Calcium carbonate supplementation causes memory impairment in mice
2018-11-14YasushiHasegawaTatsurouInoueTatsuyaFuji
Yasushi Hasegawa, Tatsurou Inoue, Tatsuya Fuji
College of Environmental Technology, Muroran Institute of Technology, Japan
Keywords:Calcium carbonate Memory impairment CREB
ABSTRACT Objective: To investigate the influence of calcium carbonate supplementation on cognitive function in mice. Methods: Mice were fed diets containing 1.0% calcium carbonate for 8 weeks, following which they were evaluated for memory function using object recognition,Y-maze, and Barnes maze tests. Next, the expression levels of cAMP response element binding protein (CREB) and phosphorylated CREB, which is involved in the memory process were investigated in both the hippocampus and cerebral cortex using western blotting methods.Results: Mice fed on a diet containing calcium carbonate showed memory impairments in object recognition, Y-maze, and Barnes maze tests with respect to the mice that were on a control diet. Further, mice that were fed a diet containing calcium carbonate and a nimodipine(an L-type calcium channel antagonist), reversed calcium carbonate-induced memory impairments, thus suggesting that excessive entry of calcium in cells may cause memory impairments. A study using western blot revealed that expression of CREB and phosphorylated CREB in hippocampus and cerebral cortex was significantly lower in the calcium carbonatefed mice than in the control-diet-fed mice. Conclusions: These results suggest that a calcium carbonate diet may cause memory impairment by decreasing CREB expression. This is the first report of calcium carbonate supplementation causing memory impairment. This simple animal model may be useful as a novel cognitive impairment model for drug development.
1. Introduction
Calcium is a secondary messenger that regulates various cellular mechanisms and plays a key role in synaptic plasticity and memory formation[1-4]. Although calcium is necessary for neuronal functioning, a marked rise in intracellular calcium causes neuronal cell death and synaptic dysfunction[5,6]. Dysregulation of calcium homeostasis in the brain has been suggested to lead to deficits in cognitive functions. An increase in intracellular calcium can occur through the influx of calcium from glutamate receptors or various voltage-dependent calcium channels, or its release from intracellular calcium stores. An increased intracellular calcium is buffered by binding to calcium-binding proteins in the cytosol,extrusion through calcium pumps, or uptake into the endoplasmic reticulum or mitochondria. However, aging has been reported to cause alternations in the expression and function of many proteins involved in calcium signaling. For example, aged neurons show a decline in the function of glutamate receptors, an increase of L-type calcium channel expression, and a decrease in the expression of cytosolic calcium-binding proteins such as calmodulin and parvalbumin[7-9]. Disturbances in calcium homeostasis in the brain have also been reported in Alzheimer’s disease[10,11].
Hanahisa and Yamaguchi[12] reported that the administration of calcium (500 mg/kg) significantly increased the serum calcium concentration and brain calcium content. They suggested that calcium supplementation may influence neuronal function in brain.
There are several studies about association of calcium intake and memory. Whiting and Wood have demonstrated that there is an inverse relationship between serum calcium concentration and cognitive functioning[13,14]. Kim et al showed that women who used calcium supplements with a prior history of stroke were seven times more likely to develop dementia than women who did not take any calcium supplements[15]. Moreover, the Rotterdam study demonstrated that individuals aged 75 and older with higher serum calcium show worse and a faster decline of cognitive function.Conversely, the Hisayama study[16] showed that higher dietary supplementations of calcium, magnesium, and potassium reduced the risk of dementia in a Japanese population. There are currently no studies that focused on the effect of calcium supplementation on memory in rodents. As it remains unclear whether calcium supplementation affects cognitive function, in the present study, we investigated the effect of calcium carbonate supplementation on memory in mice.
2. Materials and methods
2.1. Experimental mice and diets
Male or female ICR mice (that were 6 weeks old) were purchased from CLEA (Tokyo, Japan). The mice were randomly assigned to three groups of six mice each and fed either a high-fat diet or normal diet containing different amounts of mineral mixture and calcium carbonate, for 8 weeks. The diet composition is shown in Table 1.Calcium carbonate is widely used as a food additive and was the focus of investigation in this study. The mice were given a fixed amount of food (4 g/day) with free access to water. The mice were weighed every week and their serum calcium concentrations were measured using a Calcium Assay kit (Cayman chemical) after 9 weeks. All mice were monitored by daily health checks. At the end of the experiment, mice were euthanized under isoflurane anesthesia;brains were quickly excised, and hippocampus and cerebral cortex were isolated and stored at 80 ℃. Animal experiments were performed in accordance with the Muroran Institute of Technology Guidelines. These experiments were approved by the Animal Care and Use Committee of Muroran Institute of Technology.
2.2. The Y-maze test
The Y-maze test was performed according to the method described previously[17]. Briefly, the apparatus was a Y-shaped three arm maze with equal angles between the arms (30 cm long and 20 cm high).The test began by placing a mouse in the center of a Y-maze; they were then allowed to explore for 10 min while the sequence and number of arm entries were recorded. Spontaneous alternation was defined as a consecutive entry into different arms. The alternation was calculated as “number of alternations” divided by “total arm entries minus 2.” Locomotor activity was estimated by number of arm entries.
2.3. The Barnes maze test
Barnes maze test was performed according to the method described previously[17]. The apparatus was a circular platform (1.15 m in diameter) with 12 equally spaced holes (10 cm in diameter) around the edges. A darkened escape hole was maintained under one of the holes. This escape hole was placed in a constant position throughout the session.
Test began by placing a mouse in the center of the platform and allowing it to search for the darkened escape hole for 120 s during the training trials. If a mouse did not reach the darkened escape hole within 120 s, it was gently guided to the escape hole. Each mouse was trained once a day for 4 days. After four training trials, a probe test was performed. The probe test was performed by removing the escape hole and allowing the mouse to explore for 60 s. The time taken to search for the area surrounding the darkened escape hole(within 20 cm of the escape hole) was evaluated.
2.4. The novel object recognition test
The test was performed according to a method described previously[18] in an open field box with a diameter and height of 32 and 38 cm, respectively. Two objects with different shapes and colors (9 cm × 5 cm × 9 cm) were placed in the box, 30 cm apart.At a training session, each mouse was allowed to explore the objectsfor 5 min and mice were returned to a cage. After 24 h, a novel object replaced one of the objects used in the training session and exploration time for the novel object was then recorded for 5 min(test session). The exploration time was defined as mice sniffing,biting, or facing the object. Time spent exploring any one of the two objects in test session or the novel object in training session was measured and expressed as the relative exploration time.
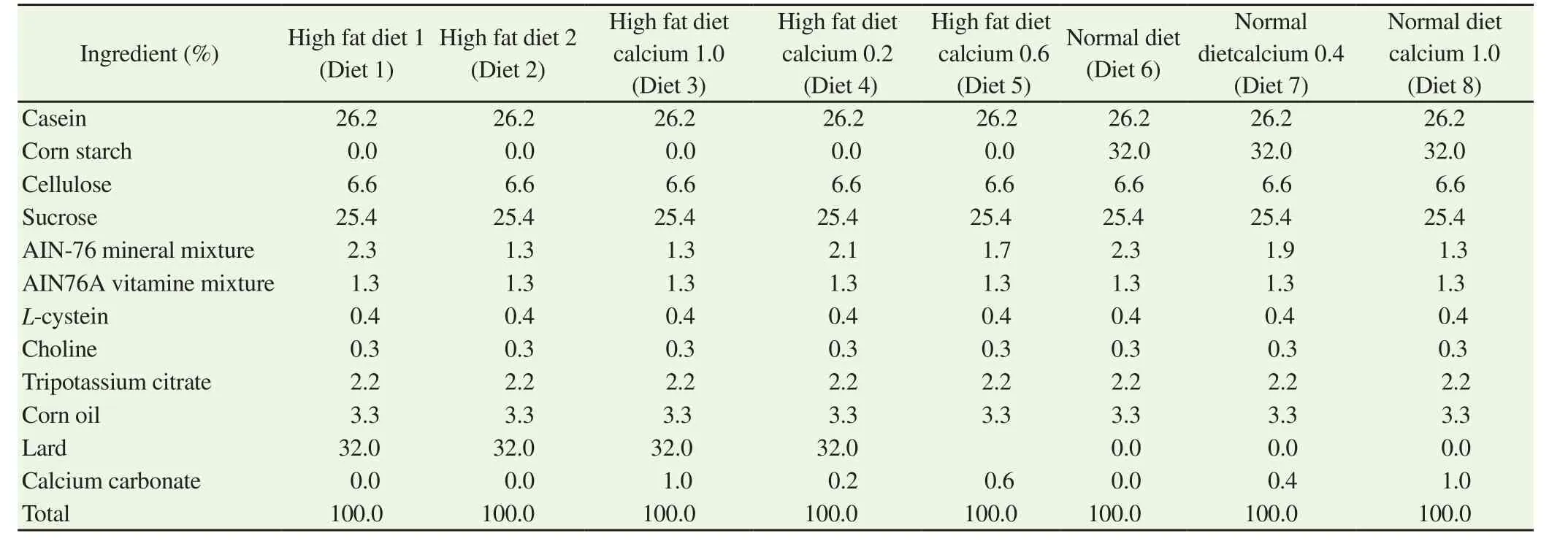
Table 1 Diet composition of the eight tested experimental diets offered to ICR mice.
2.5. Social interaction test
The test was performed in an open field box with a diameter of 32 cm and height of 38 cm. Mice were separately fed with either the calcium carbonate diet or control diet. A pair of mice, one fed with calcium carbonate and the other with control diet, was placed into the box.The time of active contact with the other (e.g., sniffing, following, and grooming the partner) over 10 min was measured.
2.6. Semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated using an RNAiso Plus kit (Takara, Shiga,Japan) from the hippocampus and cerebral cortex at the end of each experiment. After reverse transcription, PCR was performed using specific forward and reverse primers of actin, potassium inwardly rectifying channel, CCAAT-enhancer-binding protein homologous protein (CHOP), and neuropeptide Y2 receptor. The primer sequences forβ-actin were: forward, 5′- GGCTGTATTCCCCTCCATCG -3′,and reverse, 5′- CCAGTTGGTAACAATGCCATGT -3′; for CHOP,forward, 5′- TCACTACTCTTGACCCTGCG -3′, and reverse, 5′-ACTGACCACTCTGTTTCCGT -3′; for neuropeptide Y2 receptor forward, 5′- CGCCATCGTTGCATTGTCTA -3′, and reverse, 5′-AGGGTGGAAAGGCTGTAGAC -3′; for potassium inwardly rectifying channel, forward, 5′- AGGGCAGTGACTCAGAGAAC-3′, and reverse, 5′- TGCAGTCTGGTTTGTAAGGC -3′. The intensities of the bands of the PCR products were quantitated using ImageJ software and normalized with respect toβ-actin. The PCR cycles were selected on the basis of the relationship between the number of cycles and amount of PCR product.
2.7. Western blotting
The hippocampus and cerebral cortex were excised at the end of each experiment and were homogenized in a solution containing 0.2% SDS, 20 mM Tris-HCl (pH 7.5) and bromophenol blue. After SDS polyacrylamide gel electrophoresis[19], the proteins were electrotransferred onto a polyvinylidene difluoride (PVDF) membrane.After blocking the membrane with 5% skim milk (w/v) in a solution containing 0.5 M NaCl, 20 mM Tris-HCl (pH 7.5), and 0.05%Tween 20 (solution A) for 2-6 h at room temperature, antibodies against β-actin, p-CREB, or CREB were incubated overnight.After washing with solution A, the membrane was treated with an alkaline phosphatase-conjugated secondary antibody for 2 h and visualized with 5-bromo-4-chloro-3-indoyl phosphate and nitroblue tetrazolium. The band intensities were estimated using Image J.
2.8. Histochemistry
The cerebrum of brain at the end of experiments were quickly excised and fixed in 4% neural buffered formaldehyde. The tissues were embedded with paraffin, sectioned, and stained using Nissl staining.
2.9. Statistical analyses
Data were indicated as the mean and standard deviation (SD).Student’s t-test or one-way analysis of variance (ANOVA)followed by Tukey’s multiple-comparison test was used to estimate significance of difference (P<0.05).
3. Results
3.1. Effect of calcium supplementation on memory, as determined by Y-maze, object recognition, and Barnes maze tests
Excess supplementation of high-fat diet is known to cause memory impairment. To examine the influence of dietary calcium carbonate on memory, mice were fed a high-fat diet containing different amounts of calcium carbonate and minerals (Table 1) for 8 weeks.Diet 1 and Diet 2 contained 2.3% and 1.3% mineral mixture,respectively. Diet 3 contained 1.3% mineral mixture and 1.0%calcium carbonate. The calcium content of Diet 3 was approximately 3.5-fold and 2.0-fold of Diets 1 and 2, respectively. There were no differences noted in general appearance, behavior, and weight change between the groups (Table 2). Further, no changes were observed in serum calcium level after 8 weeks of feeding [Serum calcium concentration: (1.92±0.21) nM vs. (1.96±0.15) nM vs.(2.10±0.19) nM]. The effect of calcium carbonate supplementation on short-term memory was assessed by studying spontaneous alternation in the Y-maze test. Spontaneous alternation in mice fed with Diet 2 did not differ from that of mice fed Diet 1 (Table 3),suggesting that the decrease in amount of mineral mixture did not induce short-term memory impairment. Conversely, mice fed with Diet 3 had significantly lower spontaneous alternation than those fed with Diets 1 and 2. These results demonstrate that calcium carbonate supplementation induces short-term memory impairment. Moreover,although mice fed with Diet 3 tended to have more arm entries(Table 3), this change was not significant.
Next, we investigated the effect of dietary calcium carbonate on memory using the novel object recognition test. In the training session, the exploration times for the two objects among the groups that were on Diets 1, 2, and 3 did not change (Table 3). In the test session, mice fed with Diets 1 or 2 explored novel objects for a greater time than familiar objects. In contrast, the Diet 3 group spent less time exploring the novel object, showing that calcium carbonate supplementation resulted in memory impairment for the familiar object.

Table 2 Weight gain of mice fed with Diet 1, Diet 2, and Diet 3 (g).
The Barnes maze test was performed to estimate the influence of calcium carbonate supplementation on spatial memory. Following training trials of 4 days which make mice remember the location of the target hole, a probe trial was performed. During the probe trial,the duration spent searching around the target hole was measured(Table 3). This duration was significantly lower in the Diet 3 group than in Diet 1 and 2 groups. This once again suggested that calcium carbonate supplementation causes spatial memory impairment.
3.2. Dose of calcium carbonate causing memory impairment
To determine the dose of calcium carbonate that causes memory impairment, mice were fed diets containing 0.2% calcium carbonate(Diet 4), 0.6% calcium carbonate (Diet 5), and 1.0% calcium carbonate (Diet 3) as outlined in Table 1. The Y-maze test and object recognition test were then performed (Table 4). The diet containing 0.2% (Diet 4) calcium carbonate showed a tendency to decrease the percent alternation in the Y-maze test, while the diet containing 0.6%calcium carbonate significantly decreased the percent alternation in the Y-maze test. Further, in the object recognition test, the diets containing 0.2% and 0.6% calcium carbonate showed a tendency to decrease time spent exploring a novel object. These results demonstrated that the addition of 0.2% calcium carbonate may be sufficient to cause memory impairments.
3.3. Effect of calcium channel blocker on calcium carbonate supplementation-induced memory impairment
To investigate the action mechanism of calcium carbonate-induced memory impairment, the effect of an L-type calcium channel blocker, nimodipine, was investigated. Diet 3, which contained 0.1% nimodipine or 1.0% nimodipine, was fed to mice for 8 weeks before they underwent any behavioral testing (Table 5). The addition of 0.1% and 1.0% nimodipine significantly recovered the calcium carbonate-induced decrease of spontaneous alternation in the Y-maze test. The addition of nimodipine (1.0%) also considerably recovered the calcium carbonate-induced memory impairment in the object recognition and Barnes maze tests; however, these differences were not statistically significant in the Barnes maze test. These results suggested that calcium entry through L-type calcium channel may be involved in calcium carbonate-induced memory impairment.
3.4. Effect of calcium carbonate in normal diet
To remove any influence of high-fat on calcium carbonateinduced memory impairment, normal diets (Diet 6), Diet 7 which contained 0.4% and Diet 8 containing 1.0% calcium carbonate,were fed to mice for 8 weeks, before the Y-maze, object recognition,and Barnes maze tests were performed (Table 6). Similar to highfat diet, the intake of normal diets containing 0.4% and 1.0%calcium carbonate also caused memory impairment. These results suggested that memory impairment is caused by calcium carbonate supplementation alone and that high-fat content did not influence the memory impairment observed.

Table 3 Behavioral analysis of mice fed with either Diet 1, Diet 2, or Diet 3.
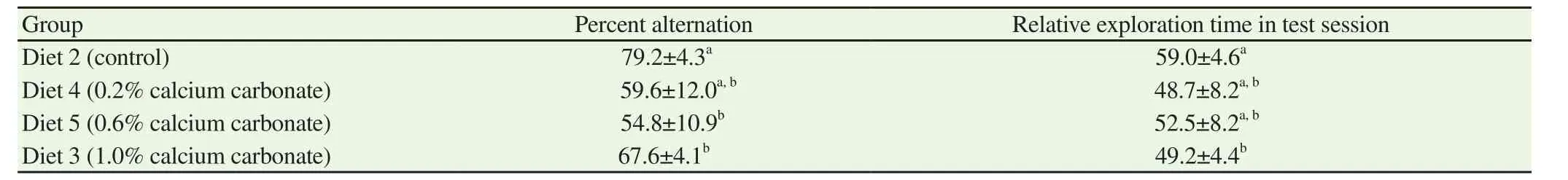
Table 4 Dose-dependent memory impairment of calcium carbonate (%).

Table 5 Effect of nimodipine against calcium carbonate-induced memory impairment.
3.5. The social interaction test
Alzheimer’s disease is known to cause behavioral and psychological symptoms of dementia (BPSD) such as aggression, anxiety,and circadian rhythm abnormalities with cognitive impairment.Mouse models of Alzheimer’s disease, such as APP transgenic mice and amyloid beta injected mice, show BPSD-like behavioral abnormality[20-22]. We investigated whether mice fed with calcium carbonate would also show such abnormal behavior, using social interaction and elevated plus maze tests (Table 7). In the social interaction test, we measured the time with active contact such as sniffing and following. Mice fed with calcium carbonate spent a shorter time in social interaction though significant difference was not observed; aggressive behavior, such as wrestling, kicking, and biting was also not observed in mice fed with calcium carbonate.Next the elevated plus maze test was performed to estimate anxiety.No significant differences were observed in the number of entries into open arms and the time spent in open arms.
3.6. Expression level of mRNA and protein involved in memory in hippocampus and cerebral cortex
We investigated the expression level of CREB and phosphorylated CREB, which are involved in neuronal plasticity and memory formation using western blotting. The expression level of CREB and phosphorylated CREB, in both the hippocampus and cerebral cortex, were lower in mice fed with calcium carbonate (Diet 3)than in those fed the control diet (Diet 2) (Figure 1). Next, we investigated changes in expression levels of memory-related mRNAs in the hippocampus and cerebral cortex[23-25]. We found that mRNA expression levels of potassium inwardly rectifying channel, CHOP,and neuropeptide-Y2 receptor in cortical neurons, and neuropeptide-Y2 receptor in the hippocampus significantly decreased in mice fed calcium carbonate (Diet 3) (Table 8).

Figure 1. Expression level of cAMP response element binding protein(CREB) and phosphorylated CREB in the brain of mice fed either Diet 2 or Diet 3.
3.7. Histology
Finally, Nissl staining was used to investigate the degeneration of neurons in the hippocampus. Although a decrease in the stainingstrength was detected in some mice that were fed diets containing calcium carbonate, a distinct neuronal change, such as cell shrinkage or neuronal loss, was not observed in the hippocampus (Figure 2).

Table 6Effect of calcium carbonate-induced memory impairment in normal diet (Diet 6).

Table 7 Behavioral and psychological symptoms of dementia (BPSD) of calcium carbonate fed mice.
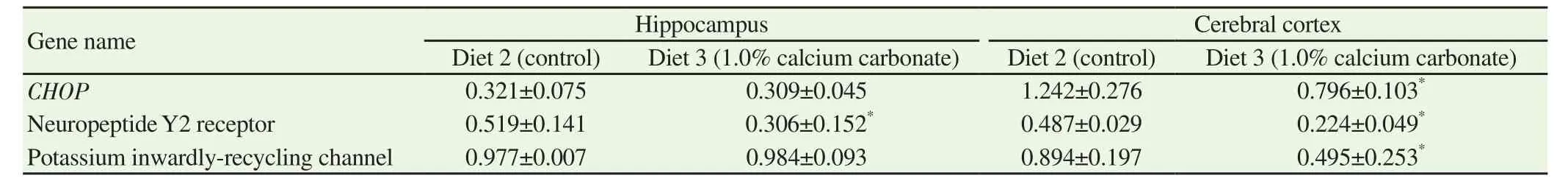
Table 8 Relative expression level of mRNAs of CHOP, neuropeptide Y2 receptor, and potassium inwardly-recycling channel.
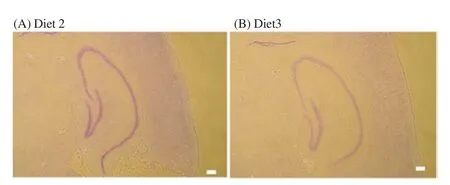
Figure 2. Histological study of the hippocampus of mice fed either Diet 2(A) or Diet 3 (B).
4. Discussion
In the current study, using a high-fat diet, we assessed the effect of calcium carbonate supplementation, which is known to cause memory impairment through inflammation and oxidative stress[26].Under our experimental conditions, the high-fat diet did not cause memory impairment (data not shown) with respect to the effects of the control diet. The high-fat content of the diet did not seem to influence calcium carbonate-induced memory impairment, since calcium carbonate supplementation of a normal diet caused similar memory impairment.
The results of the Y-maze, object recognition, and Barnes maze tests in the present study demonstrated that calcium carbonate supplementation,and not the high-fat diet in itself caused memory impairment. The calcium content used in Diets 1, 2, and 3 was 9.66, 5.46, and 17.46 mg per 3 g of diet, respectively. The calcium content of the AIN93 basal diet that is generally used is 14.3 mg per 3 g of diet. Therefore, the calcium content of Diet 3 used in the present study was never excessive.The mineral mixture content of Diets 2 and 3 was approximately 56%of Diet 1. The mineral mixture contained zinc and selenium, which are known to be involved in memory impairment. However, we did not observe any differences in memory between groups fed with Diet 1 and Diet 2, suggesting that minerals other than calcium did not play a role in memory impairment in the present study. This was also supported by the result showing memory impairment also in mice fed Diet 1 plus different calcium content compared mice fed Diet 1 (data not shown).Nimodipine reversed calcium carbonate-induced memory impairment. Nimodipine is an L-type voltage dependent calcium channel blocker that inhibits excessive influx of calcium ion. The therapeutic actions of nimodipine protect neurons by relaxing vascular smooth muscle cells against acute ischemic hemispheric stroke[27]. Lazarewicz et al.[28] proposed that nimodipine could directly act to neurons and protect not through relaxation of vascular smooth muscle. At present, it remains unclear whether the recovery effect of nimodipine against calcium carbonate-induced memory impairment is due to the relaxing of vascular smooth muscle or its direct action on neuronal cells in the brain.
Hanahisa and Yamaguchi[12] reported that the administration of calcium (50 mg/100 g) significantly increased serum calcium concentration and brain calcium content. Kim et al.[29] demonstrated that excess extracellular calcium increased intracellular calcium through calcium channels and caused calpain activation and cell death in SH-SY5Y neuroblastoma cells. If the intraventricular calcium concentration of mice that were fed Diet 3 was higher than that in mice that were fed Diet 2, then a higher extracellular calcium in the brain may have caused neuronal injury. However, since we did not assess this, the intraventricular calcium concentration of mice fed with either Diet 2 or Diet 3 remains to be tested in future studies.
The calcium carbonate diet caused a decrease of CREB and phosphorylated CREB expression in the hippocampus and cerebral cortex. CREB regulates the expression of genes required for synaptic plasticity and memory formation[30,31]. Pharmacological studies have demonstrated that CREB is essential for memory formation including spatial and social learning[32]. Our results suggested that a calcium carbonate-rich diet causes memory impairment through the decrease of expression of CREB and phosphorylated CREB. Pugazhenthi et al.[33] reported that amyloidβ treatment of hippocampus neurons caused a 40% decrease in CREB mRNA levels, due to amyloid β-generated oxidative stress. To investigate whether oxidative stress occurred in brains of mice that were fed calcium carbonate, we measured the levels of malondialdehyde (a lipid peroxidation product) and glutathione in the hippocampus and cerebral cortex. However, we did not observe any significant changes. Further studies will be required to clarify the mechanism by which calcium carbonate reduced CREB activity.
The levels of total or phosphorylated CREB in the hippocampus have also been reported to decrease in aged mice and rats[33,34].CREB-mediated gene expression was also impaired in the brains of patients of Alzheimer’s disease and a transgenic mouse of Alzheimer’s disease. Calcium carbonate-induced memory impairment is similar to that seen with aging and Alzheimer’s disease, in terms of the decrease in CREB expression. However,it remains unclear whether changes in the brain caused by calcium carbonate elicit a similar biochemical and pathological changes to that seen in the brain in Alzheimer’s disease or during aging. More histological research to detect changes found in the brain for Alzheimer’s, such as β-amyloid deposition and tau hyperphosphorylation as well as changes in the expression of β-galactosidase and calcium binding proteins observed in an aging brain, are needed. In addition, it will be interesting to investigate whether calcium carbonate supplementation promotes β-amyloid deposition in a mouse model of Alzheimer’s disease as well as that observed with a high-fat diet[35].
5. Conclusions
Our results show that a calcium carbonate diet may cause memory impairment by decreasing CREB expression. To our knowledge,this is the first report that a calcium carbonate diet causes memory impairment. This new animal model may be useful for developing new drugs for dementia.
Conflict of interest statement
We certify that there is no conflict of interest with any financial organization.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Acute kidney injury in leptospirosis: Overview and perspectives
- In vitro antiproliferative and apoptosis inducing effect of a methanolic extract of Azadirachta indica oil on selected cancerous and noncancerous cell lines
- Efficacy of voriconazole on leishmaniasis by Leishmania major: An in vitro and in vivo study
- Anti-schistosomal activities of Echinops kebericho Mesfin root and Hagenia abyssinica (Bruce) J.F Gmel flower part crude extracts in Swiss albino mice
- In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines
- Investigation of cryptic diversity and occurrence of echinostome metacercariae infection in Anentome helena (von dem Busch, 1847)
