苹果MdWRKY18和MdWRKY40参与盐胁迫途径分子机理研究
2018-12-12许海峰杨官显张静邹琦王意程曲常志姜生辉王楠陈学森
许海峰,杨官显,张静,邹琦,王意程,曲常志,姜生辉,王楠,陈学森
苹果MdWRKY18和MdWRKY40参与盐胁迫途径分子机理研究
许海峰,杨官显,张静,邹琦,王意程,曲常志,姜生辉,王楠,陈学森
(山东农业大学园艺科学与工程学院/作物生物学国家重点实验室,山东泰安 271018)
【目的】研究苹果WRKY转录因子MdWRKY18和MdWRKY40蛋白结构、表达水平及在盐胁迫中的功能,为进一步完善盐胁迫分子机理的研究提供参考。【方法】以‘红脆2号’苹果为试材,克隆和,对其蛋白结构进行分析;采用qRT-PCR测定该基因在盐胁迫条件下的表达水平,并通过GUS染色分析它们启动子活性,利用酵母双杂分析其互作关系,并通过转基因验证其功能。【结果】蛋白结构分析表明MdWRKY18和MdWRKY40均含有一个WRKY、Cx5C以及HxH结构域;和表达水平和启动子活性受150 mmol·L-1NaCl诱导,酵母双杂交试验表明,MdWRKY18和MdWRKY40能够和自身互作形成同源二聚体,且MdWRKY18也能和MdWRKY40互作形成异源二聚体;在‘王林’愈伤中分别过表达和时,能够促进和的表达,并提高‘王林’愈伤在盐胁迫处理下的生长量,在‘王林’愈伤中共表达和时,同样能够促进和的表达,但对提高‘王林’愈伤的生长量要高于分别过表达和。【结论】苹果和受盐胁迫的诱导,可以形成同源或异源二聚体,并增强‘王林’愈伤对盐胁迫的耐性。
苹果;WRKY转录因子;盐胁迫;GUS染色;酵母双杂交
0 引言
【研究意义】土壤盐渍化是对植物生长和作物产量产生不利影响的主要非生物胁迫之一[1-2],了解植物响应盐胁迫信号及抗盐胁迫反应的机制,对于提高作物抗盐能力至关重要。因此,研究WRKY家族中MdWRKY18和MdWRKY40在盐胁迫中的功能,对完善盐胁迫代谢机理具有重要意义。【前人研究进展】许多物理和化学方法能够提高植物的耐盐性,然而越来越多的研究都集中在培育新的耐盐品种,目前,耐盐机制在很多作物(玉米[3]、水稻[4-5]、小麦[6]、大豆[7]、草莓[8]、葡萄[9]和苹果[10]等)中得到广泛研究。在植物细胞中,盐胁迫会增加Na+和Cl-浓度,干扰K+浓度,导致离子毒性,影响离子稳态和代谢紊乱,植物通过自身生理和生化变化来抵抗盐胁迫[11-12],如,质膜上的Na+/H+逆转蛋白SOS1,定位于液泡膜的Na+/H+交换蛋白NHX1[13]。此外,越来越多的转录因子参与盐胁迫代谢,包括MYB家族、bHLH家族、bZIP家族和WRKY家族[14-17],其中,AtbHLH92能够影响活性氧介导的信号途径适应高盐胁迫[18],OsMYB91可以协调水稻的植物生长和耐盐性[5]。【本研究切入点】WRKY家族在植物非生物胁迫代谢调控中发挥重要作用,OsWRKY11能够提高水稻对热胁迫和干旱的耐性[19],AtWRKY57参与拟南芥中ABA和干旱胁迫的响应[20],AtWRKY25和AtWRKY33参与拟南芥中盐胁迫代谢调控[21],但苹果WRKY家族参与盐胁迫代谢的研究尚未见报道。【拟解决的关键问题】本研究通过克隆和,对其蛋白结构进行分析;并采用qRT-PCR及GUS分析测定该基因在盐胁迫条件下的表达,通过酵母双杂交验证其互作关系,并通过转基因验证其功能,旨在为进一步完善盐胁迫分子机理的研究提供参考。
1 材料与方法
1.1 植物材料与处理
植物材料为从新疆红肉苹果(f.)中的‘塔尔阿尔玛’与‘烟富3号’(cv. Fuji)杂种一代选育出的‘红脆2号’优株果实及‘王林’苹果愈伤组织。
1.2 总RNA的提取及qRT-PCR分析
参考Xu等[22]方法提取RNA及qRT-PCR分析。RNA提取试剂盒(DP432)、反转录试剂盒(KR106)、SYBR染料(FP205)均购自北京天根公司,以苹果为内参,3次重复,采用2-ΔΔCT方法进行数据分析[23]。
1.3‘王林’苹果愈伤组织的转化
用引物(MdWRKY18F:5′-gtcgacATGGACTCAA CGTGGGTGA-3′和MdWRKY18R:5′-ggatccTCATGA GTGGTCTGAAATTCTTC-3′及MdWRKY40F:5′- ccatggATGGACCATTCAGCTGCAT-3′和MdWRKY40R:5′-gctagcTTAG TAAGTATTGTGTTGAAGTATTC-3′,小写字母分别为Ⅰ、HⅠ、Ⅰ和Ⅰ位点)分别扩增和,电泳检测,回收、连接、转化。构建重组表达载体pRI101- MdWRKY18和pCAMBIA1301-MdWRKY40。将重组质粒导入农杆菌LBA4404,得到重组农杆菌,28℃,用30 mL含50 μg·mL-1卡那霉素和50 μg·mL-1利福平的YEP液体培养基培养重组农杆菌至OD600nm=0.6,12 000 r/min离心收集菌体,用30 mL ddH2O悬浮,加入乙酰丁香酮并使其浓度为100 μmol·L-1,得到侵染液。取生长2周龄的‘王林’愈伤组织浸到侵染液中,室温振荡25 min,取愈伤组织置于含0.5 mg·L-16-BA+1 mg·L-12,4-D的MS固体培养基上,28℃暗培养2 d。随后转移到含0.5 mg·L-16-BA+1 mg·L-12,4-D+50 μg·mL-1卡那霉素+250 μg·mL-1羧苄青霉素的MS固体培养基暗培养5周左右。
1.4 GUS表达载体构建、转化及GUS染色
用引物(MdWRKY18-1F:5′-tctagaACCTTACCAC ATGGCAAGTT-3′和MdWRKY18-1R:5′-ccatggTGTT GATGAAGATCAAAAGGCT-3′及MdWRKY40-1F:5′- tctagaCAGCAGAGGCTTGACAATTC-3′和MdWRKY40- 1R:5′-ccatgg CCTTTGAAAGTAACAACACTAGTTC- 3′)分别扩增和的启动子序列。GUS表达载体为pCAMBIA1305,构建重组表达载体及‘王林’苹果愈伤组织的转化,方法同1.3。染色液配置为100 mmol·L-1磷酸钠、10 mmol·L-1EDTA、1 mmol·L-1X-Gluc和0.1% Triton X-100。取1 g过表达或启动子的‘王林’愈伤置于5 mLGUS染色液中,37℃染色10—12 h。
1.5 酵母双杂交试验
用引物(MdWRKY18-2F:5′-catatgATGGACTCA ACGTGGGTGA-3′和MdWRKY18-2R:5′-gtcgacTCAT GAGTGGTCTGAAATTCTTC-3′及MdWRKY40-2F:5′-catatgATGGACCATTCAGCTGCAT-3′和MdWRKY40 -2R:5′-TTA GTAAGTATTGTGTTGAAGTATTC-3′)分别扩增和的编码框序列。分别构建pGADT7-MdWRKY18、pGBKT7- MdWRKY18、pGADT7-MdWRKY40和pGBKT7-MdWRKY40重组载体,方法如1.3。按照YeastmakerTMYeast Transformation System 2试剂盒(Clontech)说明书方法,将重组质粒共转化酵母Y2H感受态细胞,先在-T-L选择性培养基(-Leu/Trp,Clontech)上培养,然后将生长的细胞在-T-L-H-A选择性培养基(-Ade/- His/-Leu/-Trp,Clontech)上培养,最后用X-α-gal作为底物添加到-T-L-H-A培养基中检测β-galactosidase。
1.6 数据分析
实时荧光定量分析数据用Excel 2007进行作图和标准差分析,用DPS 7.05软件(http://www.chinadps. net)进行显著性检验,显著性水平用i、ii、iii和iv来表示。
2 结果
2.1 MdWRKY18和MdWRKY40蛋白结构分析
根据WRKY结构域的数量和锌指结构的特征可以将WRKY转录因子家族大体分为3类。利用SMART网站(http://smart.embl-heidelberg.de/smart/ set_mode.cgi?GENOMIC=1)对蛋白结构功能域分析,发现MdWRKY18和MdWRKY40与拟南芥AtWRKY18和AtWRKY40结构类似,都含有1个WRKY、Cx5C以及HxH结构域(图1),依据进化树、保守结构域及内含子位置将WRKY家族重新分类,MdWRKY18、MdWRKY40和AtWRKY18、AtWRKY40都属于Ⅱa+Ⅱb类[24]。

图1 MdWRKY18和MdWRKY40蛋白结构分析
2.2 盐胁迫诱导MdWRKY18和MdWRKY40的表达
通过对生长2周龄的‘王林’愈伤组织进行150 mmol·L-1NaCl处理(图2),结果显示,MdWRKY18和MdWRKY40的表达水平受盐胁迫诱导,在24 h达到最大,32 h略有下降;对过表达MdWRKY18和MdWRKY40启动子的‘王林’愈伤进行GUS染色,150 mmol·L-1NaCl处理的转基因愈伤要比不处理的颜色要深(图3)
2.3 MdWRKY18和MdWRKY40之间蛋白相互作用分析
酵母双杂交结果如图4所示,WRKY18-BD和WRKY18-AD共转Y2H后,在二缺、四缺及四缺+X- α-gal均生长,说明MdWRKY18能与自身互作形成同源二聚体,并且MdWRKY18也能和MdWRKY40互作形成异源二聚体,MdWRKY40同样也可以与自身互作形成同源二聚体。
2.4 MdWRKY18和MdWRKY40在‘王林’苹果愈伤中超表达后的功能分析
通过对‘王林’苹果愈伤的不同转基因进行处理(图5和图6),发现未处理的4种愈伤重量基本无差异,而150 mmol·L-1NaCl处理后,过表达和过表达的愈伤之间重量基本无差异,但显著高于‘王林’愈伤,显著低于共表达和的愈伤。
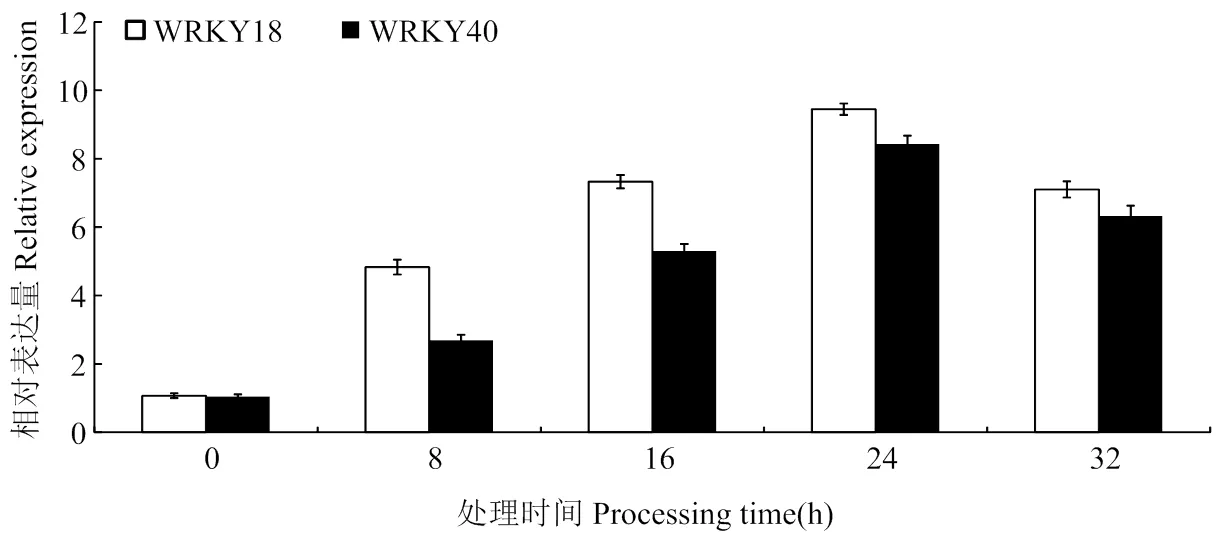
图2 150 mmol·L-1 NaCl处理下MdWRKY18和MdWRKY40的表达水平

图3 150 mmol·L-1 NaCl处理条件下MdWRKY18和MdWRKY40启动子的GUS染色分析
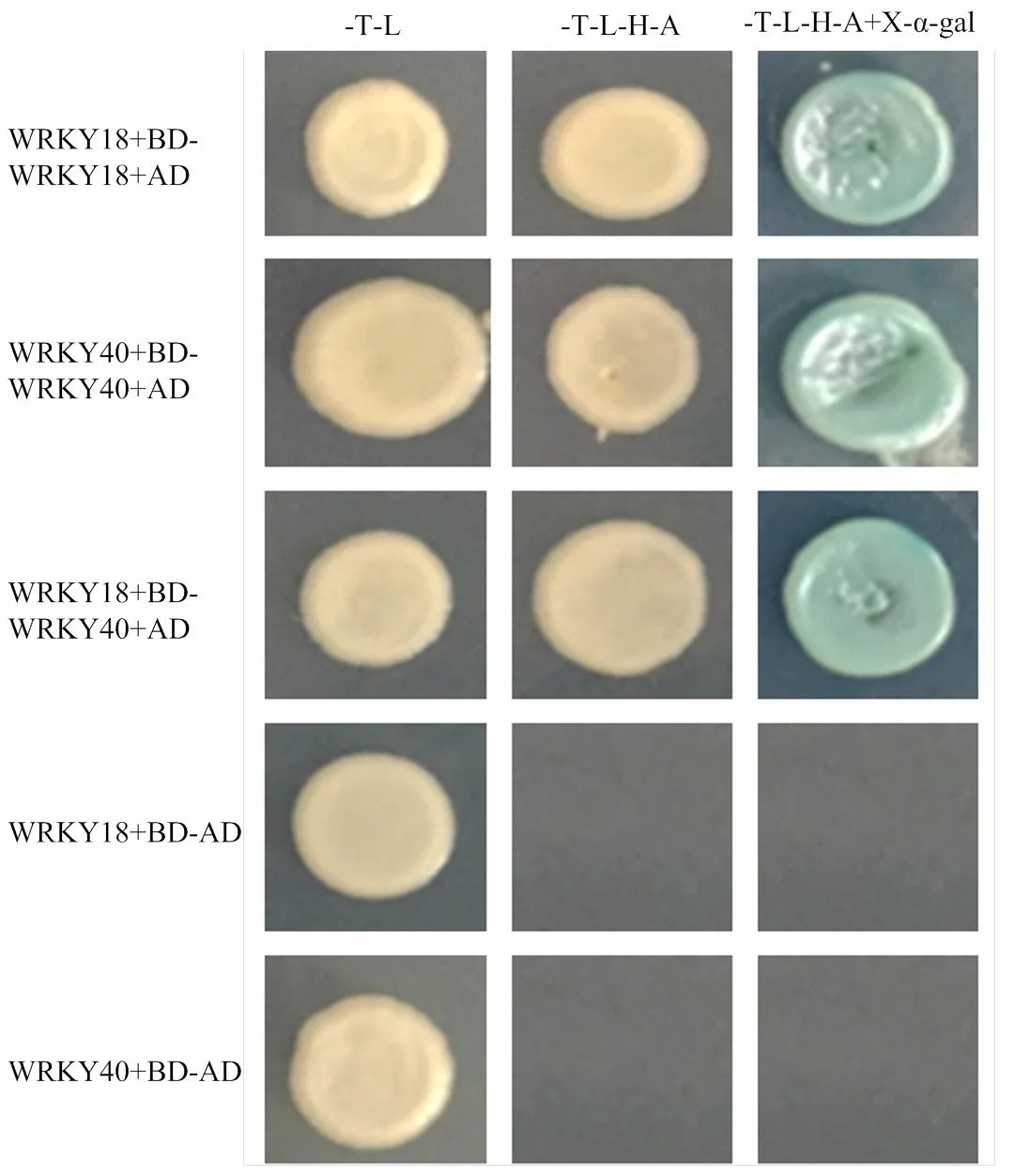
图4 MdWRKY18和MdWRKY40酵母双杂交分析
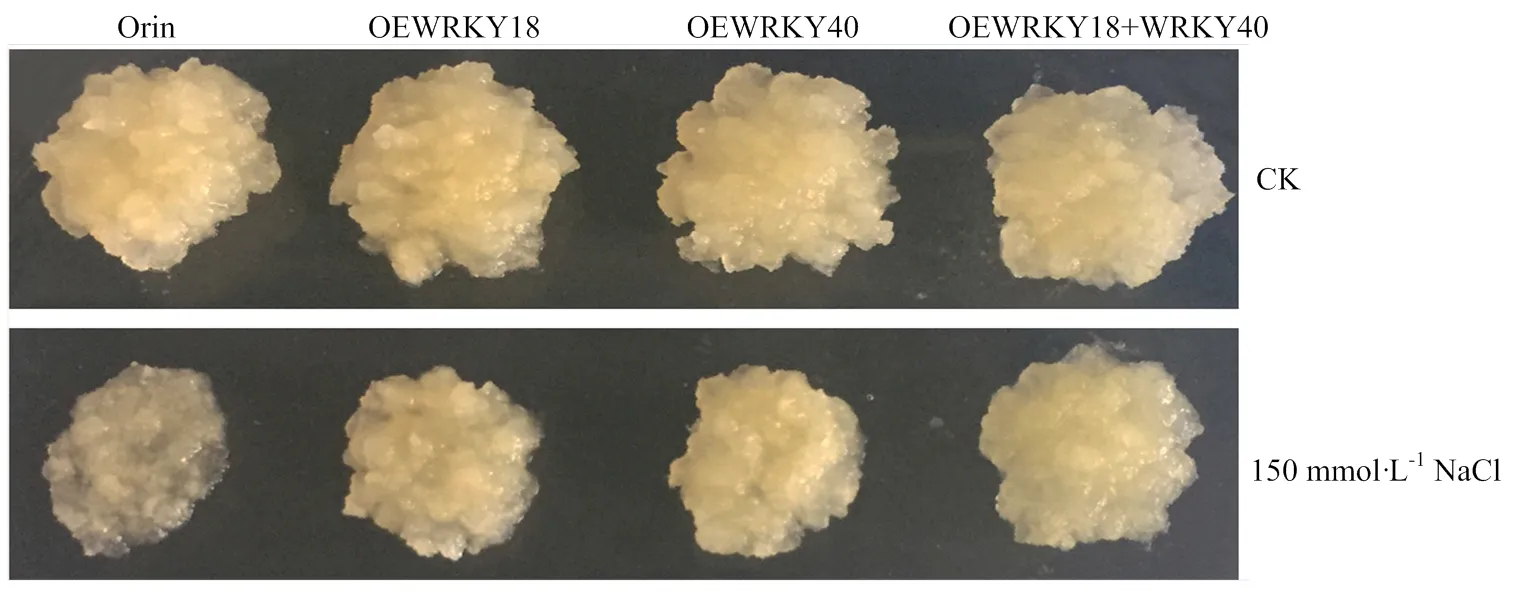
图5 150 mmol·L-1 NaCl处理条件下过表达MdWRKY18和MdWRKY40的‘王林’愈伤形态
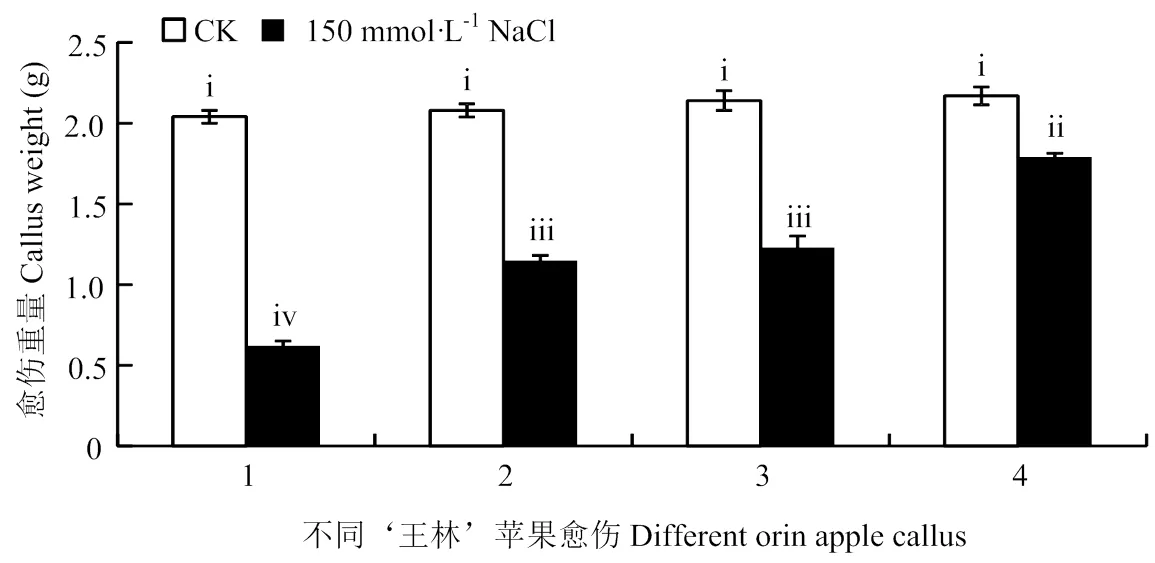
1:Orin;2:OEWRKY18;3:OEWRKY40;4:OEWRKY18+OEWRKY40
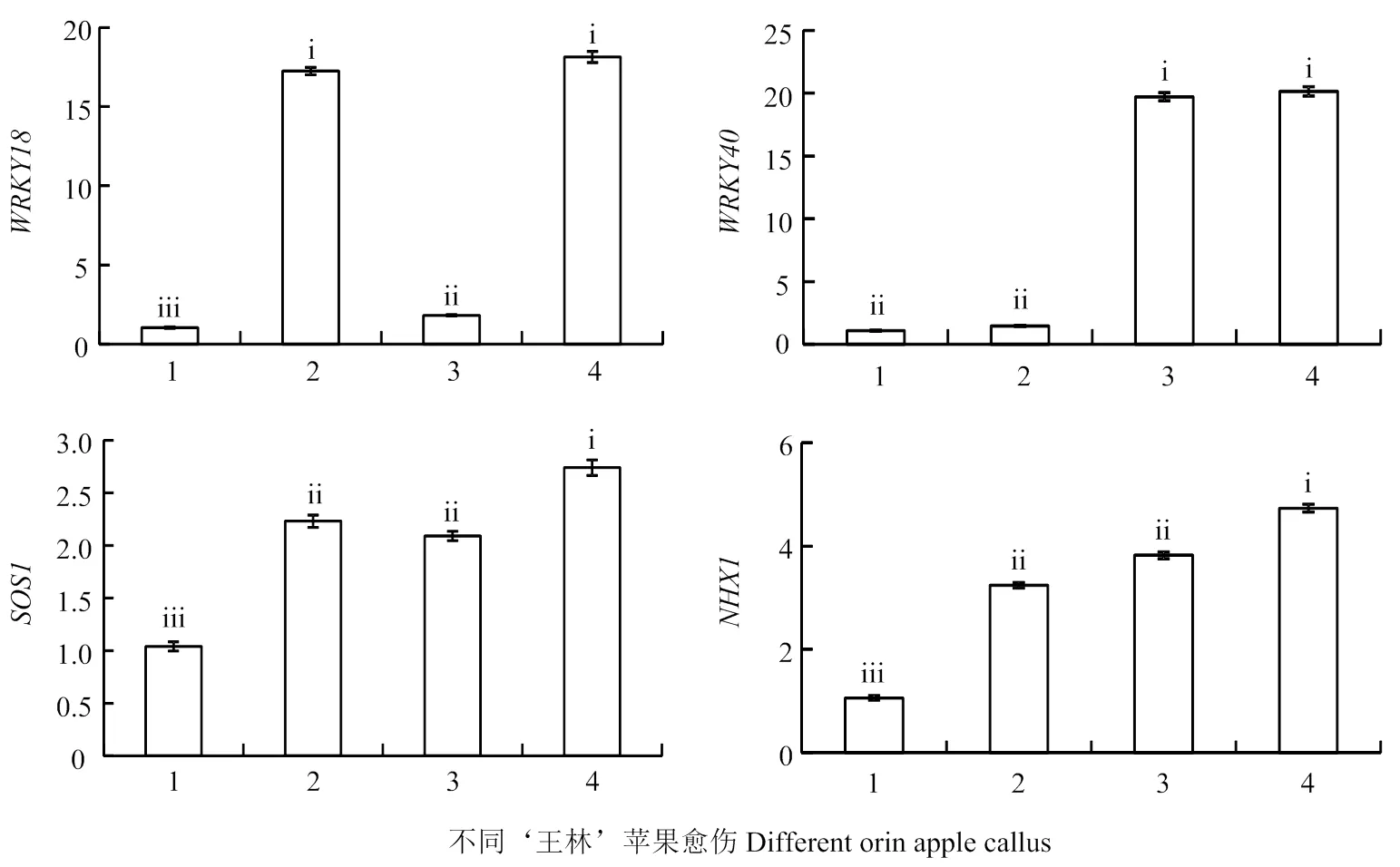
2.5 4种愈伤相关基因表达水平的分析
由图7可得,过表达的愈伤,的表达水平是‘王林’愈伤的16.58倍,但对的表达水平没有影响;过表达的愈伤,的表达水平是‘王林’愈伤的18.08倍,但对的表达水平没有影响;共表达和的愈伤,和的表达水平分别是‘王林’愈伤的17.43倍和18.47倍;与‘王林’愈伤相比,过表达的愈伤,过表达的愈伤以及共表达和的愈伤中和的表达水平均上调表达。
3 讨论
3.1 苹果MdWRKY18和MdWRKY40受盐胁迫诱导并提高‘王林’愈伤的盐耐性
WRKY蛋白是最近发现的一类序列特异性DNA结合转录因子,因其具有高度保守的WRKY结构域而得名[25],在拟南芥中发现了70多个WRKY蛋白,在水稻中发现了100多个WRKY蛋白[24,26]。WRKY蛋白能够调节植物发育与繁殖,酚类化合物生物合成,激素信号传导或衰老有关的多个过程[27-29],然而,WRKY蛋白的基础性作用在于参与植物防御信号传导以及非生物胁迫耐受性[30-31]。盐胁迫是重要的非生物胁迫之一,在大豆中,64个测试的WRKY基因中,有25个响应盐胁迫[32],并且在拟南芥,小麦和水稻等中也已经报道了类似的结果[33-35]。在许多植物物种中进行了WRKY蛋白响应盐胁迫的功能研究,小麦TaWRKY2、TaWRKY19、TaWRKY44和TaWRKY93能够通过增强渗透保护剂(脯氨酸和可溶性糖)积累和改善氧化应激反应来提高对盐胁迫耐受性[35-36],棉花GHWRKY34在适度盐胁迫下能够增强植物的萌发和生长,减少钠和ROS积累[37],拟南芥AtWRKY18、AtWRKY40、AtWRKY25和AtWRKY33也被报道能够响应盐胁迫诱导并且提高盐耐性[38,21]。本研究发现MdWRKY18和MdWRKY40含有1个WRKY、Cx5C及HxH结构域,属于Ⅱa+Ⅱb类WRKY蛋白,150 mmol·L-1NaCl处理下能够增强和的启动子活性,诱导和的表达;在‘王林’愈伤分别过表达和以及共表达和时,均能够增强‘王林’愈伤的耐盐性,并且能够诱导和的表达,而和在维持胞质Na+浓度方面起着重要作用[13,39],因此MdWRKY18和MdWRKY40可能参与调控和的表达来调节‘王林’愈伤的耐盐性。
1:Orin;2:OEWRKY18;3:OEWRKY40;4:OEWRKY18+OEWRKY40
3.2 MdWRKY18和MdWRKY40之间蛋白相互作用
具有调节功能的蛋白质很少单独作用,它们大多以相互作用的形式在生命系统中承担生物学功能,因此,研究蛋白质之间相互作用是理解复杂分子过程的关键步骤。在过去的研究中,发现了大量与信号转录有关的植物WRKY蛋白的相互作用蛋白,WRKY相互作用蛋白在转录中可影响WRKY转录因子与DNA的结合和转录调节活性,从而调控WRKY调节的下游基因表达。在拟南芥中,3种Ⅱ类型的WRKY蛋白(AtWRKY18、AtWRKY40和AtWRKY60)能够通过N-末端的亮氨酸拉链基序彼此相互作用[40],AtWRKY6和AtWRKY42之间也能相互作用[41],此外,通过酵母双杂交系统筛选了13个Ⅲ类型的WRKY蛋白发现,AtWRKY30、AtWRKY53、AtWRKY54和AtWRKY70之间存在显著的相互作用[42]。本研究发现MdWRKY18和MdWRKY40均能够与自身相互作用形成同源二聚体,且MdWRKY18和MdWRKY40也能够互作形成异源二聚体,与在拟南芥中研究结果一致。
4 结论
盐胁迫诱导和表达,且MdWRKY18和MdWRKY40之间能够相互作用形成同源或异源二聚体,在‘王林’愈伤中,过表达和能够促进和的表达,从而提高‘王林’愈伤的耐盐性。
[1] MAHAJAN S, TUTEJA N. Cold, salinity and drought stresses: An overview., 2005, 444: 139-158.
[2] TUTEJA N. Mechanisms of high salinity tolerance in plants., 2007, 428: 419-438.
[3] DONG D, ZHANG L F, HANG W, LIU Z J, ZHANG Z X, ZHENG Y L. Differential expression of miRNAs in response to salt stress in maize roots., 2009, 103: 29-38.
[4] OHTA M, HAYASHI Y, NAKASHIMA A, HAMADA A, TANAKA A, NAKAMURA T, HAYAKAWA T. Introduction of a Na+/H+antiporter gene fromconfers salt tolerance to rice., 2002, 532: 279-282.
[5] NING Z, CHENG S, LIU X, HAO D, DAI M, ZHOU D X, YANG W, YU Z. The R2R3-type MYB gene OsMYB91 has a function in coordinating plant growth and salt stress tolerance in rice., 2015, 236: 146-156.
[6] XUE Z Y, ZHI D Y, XUE G P, ZHANG H, ZHAO Y X, XIA G M. Enhanced salt tolerance of transgenic wheat (L.) expressing a vacuolar Na+/H+antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+., 2004, 167: 849-859.
[7] ÇIÇEK N, HÇ X. Effects of salt stress on some physiological and photosynthetic parameters at three different temperatures in six soya bean (L. Merr.) Cultivars., 2008, 194: 34-46.
[8] HUSAINI A M, ABDIN M Z. Development of transgenic strawberry (×Duch.) plants tolerant to salt stress., 2008, 174:446-455.
[9] RASHEDY A. Response of two grape rootstocks to some salt tolerance treatments under saline water conditions., 2010, 2: 93-106.
[10] XUE H, ZHANG F, ZHANG Z H, FU JF, WANG F, ZHANG B, MA Y. Differences in salt tolerance between diploid and autotetraploid apple seedlings exposed to salt stress., 2015, 190:2430.
[11] SAHI C, SINGH A, BLUMWALD E, GROVER A. Beyond osmolytes and transporters: novel plant salt-stress tolerance-related genes from transcriptional profiling data., 2006, 127: 1-9.
[12] HASEGAWA P M, BRESSAN R A, ZHU J K, BOHNERT H J. Plant cellular and molecular responses to high salinity., 2000, 51:463-499.
[13] BLUMWALD E, POOLE R J. Na/H antiport in isolated tonoplast vesicles from storage tissue of beta vulgaris., 1985, 78:163-167.
[14] DING Z, LI S, AN X, LIU X, QIN H, WANG D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in., 2009, 36:17-29.
[15] ABE H, URAO T, ITO T, SEKI M, SHINOZAKI K, YAMAGUCHISHINOZAKI K.AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling., 2003, 15: 63-78.
[16] YANG O, POPOVA O V, SÜTHOFF U, LÜKING I, DIETZ K J, GOLLDACK D. Thebasic leucine zipper transcription factor AtbZIP24 regulates complex transcriptional networks involved in abiotic stress resistance., 2009, 436: 45-55.
[17] REN X, CHEN Z, LIU Y, ZHANG H, ZHANG M, LIU Q, HONG X, ZHU J K, GONG Z. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in., 2010, 63: 417-429.
[18] JIANG Y, YANG B, DEYHOLOS M K. Functional characterization of thebHLH92 transcription factor in abiotic stress., 2009, 282: 503-516.
[19] WU X, SHIROTO Y, KISHITANI S, ITO Y, TORIYAMA K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter., 2009, 28: 21-30.
[20] JIANG Y J, LIANG G, YU D Q. Activated expression of WRKY57 confers drought tolerance in., 2012, 5: 1375-1388.
[21] JIANG Y, DEYHOLOS M. Functional characterization ofNaCl-inducible WRKY25 and WRKY33 transcription factors in abiotic stresses., 2009, 69: 91-105.
[22] XU H F, WANG N, LIU J X, QU C Z, WANG Y C, JIANG S H, LU N L, WANG D Y, ZHANG Z Y, CHEN X S. The molecular mechanism underlying anthocyanin metabolism in apple using the MdMYB16 and MdbHLH33 genes., 2017, 94: 149-165.
[23] KENNETH J L, THOMAS D S. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CTmethod., 2001, 25: 402-408.
[24] ZHANG Y, WANG L. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants., 2005, 5(1): 1-12.
[25] RUSHTON P J, SOMSSICH I E, RINGLER P, SHEN Q J. WRKY transcription factors., 2010, 15: 247-258.
[26] WU K L, GUO Z J, WANG H H, LI J. The WRKY family of transcription factors in rice andand their origins., 2005, 12: 9-26.
[27] GUO D, ZHANG J, WANG X, HAN X, WEI B, WANG J. The WRKY transcription factor WRKY71/EXB1 controls shoot branching by transcriptionally regulating RAX genes in., 2015, 27: 3112-3127.
[28] SCHLUTTENHOFER C, YUAN L. Regulation of specialized metabolism by WRKY transcription factors., 2015, 167: 295-306.
[29] ZHANG L, GU L, RINGLER P, SMITH S, RUSHTON P J, SHEN Q J. Three WRKY transcription factors additively repress abscisic acid and gibberellins signaling in aleurone cells., 2015, 236: 214-222.
[30] CHEN L, SONG Y, LI S, ZHANG L, ZOU C, YU D. The role of WRKY transcription factors in plant abiotic stresses., 2012, 1819: 120-128.
[31] BANERJEE A, ROYCHOUDHURY A. WRKY proteins: signaling and regulation of expression during abiotic stress responses., 2015, 2015: 3-24.
[32] ZHOU Q Y, TIAN A G, ZOU H F, XIE Z M, LEI G, HUANG J, WANG C M, WANG H W, ZHANG J S, CHEN SY. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenicplants., 2008, 6: 486-503.
[33] JIANG Y, DEYHOLOS M K. Comprehensive transcriptional profiling of NaCl-stressedroots reveals novel classes of responsive genes., 2006, 6: 25.
[34] BERRI S, ABBRUSCATO P, FAIVRE-RAMPANT O, BRASILEIRO A C, FUMASONI I, SATOH K, KIKUCHI S, MIZZI L, MORANDINI P, PÈ M E, PIFFANELLI P. Characterization of WRKY co-regulatory networks in rice and., 2009, 9: 120.
[35] NIU C F, WEI W, ZHOU Q Y, TIAN A G, HAO Y J, ZHANG W K, MA B, LIN Q, ZHANG Z B, ZHANG J S, CHEN S Y. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenicplants., 2012, 35: 1156-1170.
[36] QIN Y, TIAN Y, LIU X. A wheat salinity-induced WRKY transcription factor TaWRKY93 confers multiple abiotic stress tolerance in., 2015, 464: 428-433.
[37] ZHOU L, WANG N N, GONG S Y, LU R, LI Y, LI X B. Overexpression of a cotton () WRKY gene, GhWRKY34, inenhances salt-tolerance of the transgenic plants., 2015, 96: 311-320.
[38] CHEN H, LAI Z B, SHI J W, XIAO Y, CHEN Z X, XU X P. Roles ofWRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress., 2010, 10: 281.
[39] WANG N, QU C Z, WANG Y C, XU H F, JIANG S H, FANG H C, LIU J X, ZHANG Z Y, CHEN X S. MdMYB4 enhances apple callus salt tolerance by increasing MdNHX1 expression levels., 2017, 131: 283-293.
[40] XU X, CHEN C, FAN B, CHEN Z. Physical and functional interactions between pathogen-inducedWRKY18, WRKY40, and WRKY60 transcription factors., 2006, 18: 1310-1326.
[41] CHEN Y F, LI L Q, XU Q, KONG Y H, WANG H, WU W H. The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in., 2009, 21: 3554-3566.
[42] BESSEAU S, LI J, PALVA E T. WRKY54 and WRKY70 cooperate as negative regulators of leaf senescence in., 2012, 63: 2667-2679.
Molecular Mechanism of Apple MdWRKY18 and MdWRKY40 Participating in Salt Stress
XU HaiFeng, YANG GuanXian, ZHANG Jing, ZOU Qi, WANG YiCheng, QU ChangZhi, JIANG ShengHui, WANG Nan, CHEN XueSen
(College of Horticulture Science and Engineering, Shandong Agricultural University/State Key Laboratory of Crop Biology, Tai’an 271018, Shandong)
【Objective】In order to improve the molecular mechanism of salt stress, we studied several aspects of MdWRKY18 and MdWRKY40 in apple WRKY transcription factors, including the protein structure, the expression level and the function in salt stress. 【Method】We cloned theandgenes in ‘Hongcui No.2’ apple and analysed their protein structure. The expression levels ofandwere studied by the qRT-PCR under the salt stress, and their promoter activities were analyzed using the GUS staining. We analyzed the interaction relationship between MdWRKY18 and MdWRKY40 proteins by yeast two-hybrid and verified their function by transgenosis. 【Result】Analysis of protein structure showed that both MdWRKY18 and MdWRKY40 proteins contained a WRKY, Cx5C and HxH structural domains. The expression levels and promoter activities ofandwere induced by the 150 mmol·L-1NaCl. The yeast two-hybrid experiments showed that MdWRKY18 and MdWRKY40 could respectively interact with itself to form homodimers, and MdWRKY18 could also interact with MdWRKY40 to form heterodimers. Whenandwas overexpressed respectively in orin callus, they could increase the callus weight under salt stress and promote the expression ofand. Whenandwere co-overexpressed in orin callus, it could also promote the expression ofand, however, the weight of callus was heavier than the weight of callus overexpressingor. 【Conclusion】andwere induced by the salt stress, and they could form homodimers or heterodimers, overexpressingor
apple; WRKY transcription factor; salt stress; GUS staining; yeast two-hybrid
10.3864/j.issn.0578-1752.2018.23.010
2018-05-25;
2018-07-26
国家自然科学基金(31572091,31730080)、国家重点研发计划(SQ2016YFSF030011)
许海峰,E-mail:997524744@qq.com。
陈学森,E-mail:chenxs@sdau.edu.cn
(责任编辑 李莉)
