Lesson Eighty-six Slow/fast atrioventricular nodal reentrant tachycardia using the inferolateral left atrial slow pathway-role of the resetting response to select the ablation target
2018-12-12童鸿
In the majority of cases of slow/fast Atrioventricular nodal reentrant tachycardia(AVNRT),which occurs in≈90%of the patients,the anterograde slow pathway is formed by the rightward inferior extension of the atrioventricular node,which can be targeted for ablation with a low risk of atrioventricular block at the inferior triangle of Koch.In a smaller number of patients,the anterograde limb of the slow/fast AVNRT circuit is formed by the leftward inferiorextension of the atrioventricular node,which can be targeted at the roof of the coronary sinus,≈1 to 3cm from the coronary sinus ostium or from the inferior paraseptal mitral annulus.On rare occasions,the slow pathway participating in AVNRT may connect to the basal inferolateral left atrium (IL-LA),near the mitral annulus.we refer to it as IL-LA slow pathway.Because most of these patients do not exhibit retrograde conduction over the IL-LA slow pathway,and anterograde slow pathway conduction cannotbe recorded for localization,a different approach must be used to identify a safe and successful site for ablation.The aim of this study was to describe a technique to identify the ablation target by using the resetting response1elicited by delivering late atrial extrastimuli along the basal IL-LA,near the mitral annulus,to localize the atrial end of the anterograde slow pathway participating in the tachycardia.
Slow/Fast AVNRT Ablation Procedure
In patients with slow/fast AVNRT and no prior failed ablation procedure,the slow pathway at the inferior triangle of Koch was empirically targeted for ablation.Ablation sites always remained below the level of the roof of the coronary sinus ostium.When ablation at the inferior triangle of Koch failed to eliminate the tachycardia,the leftward inferior extension of the atrioventricular node was targeted by delivering a radiofrequency application along the roofofthe coronary,1 to 3cm from the coronary sinus ostium.In cases where ablation,which targeted both the rightward and leftward inferior extensions of the atrioventricular node,failed to eliminate the tachycardia,we considered that the IL-LA slow pathway may form the anterograde limb of the AVNRT circuit.Transseptal puncture was performed,and the resetting response to late extrastimuli during AVNRT was used to select the ablation target.
Radiofrequency applications(15-30 Watts with a nonirrigated catheter and 30-35 Watts with an irrigated catheter)were delivered to that site.When accelerated junctional rhythm occurred, the radiofrequency application was maintained until 15 to 30 seconds after cessation (or marked slowing) of the accelerated junctional rhythm.The end point of ablation was elimination of 1:1 anterograde slow pathway conduction during decremental atrial pacing and noninducibility of the tachycardia for 1 hour in both the baseline state and during isoproterenol administration(2-4μg/min).
Resetting Technique to Localize the Atrial End of IL-LA Slow Pathway
The mapping/ablation catheter was positioned at the basal IL-LA,near the mitral annulus,at≈4:00 to 5:30 o'clock in the leftanterior oblique(LAO)projection(Figure 1).During stable slow/fast AVNRT(with minimal or no cycle length variation),a single late atrial extrastimulus was delivered to the test site,beginning near the timing of the end of the QRS complex and shortening the coupling interval in 5 or 10ms decrements,as long as the timing and morphology of the retrograde atrial potential in the His bundle electrogram were unchanged,indicating that the retrograde fast pathway was not engaged(Figure 2).A reproducible advancement of the next His bundle potential by at least 5ms,followed by resetting of the tachycardia cycle length,indicated that the atrial extrastimulus engaged the anterograde slow pathway(Figure 2).The lateness of the extrastimulus was measured from the onset of the His bundle potential for comparison between sites (Figure 2).Multiple sites were tested near the inferolateral mitral annulus.The site where the latest atrial extrastimulus advanced the next His bundle potential and reset the tachycardia was considered closest to the atrial end of the anterograde slow pathway participating in the tachycardia and was selected as the target for ablation.
In patients with a prior failed ablation procedure,we generally used the resetting response during AVNRT to identify the anterograde slow pathway participating in the AVNRT circuit.Resetting was sequentially tested at the inferior triangle of Koch,the roof of the proximal coronary sinus,and the basal IL-LA.
The clinical and electrophysiological characteristics
The resetting response to extrastimuli and the response to ablation indicate that the IL-LA slow pathway formed the anterograde limb of the reentrant circuit in 10(1.2%)of 843 patients with slow/fast AVNRT.These 10 patients include 7 of 148(4.7%)patients who had a prior failed ablation procedure.
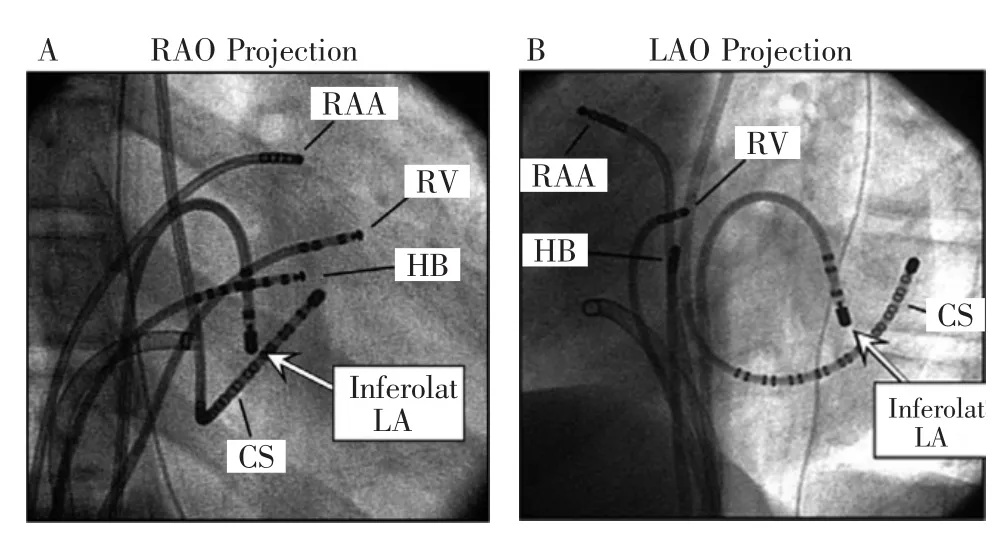
Figure 1 Radiographs in the right anterior oblique(RAO;A)and left anterior oblique(LAO;B)projections show the mapping catheter positioned at the basal inferolateral left atrium,close to mitral annulus(arrow,inferolat LA),at the site of resetting with the latest extrastimulus and successful ablation of the IL-LA slow pathway.Other catheters are positioned at the right atrial appendage (RAA),His bundle region(HB),anterobasal right ventricle(RV),and coronary sinus(CS).
There were one or both of 2 unusual characteristics noted in 5 of the patients using the IL-LA slow pathway in slow/fastAVNRT.The H-A intervalduring tachycardia was short(<30 ms)in 4 (40%)patients.The 2 for 1 response(an atrial extrastimulus resulting in simultaneous conduction over the fast atrioventricular nodal pathway and a slow pathway producing 2 His bundle/ventricular potentials)was observed in 3(30%)patients.Two patients exhibited both the shortest H-A intervals during tachycardia(0 and 15 ms)and the 2 for 1 response.Notably,these were the only 3 patients exhibiting the 2 for 1 response in the entire cohort of 843 patients with slow/fast AVNRT.
Resetting of AVNRT was initially tested at the inferior triangle of Koch in 2 of the 10 patients and along the roof of the coronary sinus in 6 of the 10 patients using the IL-LA slow pathway in slow/fast AVNRT.Extrastimuli at the inferior triangle of Koch failed to advance the His bundle potential and reset the AVNRT cycle length in both of the 2 patients,whereas extrastimuli at the roof of the coronary sinus reset the tachycardia in 3(50%)of the 6 patients.However,when successful,resetting from the roof of the coronary sinus required a relatively early extrastimulus(extrastimulus timing <35 ms after the His bundle potential)compared with the successful ablation site at the basal IL-LA(≥35ms;Figure 2).Ablation was initially performed unsuccessfully at the inferior triangle of Koch in 9 patients and in the roof of the coronary sinus in 4 patients,including the 3 patients with successful resetting from the roof of the coronary sinus.Junctional automaticity was elicited when radiofrequency current was applied to the inferior triangle of Koch,indicating injury of the rightward inferiorextension of the atrioventricular node,but neither anterograde slow pathway conduction nor the tachycardia cycle length was affected.

Figure2 Resetting technique for localizing the atrial end of the inferolateral left atrial slow pathway forming the anterograde limb of the slow/fast reentrant circuit.A,During slow/fast atrioventricular nodal reentrant tachycardia with constant cycle length 395ms,an atrial extrastimulus was delivered to the mapping catheter(roof coronary sinus[CS]) at the roof of the coronary sinus,4cm from the ostium,340ms after the right atrial appendage(RAA) potential,and 20 ms after the His bundle(HB) potential.The extrastimulus did not prevent retrograde activation of the fast pathway as the timing and morphology of atrial activation in the His bundle electrogram were not changed(A-A=395ms in the HBp electrogram).The atrial extrastimulus advanced the next His bundle potential(H2) by 10 ms (H-H2=385ms in the HBd electrogram).The advanced His Bundle potential(H2) was followed by a similar 10ms advance in atrial activation and a subsequent H2-H interval equal to the tachycardia cycle length(395ms in the HBd electrogram),indicating resetting of the tachycardia cycle length (ie , resetting the tachycardia). B, When a later atrial extrastimulus (360ms after the RAA potential and 40ms after the HB potential) was delivered from the mapping catheter in the inferolateral left atrium (LA) close to the mitral annulus(arrow,S2),after retrograde atrial activation had occurred(A-A=395ms in the HBp electrogram),the next His bundle potential(H2) was advanced by 10ms(H-H2=385ms in the HBd electrogram),thus resetting the tachycardia(10ms advance in atrial activation and a subsequent H2-H interval equal to the tachycardia cycle length of 395ms).The resetting of the tachycardia with such a late atrial extrastimulus suggests this site is located close to the atrial end of the slow pathway forming the anterograde limb of the reentrant circuit.II,V1indicates electrocardiographic leads II and V1;d,distal;and p,proximal.
Extrastimuli delivered to the basal IL-LA(Figure 1)advanced the His bundle potential by ≥10 ms and reset slow/fast AVNRT in all 10 patients(Figure 2).A median of 4 left atrial sites was evaluated in each patient.The latest atrial extrastimulus eliciting a positive resetting response was delivered at least 35ms(mean,49 ±12ms;range,35-65ms) after the timing of the onset of the His bundle potential(Figure 2).Ablation was targeted at the site of resetting by the latest extrastimulus as this was interpreted as being closest to the atrial end of the anterograde slow pathway in the reentrant circuit.A median of1 radiofrequency application eliminated slow pathway conduction and terminated the tachycardia in allpatients.The successful ablation site was located at the basal IL-LA,near the mitral annulus.Accelerated junctional rhythm with retrograde conduction over the fast pathway(IL-LA slow pathway automaticity)occurred during the successful radiofrequency application in 9 of 10(90%)patients.Anterograde fast atrioventricular nodal pathway conduction remained intact in allpatients.No procedural complications were observed in any patient.
词 汇
participate v.参与,分享,含有
empirically adj.经验性地,凭经验地
triangle n.三角形,三角,三角铁
oblique n.&adj.&v.倾斜物,斜肌;斜的;倾斜,斜行进prior adj.在先的,优先的,更早,更重要
注 释
1.resetting response指“重整反应”,是紧接重整刺激后首个心动过速激动的时间与引起重整的期前刺激联律间期之间的关系,通常有3种类型,分别是递增型、递减型和平坦型。
参考译文
第86课 基于左心房下外侧慢径路的慢快型房室结折返型心动过速:重整反应在选择消融靶点中的作用
房室结折返型心动过速(AVNRT)中的近90%为慢快型,其顺向慢径主要由房室结向右下延伸形成,位于Koch三角下方,消融此处发生房室传导阻滞的风险低。少数患者的慢快型AVNRT折返环前传支由房室结向左下延伸形成,距冠状窦口1~3cm的冠状窦顶部或二尖瓣环下间隔旁可成为靶点。极少数情况下,参与AVNRT的慢径与邻近二尖瓣环的左心房下外侧(IL-LA)基部相连接。我们称之为IL-LA慢径。由于多数患者并不表现经IL-LA慢径的逆传,且无法记录顺传慢径来定位,因此需用不同的方法来确定安全和成功的消融部位。本研究的目的是阐述一种利用沿IL-LA基部的晚期心房刺激引发的重整反应技术,来定位参与心动过速顺传慢径的心房端,从而确定消融部位。
慢快型AVNRT的消融手术
对于既往无消融失败史的慢快型AVNRT患者,于Koch三角下方行经验性消融。消融部位总是位于冠状窦口顶部水平下方。当在Koch三角下方消融失败时,选择房室结左下延伸部位为靶点,沿距冠状窦口1~3cm的冠状窦顶部进行消融。对于房室结左右侧延伸部消融均失败者,我们考虑IL-LA慢径构成AVNRT折返环的前向支。行房间隔穿刺,利用于AVNRT发作期间对晚期期外刺激的重整反应选择消融靶点。
对该部位进行射频消融(非灌注导管15~30W,灌注导管30~35W)。当出现加速性交界性节律时,维持消融直至加速性节律停止或明显减慢后15~30s。消融终点是在基础状态下或异丙肾上腺素激发下(2~4 μg/min)进行递减性心房起搏时无1:1慢径前传且无法诱发心动过速,达1h。
重整技术定位IL-LA慢径心房端
标测/消融导管位于近二尖瓣环的IL-LA基部,相当于左前斜位(LAO)上4:00~5:30钟点的位置(图1)。于稳定的慢快型AVNRT发作期间(周长微小变化或不变),发放单一晚期心房期外刺激,从接近QRS波群终点时间开始,以5或10ms的递减缩短联律间期,只要希氏束电图上的逆传房波时间与形态不变,表明未侵入逆传快径(图2)。下一希氏束电位的可重复性提前至少5 ms,随后是心动过速周长的重整,表明房性期外刺激侵入顺传的慢径(图2)。从希氏束起始开始测定期外刺激的延后时间用于不同部位的比较(图2)。对接近下外侧二尖瓣环的多个部位进行测试,能使希氏束电位前移并且重整心动过速的最晚心房期外刺激部位,认为是参与心动过速的顺传慢径心房端并确定为消融靶点。
对既往有过消融失败的患者,我们通常于AVNRT期间利用重整反应去鉴别参与心动过速的顺传慢径。于Koch三角下方、冠状窦近端顶部和IL-LA基部进行连续重整测试。
临床和电生理特征
对期外刺激的重整反应和对消融的反应表明,843例慢快型AVNRT患者中的10例(1.2%),IL-LA慢径形成折返环的前传支。这10例包含了既往有过消融失败的148例患者中的7例(4.7%)。
5例利用IL-LA慢径的慢快型AVNRT患者具有两种不同寻常特征的一种或两种表现。4例(40%)心动过速时H-A间期短(<30 ms)。3例(30%)表现为1:2反应(一次心房期外刺激同时经房室结快径和慢径传导产生2个希氏束电位/心室波)。2例同时具备心动过速时最短的H-A间期(0 and 15 ms)和1:2反应。值得注意的是这是843例慢快型AVNRT患者中仅有的表现为1:2反应的3例。
10例利用IL-LA慢径的慢快型AVNRT患者的AVNRT重整,2例开始于Koch三角下方,6例沿着冠状窦顶部。2例Koch三角下方的期外刺激均不能使希氏束电位前移和重整AVNRT周长,而6例冠状窦顶部期外刺激中的3例(50%)重整了心动过速。然而,其时的成功,与在IL-LA基部成功消融的部位比较,冠状窦顶部的重整需要相对较早的期外刺激(希氏束电位后<35ms比>35ms,图2)。9例Koch三角下方消融失败,4例冠状窦顶部消融失败,包括冠状窦顶部成功重整的3例,当对Koch三角下方消融时出现交界性节律,提示损伤到房室结右下延伸部,但慢径前传与心动过速周长均未受到影响。
所有10例IL-LA基部的期外刺激(图1)均使希氏束电位前移≥10 ms并重整慢快型AVNRT(图2)。每例患者左心房评估部位的中位数是4个。引发阳性重整的最晚房性期外刺激至少在希氏束电位起始点后35ms,平均(49±12)ms,从35~65 ms(图2)。消融靶点位于最晚期外刺激重整的部位,因为该处最接近折返环前传慢径的心房端。所有患者阻断慢径传导和中止心动过速的射频消融次数中位数是1次。成功消融部位位于近二尖瓣环的IL-LA基部。10例中的9例(90%)成功消融过程中发生加速性交界性节律通过快径逆传(IL-LA慢径自律性)。所有患者房室结快径前传无损。无并发症发生。
图1右前斜位(RAO,A)与左前斜位(LAO,B)射线照相显示标测导管位于左心房基部下外侧,接近二尖瓣环(箭标,左心房下外侧),位于最晚期外刺激和成功消融IL-LA慢径的部位。其他导管位于右心耳(RAA),希氏束区(HB),右心室前基底部(RV),和冠状窦(CS)。
图2用于定位形成慢快型折返环前传支的左心房下外侧慢径心房端的重整技术
A:在恒定周长395ms的慢快型房室结折返型心动过速期间,于距冠状窦口4cm的冠状窦(CS)顶部、右心耳(RAA)电位后340ms、希氏束(HB)电位20ms后发放心房期外刺激至标测电极。期外刺激不影响快径逆传,因希氏束电图上的心房激动时间和形态不变(希氏束近端电图A-A=395ms)。心房刺激使下一希氏束电位(H2)提前10ms(希氏束远端电图H-H2=385ms)。提前的希氏束电位(H2)尾随着相同10ms提前的心房激动,其后的H2-H间期与心动过速周长相同(希氏束远端电图395 ms),提示心动过速周长重整(即心动过速重整)。B:当从位于接近二尖瓣环(箭标,S2)的下外侧左心房(LA)标测导管发放较晚的心房期外刺激(RAA电位后360 ms、HB电位后40ms),在逆传心房激动后(希氏束近端电图A-A=395ms),下一希氏束电位(H2)提前 10ms(希氏束远端电图H-H2=385 ms),重整了心动过速(心房激动提前10ms,随后的H2-H间期与心动过速周长395ms相同)。在这一晚期心房期外刺激下心动过速重整,提示该部位接近形成折返环前传支的心房端。Ⅱ,V1指心电图Ⅱ和V1;d,远端;和p,近端。