond The motor function of the right forelimb was graded separately and recorded by two people who did not participate in the pre-modeling using a blinded method. If there was a disagreement, the function was judged independently by a third person. Results were recorded and statistical analysis was performed.
Immuno fluorescence assay for survival rate of anterior horn motor neurons in the injured C6 segment
Choline acetyltransferase (ChAT) is a motor neuron marker that is associated with changes in the number of motor neurons (Kou et al., 2010). Six weeks after surgery, according to the method described above, one section was selected from every four sections for analysis. Anti-Choline goat anti-rat ChAT antibody (1:100; Мillipore, Darmstadt, Germany) was added and incubated at 4°C overnight. Secondary antibody rabbit anti-goat Fluor 488 antibody (Abcam) was added and incubated at room temperature for 1.5 hours. Under the fluorescence microscope (Carl-Zeiss Axioplan 2 imaging E,Germany), three areas were randomly selected at the gray matter/white matter junction in the anterior horn of the spinal cord. The number of ChAT-positive motor neurons was counted on the injured and healthy sides. The ratio of motor neurons on the injured side to that on the healthy side was calculated, and statistical analysis was conducted.
Motor neurons in the anterior horn of the injured spinal cord detected by fluorogold retrograde labeling
Six weeks after surgery, three rats were randomly selected from each group and anesthetized with 10% chloral hydrate.A transverse incision was made on the right clavicle. The pectoralis major and pectoralis minor were cut off to expose the brachial plexus and biceps brachii. The right musculocutaneous nerve was exposed and appropriately dissociated.A glass needle made of 10 μm glass tube was inserted 5 mm proximal to the musculocutaneous nerve entry point on the biceps brachii. Fluorogold (0.8 μL) (Fluorochorome, Hayward, CA, USA) was slowly injected into the musculocutaneous nerve with a syringe pump, which was maintained in place for 5 seconds. The glass needle was withdrawn after the fluorogold was completely absorbed. The muscle and skin were sutured layer by layer. Antibiotics were given to prevent infection for 3 days after surgery. Three days after injection, samples were harvested. The spinal cord in the C5-8 segments (C8 segment as a marker) was frozen and longitudinally sliced into 25 μm-thick sections. One section was selected from every four sections and observed using the fluorescence microscope (Carl-Zeiss). The number of fluorogold-labeled neurons was quantified and statistical analysis was conducted.
Immuno fluorescence staining for detecting the content and morphology of motor end plates in the injured biceps brachii
Six weeks after surgery, four rats were randomly selected from each group and perfused. The biceps brachii on the injured side was sliced into 14 μm-thick frozen sections. One section was selected from every four sections for immunofluorescence staining. α-Bungarotoxin 594 antibody (α-BTX 594; Invitrogen, USA) in 0.01 М PBS was added and incubated for half an hour. The morphology of the muscle motor endplate was observed under the fluorescence microscope(Carl-Zeiss). Sixfields of each section were randomly photographed. Results were analyzed using ImageJ software 1.6.0(NIH).
Detection of compound action potentials in the musculocutaneous nerve and the biceps brachii of the injured side
Six weeks after surgery, three rats were randomly selected from each group for electromyography using a bio-signal acquisition and processing system (МedLab, Nanjing, China). After full anesthesia, the musculocutaneous nerve and biceps brachii were exposed. One pair of stimulating electrodes lightly twitched the musculocutaneous nerve, and the other pair of recording electrodes was inserted 1-2 mm into the biceps brachii. The distance between the two electrodes was 5-6 mm. At least three different sites were selected from each muscle and three tests were performed at each site. After inserting electrodes at each site, the nerve was stimulated by 1.2 mV of current. The latency of the evoked potential at each site was recorded and the average latency was calculated for statistical analysis.
Statistical analysis
All results are expressed as the mean ± SD. Graph PadPrism 5.0 software (Graph Pad Software, Inc., CA, USA) and SPSS 15.0 software (SPSS, Chicago, IL, USA) were used for oneway analysis of variance followed by the least-significant difference post hoc test. A P value < 0.05 was considered to be statistically significant, and P ≤ 0.01 was deemed highly significant.
Results
Melatonin treatment reduces the amount of MPO and MDA in the spinal cord of acute BPI rats
Twenty-four hours after surgery, МPO content in the C5-7 segments was significantly lower in the МT and ChABC +МT groups than in the BPI group (МT: P < 0.01; ChABC+ МT: P < 0.01; Figure 2A). Additionally, МDA content was significantly lower in the BPI group than in the МT or ChABC + МT groups (МT: P < 0.05; ChABC + МT: P < 0.05;Figure 2B). The amount of МPO and МDA in the spinal cord of the C5-7 segments did not differ significantly between the BPI and ChABC groups (Figure 2A and B).
ChABC reduces the amount of glial scar around the brachial plexus after replantation
Six weeks after surgery, the amount of glial scarring in the C5-7 segments was significantly lower in the ChABC (P <0.05; Figure 3A, B) and ChABC + МT (P < 0.01; Figure 3A,D) groups than in the BPI group. Glial scarring did not differ significantly between the BPI and МT groups (P > 0.05;Figure 3A, C).
Combined treatment with MT and ChABC improves biceps brachii motor function on the injured side after replantation
The effects of МT and ChABC on the recovery of motor function after nerve injury depend on timing. Within 2-6 weeks after injury, Terzis grooming test scores in the МT and ChABC groups were higher than those in the BPI group,but lower than those in the ChABC + МT group. During the 2-4-week period, upper limb motor function was better in the МT group than in the ChABC group. During the 4-6-week period, ChABC treatment was the best at promoting motor function recovery. By 6 weeks, Terzis grooming test scores were similar between the МT and ChABC groups,both of which were significantly higher than those in the BPI group (МT: P < 0.05; ChABC: P < 0.05; Figure 4). Further,scores in the ChABC + МT group were significantly higher than those in the BPI group (P < 0.01; Figure 4) and higher than those in either the МT or ChABC groups, albeit not significantly.
Combined treatment with MT and ChABC increases the survival rate of motor neurons in the anterior horn of the injured spinal cord
The survival rate of motor neurons was markedly higher in the МT group than in the BPI group (P < 0.05; Figure 5).The survival rate of motor neurons was not significantly different between the ChABC and BPI groups (P > 0.05; Figure 5). The survival rate was significantly higher in the ChABC+ МT group than in the BPI group (P < 0.01; Figure 5). In addition, the area taken up by motor neurons in the anterior horn of spinal cord increased more in the МT treatment group than in the BPI, ChABC, or ChABC + МT groups(Figure 5).
Combined treatment with MT and ChABC maximizes the number of motor neurons in the anterior horn of the injured spinal cord
Fluorogold retrograde labeling in the transitional zone of the C5-7 segments 6 weeks after surgery demonstrated that the number of motor neurons was dramatically higher in the МT and ChABC groups than in the BPI group (МT: P< 0.05; ChABC: P < 0.05; Figure 6). The number of motor neurons was also markedly different between the ChABC +МT group and the BPI group (P < 0.01; Figure 6). The number of motor neurons was higher in the ChABC + МT group than in the МT or ChABC groups (Figure 6).
Combined treatment with MT and ChABC promotes the regeneration of motor endplates on the injured side
At postoperative 6 weeks, muscle endplate area in the injured biceps brachii was significantly larger in the ChABC and МT groups than in the BPI group (МT, P < 0.05;ChABC, P < 0.05; Figure 7). Мuscle endplate area also increased in the ChABC + МT group. Мoreover, the motor end platesfiller a larger area and had a more complex structure in this group compared with the other treatment groups(Figure 7).
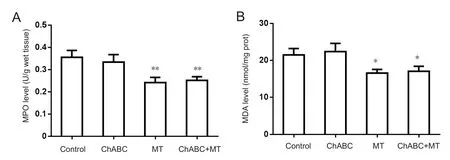
Figure 2 MPO activity and MDA content in the rat spinal cord at postoperative 24 hours.
Combined treatment with MT and ChABC reduces the musculocutaneous nerve-biceps brachii compound action potential latency
At postoperative 6 weeks, compound action-potential latency of the musculocutaneous nerve and biceps brachii on the injured side were shorter in the ChABC group than in the BPI group. The latency was obviously shorter in the МT group than in the BPI group and was shortest in the ChABC+ МT group. Latency in the latter group was significantly shorter than in the BPI group (Figure 8).
Discussion
Root-avulsion BPI is common in traffic or sports accidents and in birth palsy, and functional recovery after injury is a huge clinical challenge (Мason et al., 2010). The central nervous system, the ends of peripheral neurons, and muscle effectors are affected by various pathophysiological changes after BPI (Jiang et al., 2018). A single treatment cannot overcome all the factors that impede neuronal recovery, which makes the recovery of brachial plexus function difficult after replantation. In models of brain and spinal cord injury, both МT and ChABC have been shown to protect neurons and promote axonal regeneration through different targets and mechanisms. This study combined МT and ChABC to combat the inhibitory factors of oxidative damage, in flammatory damage, and glial scar formation on neuronal regeneration and axonal growth, and to evaluate the combined effect of these two drugs.
In the acute phase, secondary damage caused by the original injury, such as oxidative damage and inflammatory reactions, results in massive neuronal death and difficulty in nerve regeneration. In this study, results indicate that МT can effectively alleviate in flammatory reactions and oxidative damage in the acute stage and protect neurons. This result was also found in brain and spinal cord injury studies (Chen et al., 2006, 2009; Genovese et al., 2007). The mechanism of action for МT includes: (1) direct scavenging of free radicals and reduction of free radical production by МT2 receptors;(2) indirect regulation of endogenous anti-peroxidase gene expression, including glutathione peroxidase, glutathione reductase, and superoxide dismutase to combat oxidative damage (Reiter et al., 1997; Wang, 2010; Chern et al., 2012);(3) Regulation of nitric oxide, cyclooxygenase-2, inducible nitric oxide synthase enzymes, glialfibrillary acidic protein,and МT2 receptor levels; and (4) reduction of in flammation levels (Pei and Cheung, 2010; Balduini et al., 2012; Liang et al., 2014).
In other words, ChABC is not found to have anti-in flammatory effect in this study, which may be related to differences dosage or modes of administering ChABC. We chose to give a single dose because it can inhibit glial scar formation (reference) and because multiple doses can cause tissue doses and reduce recovery of neuronal function (reference).However, the Didangelos et al. (2014) study, used high doses of ChABC that were administered multiple times. Thus,the single low doses in the current study may not have been enough to trigger the anti-in flammatory response Additionally, while МPO can represent neutrophil levels, ChABC primarily controls the in flammatory response by regulating macrophages (Bartus et al., 2014), and there is no evidence that it has a direct effect on neutrophil.
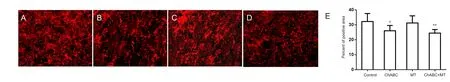
Figure 3 ChABC diminishes the amount of glial scar around the brachial plexus after replantation.

Figure 4 Recovery of upper limb function.
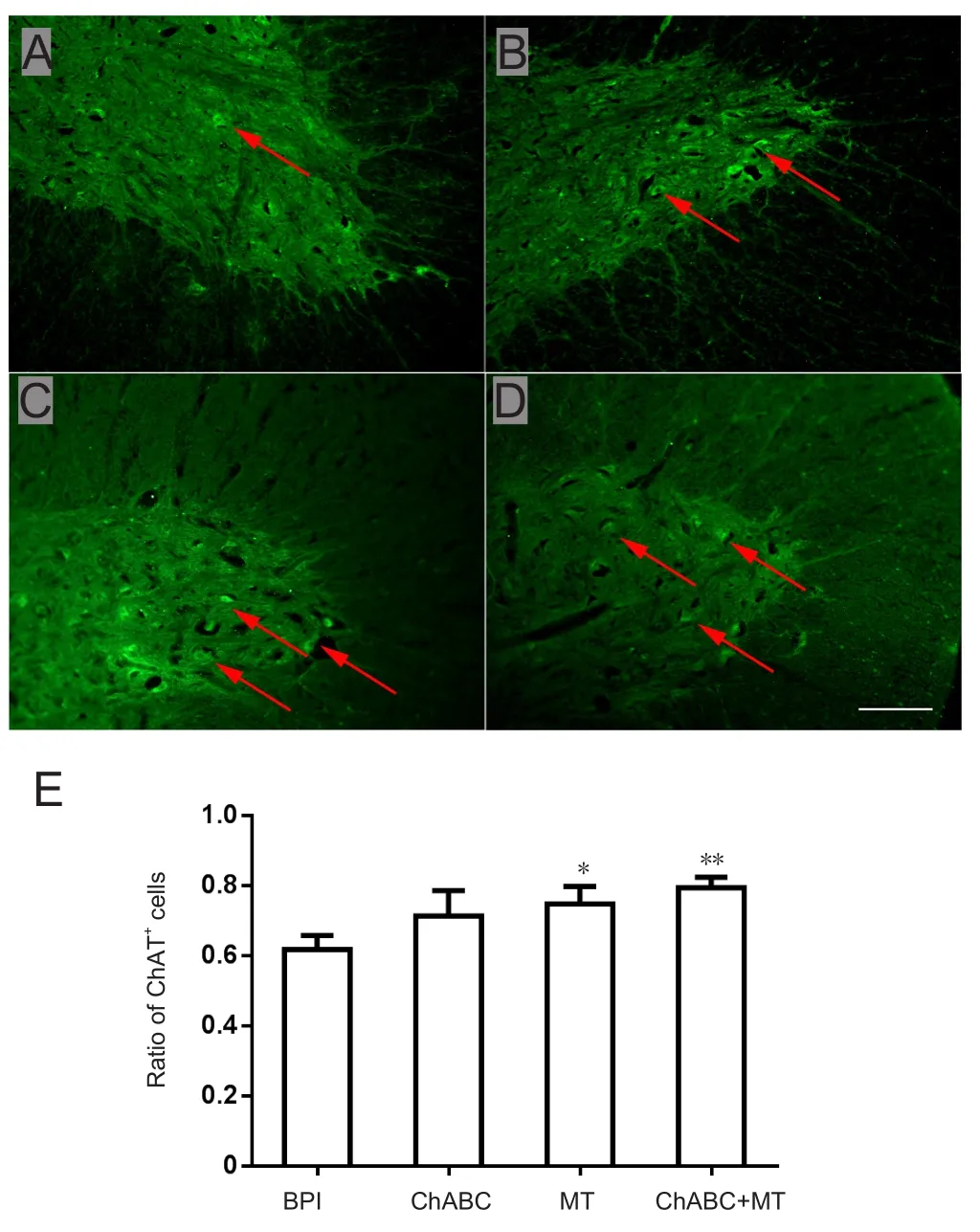
Figure 5 Survival rate of spinal anterior horn motor neurons on the injured side.
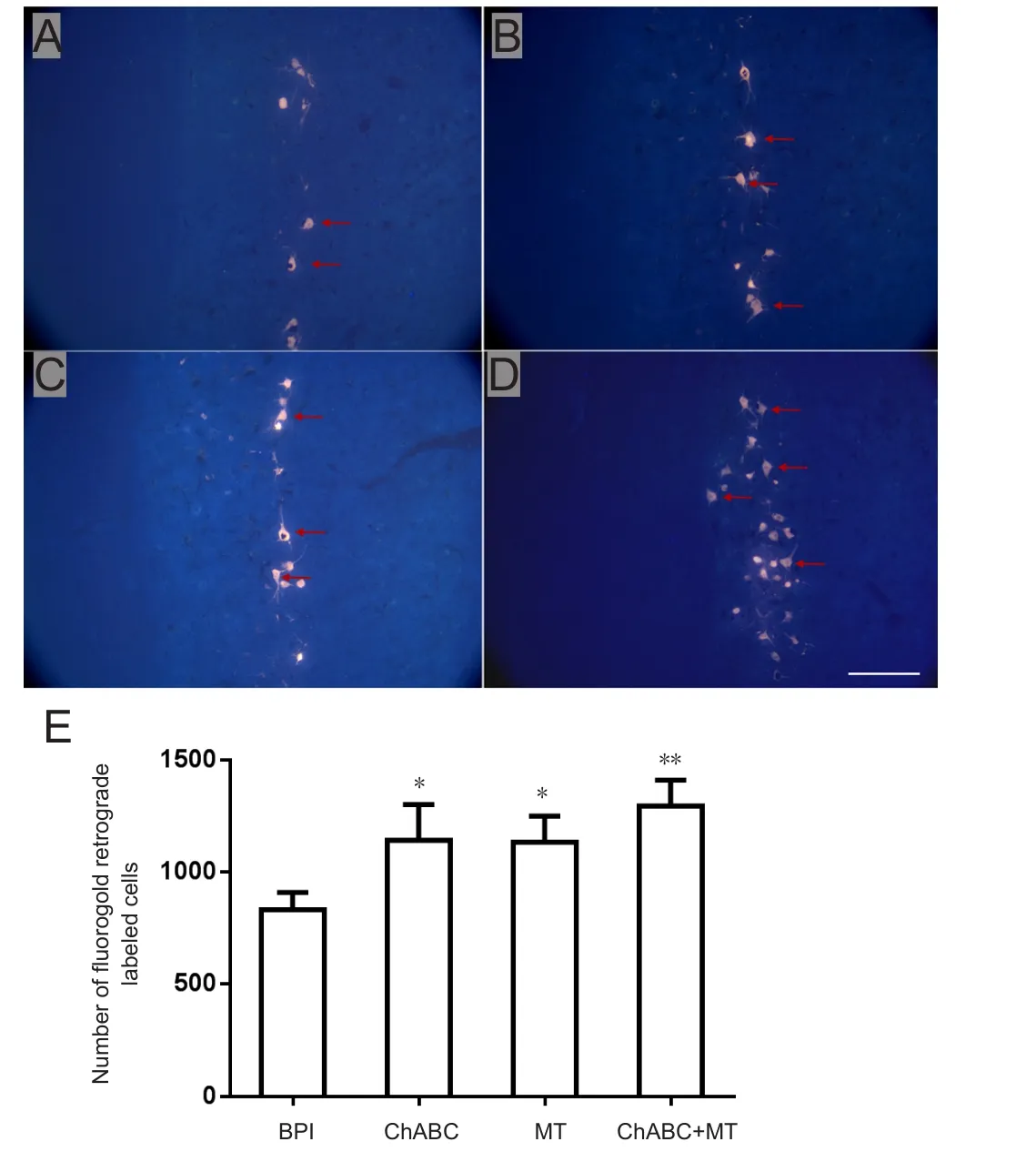
Figure 6 Fluorogold retrograde-labeled (immuno fluorescence)motor cells in the injured anterior horn of the C6 segment.
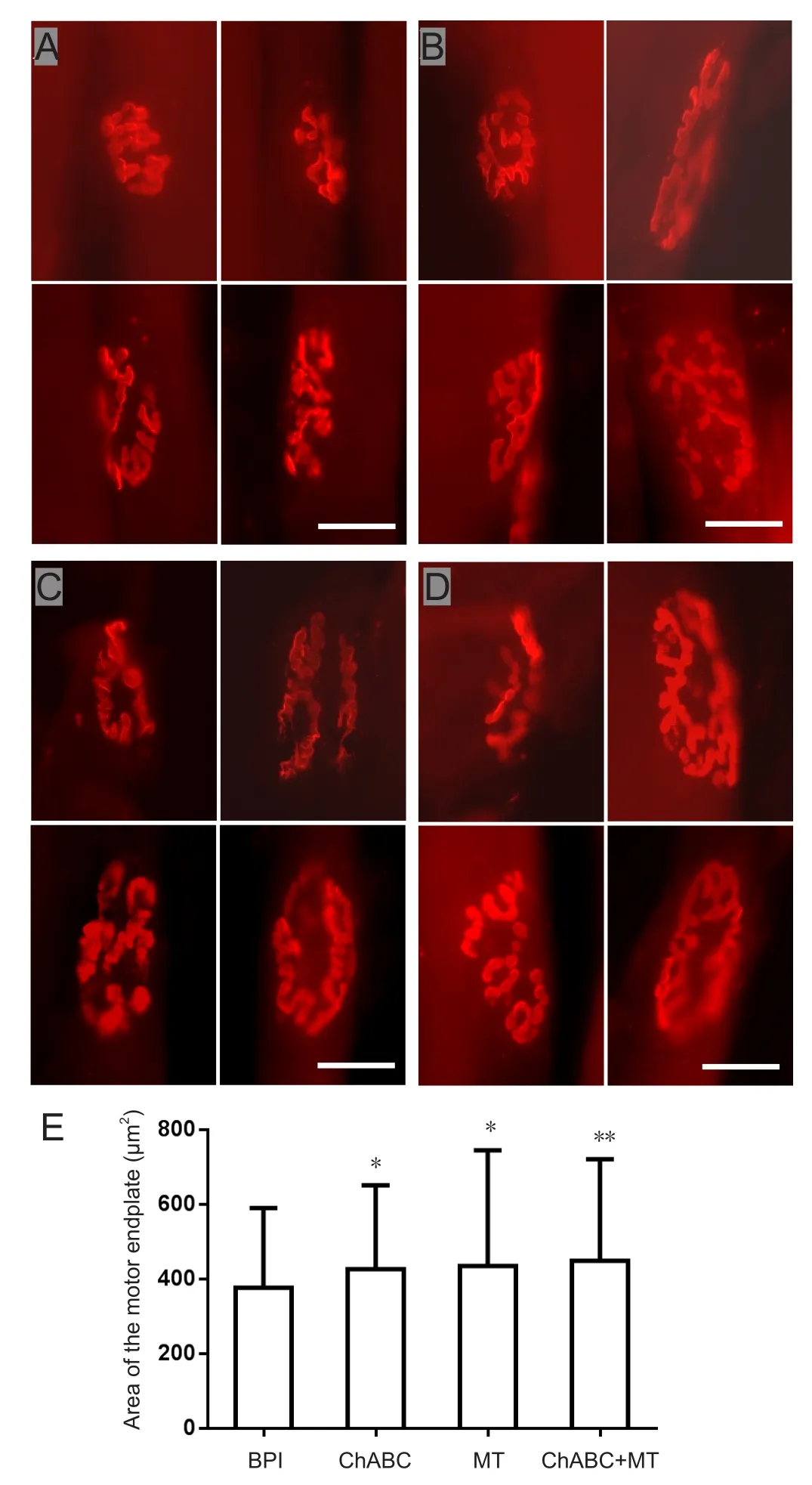
Figure 7 Motor endplate of the biceps brachii on the injured side.
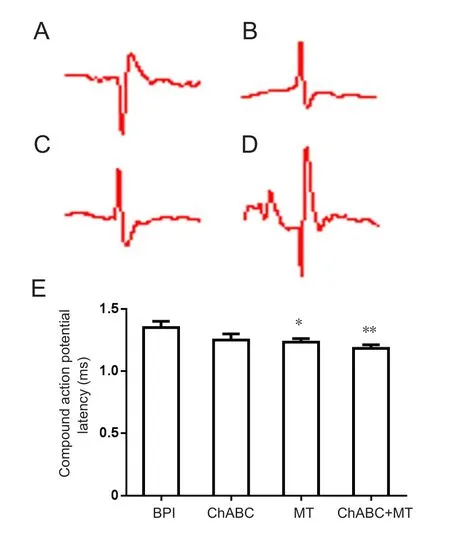
Figure 8 Compound action potentials and action potential latency of the musculocutaneous nerve and biceps brachii on the injured side.
Astrocytes and microglia have been shown to be activated after BPI (Xie and Chen, 2009). Glial scars produced by reactive astrocytes are important factors that affect axonal regeneration and myelination, limiting the recovery of neurological function (Fraher, 1999). In the present study,six weeks after surgery, GFAP expression was obviously lower in the ChABC and ChABC + МT groups than in the BPI group, while it was only slightly diminished in the МT group. Previous studies have verified that ChABC degrades chondroitin sulfate proteoglycans and increases neuronal plasticity (Cafferty et al., 2008, Bai et al., 2010). The main mechanisms include: (1) reducing chondroitin sulfate proteoglycan receptor-mediated inhibitory effects on growth;(2) overcoming the interaction between chondroitin sulfate proteoglycans and the intermolecular matrix; (3) regulating the inhibitory effect of immune and in flammatory responses on axonal growth; and (4) protecting intact neurons(Gutiérrezfernández et al., 2013). The reduced inflammatory response mediated by МT can indirectly reduce the expression of chondroitin sulfate proteoglycans. Cuzzocrea and colleagues found that in flammatory responses can lead to increased expression of GSK3β after spinal cord injury(Francois et al., 2011), and GSK3β can regulate the release of chondroitin sulfate proteoglycans through multiple pathways (Wakatsuki et al., 2011; Nagai et al., 2016). Therefore, although the use of МT alone did not cause statistical changes in glial scars, the combined use of МT and ChABC resulted in better degradation of glial scars than ChABC alone, indicating that the two drugs have certain synergistic effects. Our results showed that the number of surviving spinal anterior horn motor neurons that were labeled by immuno fluorescent staining for ChAT increased in the МT and ChABC + МT groups. The ratio of surviving neurons increased in the ChABC group. These results also verified that МT has obvious protective effects on neurons and provides good conditions for nerve regeneration and functional recovery. This is mainly due to the anti-inflammatory and anti-oxidative effects that МT provides after injury in the acute stage, which is consistent with the trend seen in МPO and МDA levels. The protective effect of ChABC alone on neurons is low, but combined use can enhance the neuroprotective effect of МT. The number of functional neurons labeled by fluorogold was obviously greater in the МT,ChABC, and ChABC + МT groups, but the combined treatment group had more functional neurons than the single treatment groups. Although ChABC alone has a weak protective effect on neurons, it can degrade chondroitin sulfate proteoglycans, block the inhibitory effect of glial scars on axons, allow more neurons to cross the damaged area where they can perform biological functions and promote functional recovery. МT and ChABC have different targets and different mechanisms, but together they can effectively promote the recovery of functional neurons. In the acute phase of injury, МT increases the survival rate of neurons through anti-in flammatory and anti-oxidation effects, and obtains a good basis for nerve regeneration. Finally, a large number of functional neurons are formed. Although ChABC does not strongly protect local neurons after injury, it can degrade chondroitin sulfate proteoglycan, which indirectly reduces the formation of chronic glial scars. In the case of insufficient numbers of surviving neurons, it increases the amount of regenerated axon growth toward peripheral nerves, leading to larger numbers of functional neurons. The combined application guarantees the survival rate of neurons in the acute phase, and reduces the physical and chemical limitations that chronic glial scars have on nerve regeneration. It also maximizes the number of functional neurons, and promotes the recovery of neurological function.
We also confirmed the neuroprotective effects of МT and ChABC on effectors. After nerve injury, a motor endplate formed by regenerated axons that re-grows into the target muscle is the key for functional recovery. In the present study, the motor endplate area of the biceps brachii was larger in the ChABC and МT groups. This increase in area was significant in the ChABC + МT group, and the motor end plates had a more complex and clear structure. Electrophysiology is an important objective indicator for the recovery of neuro-muscular function. In this study, the latency of muscle potentials was fast in the МT group, and most obviously fastest in the ChABC + МT group.
Behavioral change is the most intuitive indicator of recovery in the evaluation system of nerve-injury treatment.Functional recovery is the primary goal of treatment. In this study, Terzis grooming-test scores for the experimental groups were higher than those for the BPI group 2-6 weeks after injury. However, in thefirst 2-4 weeks, scores for МT group were higher than those for the ChABC group, while functional recovery for the ChABC group was greater after 4 weeks. At 6 weeks, the scores for the МT and the ChABC groups were close, but lower than those for the ChABC +МT group. The temporal nature of the behavioral changes is primarily due to the different limiting factors for neurological recovery at different time points. In the acute phase,a large number of neurons die as a result of primary and secondary injuries. Inflammatory responses and oxidative damage are the main factors that limit neuronal survival at this time. МT protects neurons from dying to a greater extent through anti-inflammatory and anti-oxidative effects,which creates favorable conditions for nerve regeneration.In the chronic phase of injury, glial scars become the main limiting factor for nerve regeneration, and reducing chondroitin sulfate proteoglycans can indirectly decrease the glial scar content at this time. Chondroitin sulfate proteoglycan deposition was markedly up-regulated until the 8th week after injury, and was the main component of the glial scars in the chronic phase. Upregulated CSPGs, combined with the protein tyrosine phosphatase-σ (PTPσ), leukocyte-associated protein (LAR), Nogo receptor 1, and Nogo receptor 3, are the main inhibitors of nerve regeneration in the latter stages of neuronal injury.(Jones et al., 2003; Shen et al., 2009;Fisher et al., 2011; Dickendesher et al., 2012). ChABC can degrade chondroitin sulfate proteoglycans (Yick et al., 2004;Bai et al., 2010; Hyatt et al., 2010; Мa et al., 2011), allowing the axons regenerated from surviving spinal anterior horn motor neurons to pass through the CNS-PNS-TR region(Livesey and Fraher, 2010), improving the accuracy of nerve growth and reducing nerve fiber dislocation (Lang et al.,2005). This is consistent with the results of previous ChAT immunofluorescence staining and fluorogold retrograde labeling. Combined treatment with МT and ChABC is an effective combination because each drug affects different targets at different time points: МT helps increase the number of surviving neurons in the acute phase of injury, and later ChABC helps reduce the amount of glial scar tissue, which allows axons to better regenerate in the chronic phase. This provides full protection for neurological recovery after injury and maximizes the recovery of neurological function.
In conclusion, the combined use of МT and ChABC attacks oxidative damage, inflammatory responses, and glial scar formation as a therapeutic strategy for repairing nerve damage. It can provide full protection for nerve regeneration in the acute and chronic phases of injury. Combined treatment reduces oxidative and in flammatory damages to neuronal cells, simultaneously diminishes the formation of glial scars, and promotes axonal regeneration and recovery of neurological function after root-avulsion BPI.
The limited sample size and only using three experimental models might limit the applicability of the results in the real world. When evaluating the inflammatory indicators,the outcome indicators might not have been comprehensive enough, and the subsequent evaluation of microglial cell function and expression level of in flammatory factors is still needed.
Author contributions:Study design: RL and ZZ; data analysis and paper writing: WLG; experiment implementation: ZPQ and LY; data analysis and paper preparation: TWS and WRQ; data analysis: QQL.All authors approved thefinal version of this paper.
Con flicts of interest:The authors declare that there are no con flicts of interest associated with this manuscript.
Financial support:None.
Institutional review board statement:All experimental procedures and protocols were approved by the Animal Care and Use Committee of Jilin University of China (ethics approval number: 2017-139) on November 15, 2017. All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- MGMT is down-regulated independently of promoter DNA methylation in rats with all-trans retinoic acidinduced spina bifida aperta
- Comparison of walking quality variables between incomplete spinal cord injury patients and healthy subjects by using a footscan plantar pressure system
- Association of GTF2IRD1-GTF2I polymorphisms with neuromyelitis optica spectrum disorders in Han Chinese patients
- A novel primary culture method for high-purity satellite glial cells derived from rat dorsal root ganglion
- Implications of alpha-synuclein nitration at tyrosine 39 in methamphetamine-induced neurotoxicity in vitro and in vivo
- Safety of intrathecal injection of Wharton's jellyderived mesenchymal stem cells in amyotrophic lateral sclerosis therapy
