In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines
2018-11-14SalyGhedaMostafaElSheekhAlaaAbouZeid
Saly Gheda, Mostafa El-Sheekh, Alaa Abou-Zeid
Botany Department, Faculty of Science, Tanta University, Tanta, Egypt
Keywords:Marine algae Antioxidant activity Cytotoxicity Apoptosis Lactate dehydrogenase Polymerase chain reaction
ABSTRACT Objective: To evaluate the potential role of the polysaccharides of the marine algae as an anticancer agent in vitro against colon cancer cell line (CoCa2) and breast cancer (MCF7) cell lines and to measure lactate dehydrogenase enzyme (LDH) activity as biomarker of membrane integrity of the cells. Methods: The cells of breast cancer (MCF7) and colon cancer (CoCa2)were used to evaluate the potential anticancer role of the polysaccharides of marine algae. Antiproliferative activity against MCF7 and CoCa2 cell lines were evaluated in vitro by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Results: The in vitro assay of the antioxidant activity of eight marine seaweed species showed that the red seaweed Jania rubens (J. rubens) had the highest DPPH (2,2 diphenyl-1-picrylhydrazyl) free radical scavenging activity. The extracted polysaccharides with concentrations 0.1-40.0 mg/mL from J. rubens were tested for its anticancer potentiality and cytotoxic effects against the cell lines of human breast (MCF7) and colon cancer (CoCa2) cell lines by MTT assay. The inhibitory concentration at 50 (IC50) value the of J. rubens polysaccharide extract was 0.312 5 mg/mL for MCF7 and 20 mg/mL for CoCa2. LDH activity and annexin Ⅴ concentration were higher in the treated MCF7 and CaCo2 cells than in the untreated ones. Quantitative polymerase chain reaction technique indicated that the polysaccharide treatments caused up-regulation of Bax, caspase 8 and P53 genes expression in CoCa2 cells, and up-regulation of caspase 3 and down-regulation of Bcl2 genes expression in MCF7 cells. Conclusions: The polysaccharides of the red marine alga J. rubens could be a potential candidate for the natural compounds as antioxidant and anticancer therapy.
1. Introduction
Cancer causes the highest mortality around worldwide,particularly in undeveloped countries. Chemotherapy drugs are still standard cancer treatments in spite its toxicity against normal cells and tissues. In contrast, natural products for cancer treatments have relatively few side effects on normal cells and tissues[1] and have become an essential goal in many studies of immunopharmacology[2].
Marine macroalgae (seaweeds) have significant contents of bioactive compounds which work as anti-inflammatory,antimicrobial, antiviral and antitumor drugs[3-5]. In vitro, the antioxidant activities of the polysaccharides substances extracted from seaweeds inhibit the proliferation of cancer cells[6,7]. In vivo,the action of polysaccharide substances of seaweeds decrease tumor growth[8]. The seaweed polysaccharides decrease tumor growth through restraining the expansion of cancer cells by apoptosis.This occurs through the stimulation of cytotoxic cytokines on gene expression, by stopping the development of tumors essential to the blood supply or by cellular differentiation[8,9].
The sulfated polysaccharides, fucoidan of the brown seaweeds[10],carrageenan and agar from red seaweeds[11,12] have better antitumor activities if compared with other polysaccharide types because of their antioxidant and immunostimulating activity[13,14]. However,the biological activity of polysaccharides depends mainly on molecular weight, monosaccharide composition, sulfate content,and composition of the main polymers[15]. Since the structure of polysaccharide is heterogeneous, many investigations of biological activities were done utilizing moderately unrefined polysaccharide preparations.
Zandi et al. revealed that the red marine alga Gracilaria corticata has anticancer activity against human leukemic cell lines[16].Additionally, Namvar et al. reasoned that red kelp Gracilaria corticata is a good resource of likely integral and option useful substances for the treatment of breast tumor[17].
Herein, we aimed to evaluate the antioxidant activity of polysaccharide extracted from eight species of seaweeds [Ulva lactuca, Padina pavonica, Sargassum vulgare, Petrocladia capillacea,Jania rubens (J. rubens), Corollina elongate, Cystoceira compressa and Sargassum latifolium]. The seaweed polysaccharide with the highest antioxidant activity will be tested as an anticancer agent in vitro against colon cancer cell line (CoCa2) and breast cancer (MCF7) cell lines. Lactate dehydrogenase (LDH) enzyme activity as a biomarker of membrane integrity of the cells and the expression of some genes level as a biomarker for apoptosis will be measured.
2. Materials and methods
2.1. Collection and identification of seaweeds
Seaweed sampling was carried out in June 2013, from Abo-Qir bay Alexandria [Ulva lactuca (Chlorophyceae), Padina pavonica, Sargassum vulgare (Pheaophyceae), Petrocladia capillacea, J. rubens and Corollina elongate (Rhodophyceae)] and from Ras Sadr, Sinai [Cystoceira compressa and Sargassum latifolium (Pheaophyceae)]. The marine macroalgae after being collected were kept in plastic bags containning sweater and transferred immediately to the laboratory to prevent evaporation. Seaweeds were washed twice with fresh water to remove epiphytes, debris and surface salts. A portion of the seaweed samples were prepared as herbarium example gathering, for taxonomical distinguishing proof and the other part was air-dried in the unmindful at room temperature (25-30 ℃) on absorbent paper. The dried examples were crushed to fine powder and stock up at -20 ℃ until utilize.Taxonomic identification of the collected seaweeds was carried out according to other studies[18-22].
2.2. Extraction of polysaccharides from seaweeds
Polysaccharides were extracted from the seaweeds of study,according to methods described by Sudo et al[23]. The seaweed samples were boiled with water for 1 h and then centrifuged at 4 000 r/min for 20 min. The clear supernatant containing polysaccharides was collected. The extracellular cell wall polysaccharides were precipitated by adding double volumes of cold absolute ethanol to a known volume of the clear supernatant under continuous stirring,and the solution was allowed standing overnight at 4 ℃. After centrifugation at 6 000 r/min for 10 min, the white material of crude polysaccharide (EPS) was collected. It was then washed out by dialysis against cool distilled water for 48 h, changed twice day by day. At that point after, the dialyzed samples were then recouped by lyophilization or dried at 37 ℃ with a specific end goal to get a fibrous powder of the separated polysaccharides.
2.3. Cell lines and culture
Cells of breast cancer (MCF7) and colon cancer (CoCa2) were purchased for the Medical Technology Center-Alexandria University.The cells were suspended in a medium containing 10% fetal bovine serum (RPMI 1640) and incubated at 37 ℃ in atmospheric 5% CO2.
2.4. Measurement of seaweed antioxidant activity
The antioxidant activities of the seaweeds were assayed according to the methods adopted by Blois[24]. In this method, the seaweed extracts were dissolved in methanol, where the metabolic 2,2 diphenyl-1- picryl hydrazyl (DPPH) was used as a control. A mixture of 5 mL DPPH solution (10, 20, and 40 mg/mL) was used,and the absorbance was measured spectrophotometrically at 517 nm after 30 min. The free radical scavenging activities of the seaweed extracts were indicated by the degree of decolorization of DPPH.The decolorization % was calculated using the following equation:
Free radical scavenging % = (Ac-As)/Ac× 100.
Ac= Absorbance of control and As= Absorbance of the sample.
Ascorbic acid was used as a reference free radical scavenger.
2.5. In vitro anticancer activity
2.5.1. Cell viability (cytotoxicity) assay
Anti-proliferative activity against MCF7 and CoCa2 cell lines were evaluated in vitro by the methods reported by Chen et al[25]. The MCF7 and CoCa2 cells (5×104cells/mL) were incubated at 37 ℃ in 96-well plates containing 0.1 mL of the culture medium in each well.This took place in a humidified atmosphere with 5% CO2. The cells were incubated for 24 h to allow them to adhere, and then 100 μL of the different concentrations of polysaccharides in medium (0.1-40.0 mg/mL) were added to each well. After 24 h, this medium was washed down with 0.1 mL of phosphate saline buffer and replaced with fresh medium. Then after, the cells in each well were moved in culture medium with 0.02 mL of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) solution (5 mg/mL) for 4 h. After the media were removed, 0.15 mL of DMSO was added to each well. Absorbance at 490 nm was determined by a BIORAD Micro-plate ELISA Reader.
The viability percent was calculated according to the formula below:
Viability percent = (1-Absorbance of experimental group/Absorbance of blank control group) × 100%.
2.5.2. Apoptosis assay
Measurement of apoptosis of colon and breast cancer cell lines was performed using human annexinⅤ-Platinum ELISA kit(eBioScience). The mixture was incubated to allow streptavidin horseradish peroxidase to bind to the biotin-conjugated antihuman annexin Ⅴ antibody. After incubation, the unbound streptavidin horseradish peroxidase was removed by washing and the substrate solution reactive with horseradish peroxidase was added to the wells. As a result of the reaction, the colored product was created in ratio to the amount of human annexin v in the sample or standard.The human annexin Ⅴ concentration was detected by measuring the absorbance of the reaction product at 450 nm. Different concentrations of human annexin Ⅴ and human annexin Ⅴ samples were used for the preparation of the standard curve.
2.6. Assessment of membrane integrity by measuring LDH activity
The integrity of the cell membrane of colon and breast cells treated with seaweed polysaccharide was assessed by determining the activity of LDH leaking out of the cell[26]. LDH activity in the cell medium was determined using LDH kit (Biovision, UK)after the cell was treated. LDH quantification assay depends on the measurement of the colour produced from reduction of NAD to NADH by LDH at 450 nm.
2.7. Polymerase chain reaction (PCR)
Real time PCR was completed with the sybr Green Supermix RT-PCR kit using the real-time PCR identification framework,following the instructions of the manufacturer (Bio-Rad, Hercules,USA). RT-PCR with sybr green was used to measure the expression of mRNAs of target genes in the treated MCF7 and CoCa2 cells,with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal reference. RT-PCR was performed using the kit Sybr Green Supermix RT-PCR kit (Bio-Rad), according to instruction manual.RT-PCR reaction started with initial denaturation at 95 ℃ for 10 min and continued with 40 cycles of 15 s at 95 ℃, annealing at 60 ℃ for 30 s and extension at 72 ℃ for 30 s using the specific primers for each gene (Table 1).
The acquired results were analyzed using the following relative gene expression formula[27]:
Relative gene expression (Delta CT) = CT(Target gene in treated cell line) - CT(reference in treated cell line)
Ct(cycle threshold) indicates the partial cycle number at which the measure of intensified target achieves a settled limit[28].
2.8. Identification of J. rubens polysaccharides
The polysaccharides extracted from J. rubens were identified by Vibrational Spectroscopy Fourier Transform Infrared (FTIRATR) at the Institute of Marine Research, University of Coimbra,Portugal. The polysaccharide samples were prepared by drying at room temperature, followed by grounding. The FTIR spectra obtained on an IFS 55 spectrometer, using a Golden Gate single reflection diamond ATR framework, with no requirement for sample preparation[29]. All spectra are the middling of two counts, with 128 scans each and a determination of 2/cm.
2.9. Statistical analysis
The results are expressed as mean ± standard deviation. The oneway ANOVA method was applied to analyze the statistical variance between concentrations of seaweed polysaccharide and those of annexin v. Significant differences between means, among the control and the treated seaweed polysaccharide concentrations, were identified using the least significant difference (LSD) test at P<0.05.The statistical analyses were done by using SPSS 15.0 software SPSS[30].
3. Results
3.1. Antioxidant potential of marine algae polysaccharide
The antioxidant activities of the EPS extracted from eight species of seaweeds are shown in Table 2. The radical scavenging ability of unlike concentrations of EPS extract (10, 20 and 40 mg/mL)highly contributes to the antioxidative effect. At concentrations 10,20 and 40 mg/mL of polysaccharide, the antioxidant activity of EPS increased with the increasing concentration, respectively. The EPS extracted from the red seaweed (Petrocladia capillacea) had the lowest antioxidant activity, while those extracted from the red seaweed J. rubens have the highest antioxidant activity.

Table 1 Forward and reverse primers sequence for candidate genes.

Table 2 Antioxidant activities of polysaccharide extracted from marine seaweeds using DPPH radical scavenging assay(%).
3.2. Anticancer activity of J. rubens polysaccharides
J. rubens polysaccharide was selected to test the anticancer activity on MCF7 and CoCa2 cell lines because of its high antioxidant activity.The breast and colon cancer cell lines were cultured for 24 h in seaweed polysaccharide extracts of concentrations of (0.1-40 mg/mL)and analysed by MTT assay to test the cytotoxicity of polysaccharide extracted from seaweeds on selected cancer cell lines (Figure 1).
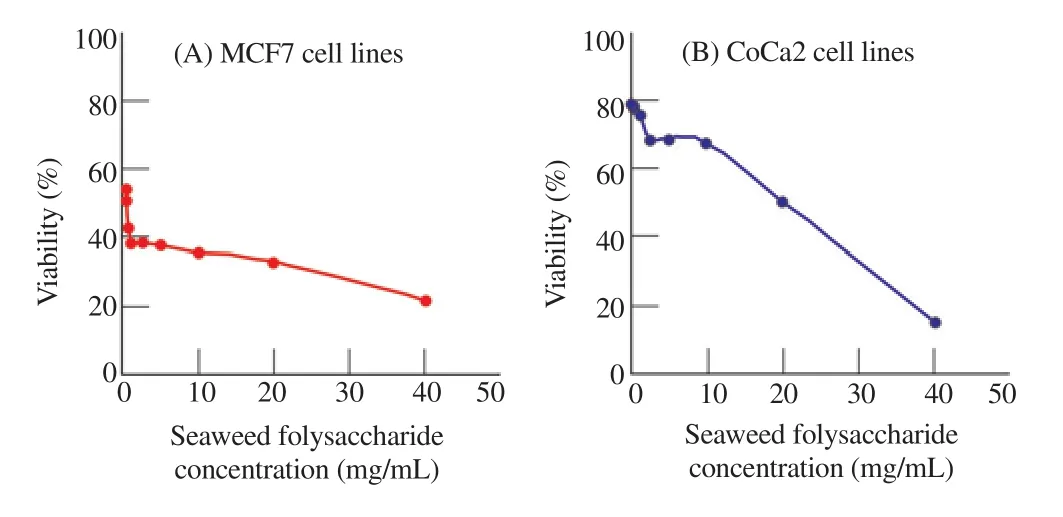
Figure 1. Cytotoxicity of J. rubens crude polysaccharides (EPS) against MCF7 and CoCa2 cells using MTT assay.
In this work, the polysaccharide extract of J. rubens showed a significant number of cell death of MCF7 and CaCo2 cells. The percentage of viable cells calculated using the formula based on which the inhibitory concentration at 50 (IC50) value of seaweed polysaccharide extract was 0.312 5 mg/mL for MCF7 cell lines and 20 mg/mL for CoCa2 cell lines (Figure 1).
3.2.1. Apoptotic assay
Generally, in the studied MCF7 and CoCa2 cell lines, annexinⅤconcentrations increased when being treated with seaweed polysaccharide when compared with the untreated ones. In addition,increases in annexin Ⅴ concentration were directly proportional to the concentration of polysaccharide. In case of MCF7, annexin Ⅴconcentration increased by 1.3 and 1.7 folds in the cells treated with 10 and 20 mg/mL seaweed polysaccharide respectively as compared with the untreated MCF7 cells. On the other hand, in case of CoCa2,annexin v concentration increased by 1.8 and 2.0 folds with cells treated with 10 and 20 mg/mL seaweed polysaccharide respectively as compared with the untreated CoCa2 cells (Table 3). AnnexinⅤ concentrations showed high significance with the two seaweed polysaccharide concentrations at (P<0.001).

Table 3 Apoptosis activity of J. rubens crude polysaccharides (EPS) against MCF7 and CoCa2 cells using annexin Ⅴ ELISA kit.
3.2.2. LDH enzyme
The release of LDH which is a soluble cytoplasmic enzyme was measured in the polysaccharide treated MCF7 and CoCa2 cells to estimate the damage of cell membrane and loss of integrity. In MCF7, LDH activity increased by 1.9 and 2.6 folds in the cells treated with 10 and 20 mg/mL seaweed polysaccharide respectively as compared with the untreated MCF7 cells. However, in case of CoCa2, LDH activity increased by 2.3 and 2.9 folds in the cells treated with 10 and 20 mg/mL seaweed polysaccharide respectively as compared with the untreated CoCa2 cells (Figure 2).
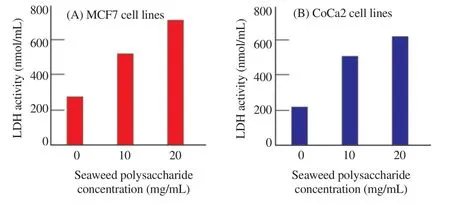
Figure 2. Lactate dehydrogenase (LDH) activity in MCF7 and CoCa2 cells treated with J. rubens crude polysaccharides (EPS).
3.3. Relative expression genes in the treated cells
As the death of treated cells is thought to be related to the gene products, total RNA isolated from both CaCa2 and MCF7 cells treated with polysaccharides of seaweeds were investigated using the real-time PCR. The quality and concentration of the extracted RNA were assessed by Nanodrop.
The results revealed up-regulation in the expression level of the apoptotic gene, Bax, Caspase 8 and P53 genes in CoCa2 cells at all treatments of the J. rubens polysaccharide. However, the regulation level differed between the investigated genes. The highest significant up-regulation was observed in P53 gene in the CoCa2 cells treated with 20 mg/g J. rubens polysaccharide, while the lowest significant up-regulation was observed in caspase 8 gene in the CoCa2 cells treated with 10 mg/g J. rubens polysaccharide (Figure 3).

Figure 3. Effect of of J. rubens crude polysaccharides (EPS) on the relative expression of apoptosis related genes in CoCa2 cells.
In contrast, in the treated MCF7 cells, the Bcl2 anti-apoptotic gene recorded a down-regulation in its expression level. However,the caspase 3 gene recorded up-regulation of expression at the two treatments of study (Figure 4).
The up-regulation of Caspase 3 gene expression was higher in MCF7 cells treated with 20 mg/mL seaweed polysaccharides than in MCF7 cells treated with 10 mg/mL seaweed polysaccharides(Figure 4).

Figure 4. Effect of of J. rubens crude polysaccharides (EPS) on the relative expression of apoptosis related genes in MCF7 cells.
3.4. FTIR spectra of polysaccharides in J. rubens
The FTIR analysis of the studied polysaccharides in J. rubens shows that the agar was the main component of this polysaccharide(absorbance band at 713/cm). However, some functional groups like C-O-SO4 were recorded on C6 of galactose at absorbance band of 870/cm. Band of 1 035/cm showed symmetric C-O vibration associated with C-O-SO3, while band of 1 082/cm showed skeleton of galactans sulphate ester band at 1 153 and 1 395/cm.
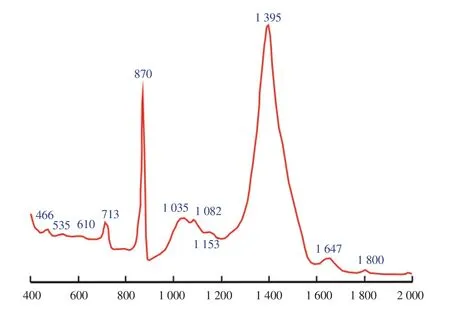
Figure 5. Vibrational Spectroscopy Fourier Transform Infrared (FTIR-ATR)spectra of J. rubens polysaccharides.
4. Discussion
The DPPH free radical is a steady free radical, which has been generally established as a means for estimation the free radicalscavenging activities of antioxidants[31]. The results of antioxidant activities of seaweeds polysaccharide obtained in the current work are consistent with those reported in the literature. Jimenez-Escrig et al. evaluate the antioxidant activity of alcoholic and aqueous extracts of seaweeds by DPPH assay and other assays[32]. Several studies have investigated the anti-proliferative activity of crude seaweed extracts in cancer cell lines, and most of these studies focused on their antioxidant activity. Water-soluble polysaccharides, such as laminarans, fucoidans, carragenans, and agar, are representative anticancer substances extracted from seaweeds[33]. However, the oxidative mechanisms and structure of seaweed polysaccharides have not been elucidated[34]. These results of anticancer activities of the polysaccharides of J. rubens are consistent with those obtained by Anastyuk et al.[35] who found selective cytotoxic activity in the methanolic extract of the red alga Amphiroa zonata. Several cytotoxic compounds such as agar and carrageanans extracted from marine red algae possess anticancer and anti-proliferative properties. Hence,many bioactive compounds extracted from marine algae have been reported against various cell lines[4]. The increase of annexin v concentration in treated cells refers to the apoptosis effect of the polysaccharide seaweed. This apoptosis effect causing the cell death is tightly regulated by a number of gene products that either promote or block the cell death at different stages of the cell cycle. However,apoptosis can be triggered also by extrinsic stimuli, such as death ligand-receptor engagement. The enhancement of cysteine-dependent aspartate-directed proteases (caspases) and nucleases as a result of intrinsic and extrinsic signals causes the destruction of the cell. It causes cell shrinkage, cytoplasmic and nuclear fragmentation, and collapse of mitochondrial potential[36,37]. The higher LDH activity levels in the treated MCF7 and CoCa2 cells were observed due to the damage of the plasma membrane. Therefore, LDH released into the extracellular space[38]. However, Jovanovic et al. showed that LDH release from the cell membrane might occur during oxidative stress or cell damage[39].
The significant increases in genes of caspase 8 and 3 in the treated cells of CoCa2 and MCF7 are consistent with the conclusions of Chang et al[40]. Chang et al. estimated that caspase-3 is an efficient death protease catalyzing the breakdown of cellular protein[40].
Waxman and Schwartz showed that caspase 8 and caspase 9 act as initiators while caspase 3 acts as an effector[41]. The initiator activates through two pathways. The first is mediated through caspase 8 involving binding of cell death ligands[42]. This subsequently causes activation of caspase 8 and caspase 3. The second pathway includes mitochondria-mediated apoptosis through caspase-9[43,44].
Another possible mechanism for the effect of polysaccharides on the cancer cells, inducing apoptosis might occur due to activation of apoptotic proteins such as gene Bax. The gene Bax (pro-apoptotic gene) promotes cell death at different stages of the cell cycle[45]. Ma et al. reported that cell apoptosis counts on the harmony between the extent of the pro-apoptotic proteins like Bax and anti-apoptotic proteins like Bcl2[46]. The pro-apoptotic proteins of Bax caused the permeabilization of the mitochondrial outer membrane, enhancing the release of pro-apoptotic molecules into the cytoplasm, This, in turn, activated the caspase cascade gene[45-48]. The mechanism by which Bcl2 family regulates apoptosis is through their effect on the permeability of the mitochondrial outer membrane[49].
The mechanism of polysaccharide pro-apoptotic activities in the treated CoCa2 and MCF7 cells was discussed based on measuring mRNA expression levels in the recorded genes using RT-PCR. It was found that anti-apoptotic Bcl2 expression level was significantly decreased, while the expression levels of caspase 3 in MCF7 cells and Bax, P53 and caspase 8 in CoCa2 cells were up-regulated. This agrees with the study of Zhang et al. who showed that the β-glucan extracted from Pleurotus tuberregium caused decrease of the antiapoptotic Bcl2 and increase in pro-apoptotic Bax in MCF-7 cells [50].
The agar and galactans polysaccharide in red seaweeds have been reported to have antioxidant activity[51] and effective antitumor activity by attacking the cancer cell directly[13] or enhancing the host immune function[52]. This finding, which agrees with many studies,suggests that the dynamic substances cooperate with particular cancer-related receptors or cancer cell special molecule, thus triggering some mechanisms that cause cancer death[34]. However,the role of seaweed polysaccharide structure in antioxidative mechanisms has not been elucidated. Therefore, it is necessary to explore new seaweed polysaccharides with antioxidant and anticancer activities and also to elucidate their mode of action[8].Many studies reported the red seaweeds can be considered as a promising anticancer agent[16,17].
5. Conclusion
The marine red seaweed J. rubens had the highest antioxidant activity among the other species of tested seaweeds. The effect of J. rubens polysaccharide had cytotoxic effects on MCF7 and CoCa2 cell lines. Annexin Ⅴ concentrations and LDH activity were increased in the polysaccharide treated MCF7 and CoCa2 cells as compared with the untreated ones.
Expression of genes was measured as a biomarker for apoptosis.All genes (Bax, caspase 3, caspase 8, and P53) except Bcl2 gene were up-regulated in the MCF7 and CoCa2 cells treated with J. rubens polysaccharide. It could be concluded that polysaccharide extracted from J. rubens was effective as anticancer agents. Further studies could be done to explore new compounds from seaweeds to develop alternative therapeutic drugs.
Conflicts of interest statement
The authors declare that they have no conflict of interests.
Acknowledgments
The authors would like to thank Prof. Leonel Pereira, Department of Life Sciences, Institute of Marine Research, University of Coimbra,Portugal for his help in identification of the algal polysaccharides by FTIR technique.
Foundation project
The study was funded by Research Project Funds from Tanta University (Tu-03-13-04).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Acute kidney injury in leptospirosis: Overview and perspectives
- In vitro antiproliferative and apoptosis inducing effect of a methanolic extract of Azadirachta indica oil on selected cancerous and noncancerous cell lines
- Efficacy of voriconazole on leishmaniasis by Leishmania major: An in vitro and in vivo study
- Anti-schistosomal activities of Echinops kebericho Mesfin root and Hagenia abyssinica (Bruce) J.F Gmel flower part crude extracts in Swiss albino mice
- Calcium carbonate supplementation causes memory impairment in mice
- Investigation of cryptic diversity and occurrence of echinostome metacercariae infection in Anentome helena (von dem Busch, 1847)
