In vitro antiproliferative and apoptosis inducing effect of a methanolic extract of Azadirachta indica oil on selected cancerous and noncancerous cell lines
2018-11-14MuhammadKashifDongwookKimGonhyungKim
Muhammad Kashif, Dongwook Kim, Gonhyung Kim
Veterinary Medical Center, College of Veterinary Medicine, Chungbuk National University, Cheongju, South Korea
Keywords:Neem oil extract Cell lines Cytotoxicity Apoptosis Caspase activity
ABSTRACT Objective: To find the cytotoxic and apoptotic effects of neem oil extract on the selected cancerous (A-549, PC-3 and DU-145) and noncancerous (NIH3T3 and CCD-18Co) cell lines.Methods: Viability and cytotoxic effect induced by the extract was measured by using MTT assay and apoptotic effect of the extract was evaluated by using Hoechst 33342 and propidium iodide dual staining through a fluorescent microscope and activity of caspases 3, 8 and 9 through colorimetric assay kits. Results: The results showed that neem oil extract significantly reduced the viability in all selected cancer cells treated with varying concentrations of extract as compared with untreated cells and had less effect on noncancerous cell lines. It significantly increased the percentage of necrotic and apoptotic cells, and caspases 3, 8 and 9 activities in all cancer cells treated with extract as compared with untreated cells whereas no effect on noncancerous cell lines. It suggested that neem oil extract exerted a higher cytotoxic effect on cancer cells than normal cells and lower concentration induced apoptosis only in cancer cells.One of the apoptosis-inducing mechanism was through the activation of caspases signaling pathways. Conclusion: Conclusively, it implies that neem oil extract may contain one or more potential agents that can be used as a safe and effective anticancer therapy.
1. Introduction
Cancer is number one cause of death all over the world. According to survey, 1 735 350 new cancer cases and 609 640 cancer deaths are projected to occur in the United States in 2018[1]. Phytotherapy has been used to treat various diseases for thousands of years[2].It is a fact that several pharmacological active ingredients are derivative of natural resources including herbs and medicinal plant[3]. Several previous studies have shown that medicinal plant like olive, black seed, curcumins, and dates are effective in cancer treatment and prevention[4,5]. The present modes of treatment of cancer, based on synthetic drugs, surgery and radiotherapy are toxic,expensive and alter the functions of the normal cell while traditional herbal medicines are being cost effective, readily available and comparatively showing less side effects[6].
Azadirachta indica (locally called neem tree) (A. indica) is an evergreen, fast-growing and resistant to high temperature and drought, native to tropical and semi-tropical climates like in India,Pakistan and Bangladesh[7]. Each part of this tree including flower,seed, leaves, root, wood, oil and their purified products have been used as traditional medicine to cure multiple human and animal diseases. It possesses antibacterial, antiviral, anthelmintic, antifungal,antidiabetic, sedative and contraceptive effects[8]. Invasion and migration of cancer cells is an important step in cancer metastasis.Neem phytochemicals suppress growth and proliferation of cancer cells, induce apoptosis and cell cycle death, inhibit angiogenesis,decrease tumor cell invasion and migration and interfere with growth factor signaling due to the presence of numerous chemical constituents in various parts of plants[9]. In a xenograft mouse model of colon cancer, administration of neem compound nimbolide at doses of 5 and 20 mg/kg intraperitoneally inhibited the survival and growth of colorectal carcinoma xenografts through modulation of expression of NF-κB regulated gene products linked to survival,proliferation, invasion and angiogenesis[10].
Neem oil derived from its seeds exhibits many biological activities and has a complex composition which is different according to the climatic conditions and nature of soil. However, in any case, it contains limonoids, terpenoids, volatile compound, glycerides and sulphur modified fatty substance[11]. In the previous studies, it was also found that polysaccharides and limonoids present in the leaves,bark and seed oil of neem inhibited lymphocytic leukemia, tumors,and cancers[12,13]. Neem oil is very effective in cancer chemotherapy and radiotherapy as an adjunct[14].
The toxicity of the compound to normal cells always becomes an issue in its therapeutic use. Therefore anticancer practitioners are always searching for drugs that selectively effects only on the cancer cell. In the previous study of our lab, we found that nimbolide derivatives of neem seed and leaves exerted cytotoxic effect and induced apoptosis selectively only in cancer cells without affecting normal cells[15]. This study aims to explore the antiproliferative effect and presence of apoptosis induced by neem oil extract on selected cancer and noncancerous cell lines.
2. Materials and methods
2.1. Preparations of methanolic extract of neem oil
Pure (100%) neem oil was purchased from (Thewitch) South Korea.Methanolic extraction of neem oil was prepared by mixing 100 mL of neem oil into 1.2 liters of methanol. The mixture was dried under vacuum conditions at low temperatue and obtained a solid sediment.The stock solution (100 mg/mL) was made by dissolving the pellet in ethanol. The stock solution prior to use was put in the dry oven for 24 h at 90 ℃ to destroy thermally unstable compounds and azadirachtin according to procedure previously described[16]. The desired concentration of the stock solution was made after dissolving it in medium before use.
2.2. Chemicals
Trypan blue, propidium iodide stain, Hoechst 33342 stain were got from Sigma Chemicals Co. (St. Louis, Mo, USA). FLICE/caspase 3, 8 and 9 colorimetric assay kits were obtained from Bio Vision (Milpitas, California, USA). Fetal bovine serum (FBS),penicillin-streptomycin, Dulbecco’s phosphate buffered saline and trypsin-EDTA were purchased from Welgene Bioscience(Dalseogu, Daegu, South Korea), DMEM and RPMI-1640 medium from Capricorn Scientific (Sujeong-gu, Seongnam, South Korea),MTT dye (Thiazolyl Blue Tetrazolium Bromide) from Solon Ind.(Pkwy, Solon, Ohio), DMSO was obtained from Junsei Chemicals(Nihonbashi-honcho, Tokyo, Japan), FITC annexin-V kit was purchased from Invitrogen (Waltham, Massachusetts, USA).
2.3. Cell lines and cell culture
The selected cancer (PC-3, Du-145, A-549) and noncancerous(NIH3T3 and CCD-18Co) cell lines were obtained from the Korean Cell Line Bank, South Korea. PC-3 and Du-145 are human prostate cancer cell lines, A-549 is human lung cancer cell line, CCD-18Co is a human colon fibroblast cell line and NIH3T3 is mouse embryonic fibroblast cell line. The cancerous cells were cultured in RPMI 1640 media and noncancerous cells were grown in DMEM according to instructions of Korean Cell Line Bank. All the cell lines were maintained in medium provided with 10% FBS and 1% antibiotics in humidified 5% CO2and 95% air incubator at 37 ℃.
2.4. Cytotoxicity (MTT) assay
MTT assay is commonly used to measure the viability of cells throughout the world, described by Mosmann in 1983[17]. It caused reduction of a yellow tetrazolium salt [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, or MTT] to measure cellular metabolic activity as a proxy for cell viability. Viable cells contain NADPH dependent oxidoreductase enzymes which reduce the MTT reagent to formazan, an insoluble crystalline product with a deep purple color. It is based on the principle that dead cells do not reduce the MTT dye, a tetrazolium compound while the live cells convert it into the purple colored product (formazan) by mitochondrial enzyme.Shortly, approximately 8 000 cells/well were grown in 96 wells tissue culture plate and treated with increasing concentrations of neem oil extract ranging 31 μg/mL to 250 μg/mL for 24, 48, 72 and 96 h (except the untreated control). After that medium was discarded and MTT solution (5 mg/mL) 100 μL was added to each well and place in 5% CO2incubator for 2 h at 37 ℃. After removal of MTT solution, the formazan crystals was dissolved in 50 μL of DMSO.The micro-ELISA plate reader was used to measure the intensity of developed color at 570 nm. Blank wells contained medium only. The percentage viability of treated cells was calculated with the help of the following formula, % viability of cells = (Average optical density of treated cells/Average optical density of control cells) ×100%,while the cell viability in the control group was considered 100%.
2.5. Morphological changes in phase-contrast microscopy
To observe the morphological changes, each cancer and noncancerous cell line was grown using 6 well tissue culture plates and treated with different concentrations of neem oil extract (31, 62 and 125 μg/mL) for 48 h (except the untreated control) and observed under phase contrast microscope[18].
2.6. Hoechst- propidium iodide dual staining
The cancerous and noncancerous cell lines were treated with 31,62, 125 μg/mL of neem oil extract (except the untreated control cells) and seeded in 6 well tissue culture plate (5×105cells/well) for 24 h and 48 h. After trypsinization and washing with PBS, cells were stained in working solution of Hoechst 33342 (10 μg/mL) and put in CO2incubator at 37 ℃ for 15 min. After that cells were mixed and counterstained by adding propidium iodide (50 μg/mL) solution and placed for 15 min in a CO2incubator. The cells were centrifuged and suspended cells were placed on a clean glass slide and observed using a fluorescence microscope. The cells that had a normal nuclei,organized structure and blue chromatin were considered normal viable cells, the cells that had bright blue stained chromatin which was fragmented or condensed were considered as early apoptotic cells, the cells that had bright pink chromatin which was fragmented or condensed were considered as late apoptotic cells and the cells that had pink chromatin with organized structure were considered as necrotic cells. The percentage of apoptosis and necrosis were quantified using following formula[19,20].

2.7. Measurement of caspases 3, 8 and 9 activities
The caspases 3, 8 and 9 activity were measured using caspases/FLICE colorimetric assays kits according to manufacturer’s instructions. Briefly, the cells treated with 125 μg/mL of neem oil extract for 24 h. After trypsinization (1×106) cells were pelleted in all groups. The cell pellets were resuspended in a chilled cell lysis buffer (50 μL) and kept on ice for 10 min. The centrifugation of lysed cells was done at 10 000 g for 1 min at 4 ℃. Lysate proteins in equal amounts from each sample were added to 96 well tissue culture plate. Afterwards 50 μL of 2 reaction buffer and 5 μl pNA-conjugated substrates were added and placed at 37 ℃ for 2 h in CO2. The released amount of pNA was measured using an ELISA microplate reader.
2.8. Statistical analysis
All the data was presented as means ± standard deviation and all the experiments were performed in triplicates. Statistically significant difference between treated and untreated control cells was analyzed through one way ANOVA using IBM SPSS Statistics 24, followed by posthoc analysis. A value of P<0.05 considered statistically significant.
3. Results
3.1. Effect of neem oil extract on the viability of selected cell lines
Neem oil extract caused a significant reduction in percentage viability as compared with control in all cancer cell lines. Whereas for noncancerous cell lines, no significant effect was found when comparing untreated cells with cell lines treated with low concentration. It exerted time and dose dependent cytotoxic effect(Figure 1). The IC50value in cancer and noncancerous cell lines were depicted in Table 1. It is suggested that neem oil extract is more toxic to cancer cells as compared with noncancerous cells.
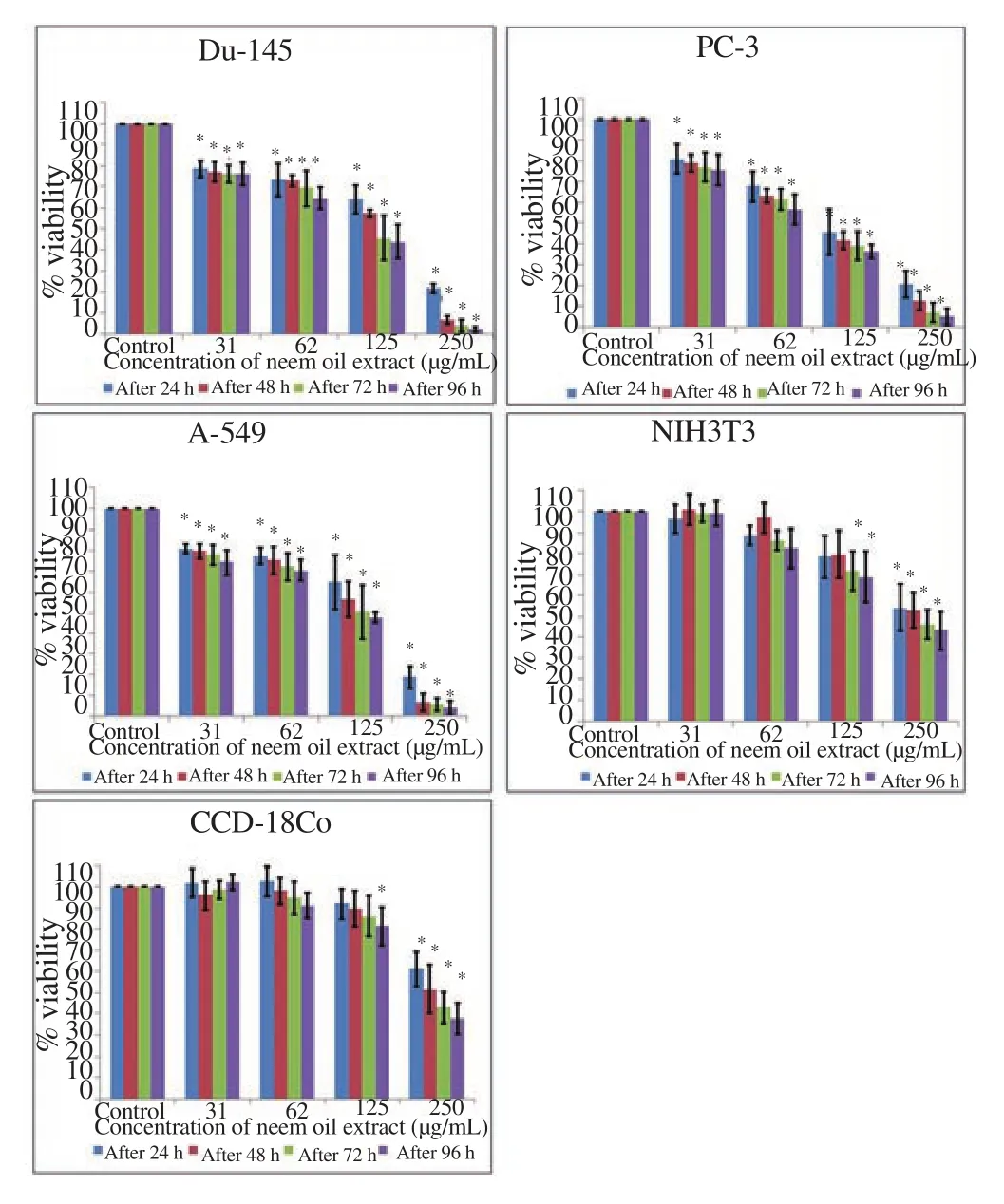
Figure 1. Neem oil extract reduced the viability of cancerous cell with minimal toxicity to the noncancerous cells measured through MTT assay.Each bar represents the values of means ± standard deviation of four independent experiments. *indicates statistically significant difference between treated and untreated control cells (P<0.05).

Table 1 IC50 values (μg/mL) were calculated through linear regression equation using Microsoft excel 2013 from the average percentage viability from four independent experiments treated with different concentration of neem oil extract for 24 h, 48 h, 72 h and 96 h.
3.2. Effect of neem oil extract on morphological changes under phase contrast microscope
Exposure of neem oil extract to cancer cells led to typical apoptotic features such as decrease number of cells, condensed cells, lose contact with adjacent cells and detached from the surface of tissue culture plate whereas, in contrast, the untreated control cells maintained original morphology form and adherent to the tissue culture plates. Noncancerous cells treated with neem oil extract did not exhibit these typical features of apoptosis and appeared in a normal shape similar to untreated control cells as shown in Figure 2.

Figure 2. Morphological changes observed in cancer (Du-145, PC-3 and A-549) and noncancerous (NIH3T3 and CCD-18Co) cell lines treated with concentrations (0, 31, 62 and 125 μg/mL) of neem oil extract for 48 h and observed under phase contrast microscope (magnification 100 ).
3.3. Effect of neem oil extract on morphological changes under fluorescence microscope
Apoptotic and necrotic cells were detected and quantified using dual staining of propidium iodide and Hoechst 33342 and through a fluorescence microscope. Results showed that cancer cells treated with different concentration of neem oil extract displayed chromatin condensation, brightly stained and multiple fragmentations of nuclei(apoptotic) and emit pink fluorescence (late apoptosis and necrosis)whereas the control (untreated) cells appeared with oval shape, were less brightly stained and had an absence of red fluorescence. The noncancerous cells treated with the same dose of neem oil extract did not show significant morphological alteration as compared with untreated control under a fluorescence microscope as depicted in Figure 3. The percentages of necrotic and apoptotic cells treated with neem oil extract were significantly higher as compared with control group in cancer cell lines whereas no significant difference was observed between treated cells and untreated control cells in noncancerous cell lines as shown in Figure 4 and 5 respectively.

Figure 3. Morphological changes detected in cancer and noncancerous cell lines with dual staining of Hoechst 33342 and propidium iodide under fluorescent microscope (magnification 400).

Figure 4. Percentage of apoptotic cells quantified by Hoechst 33342 and propidium iodide dual staining after treatment with different concentrations of neem oil extract for 24 and 48 h.
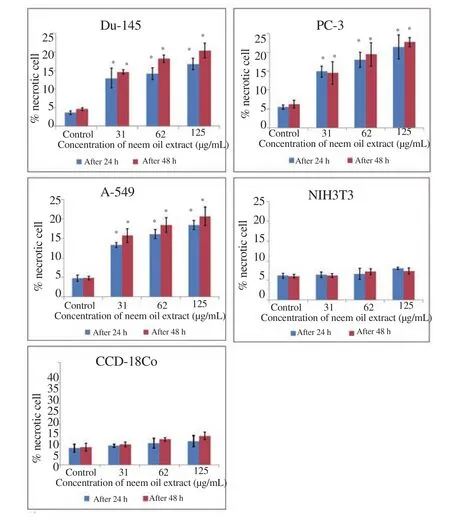
Figure 5. Percentage of necrotic cells quantified by Hoechst 33342 and propidium iodide dual staining after treatment with different concentrations of neem oil extract for 24 and 48 h.
3.4. Effect of neem oil extract on signaling pathways of caspases
To examine the mechanism of apoptosis, caspases activities were measured. The cells treated with neem oil extract significantly increased the caspase 3, 8 and 9 activity as compared with control in all cancer cells whereas in noncancerous cells no significant difference was observed between treated and untreated cells as shown in Figure 6. This suggests that neem oil extract induced apoptosis through the activation of caspase cascade signaling pathways.

Figure 6. Measurement of caspases 3, 8 and 9 activity of cancer and noncancerous cell lines treated with neem oil extract (125 μg/mL) for 24 h through colorimetric assay kits.
4. Discussion
Natural products and bioactive compounds are important sources for potential chemotherapeutic and antitumor agents. Neem oil used for centuries in herbal, ayurvedic and traditional medicines and have many potential biological activities. Some studies also explored the potential of neem oil and its purified compounds in the control of viruses and cell proliferation[21]. In the present study methanolic extract of neem oil exerted a higher cytotoxic effect on cancer cells than noncancerous cells. The IC50values were much lower in cancer cells than the noncancerous cells at various time points. It suggested its selective toxicity towards cancer cells and provided a background for the development of neem/oil components as a biosafe chemotherapeutic agent. The previous studies also showed consistent results with our results that neem extracts and its components exerted antiproliferative effect on the growth of numerous cancer cells such as human choriocarcinoma cells, squamous cell carcinoma,melanoma cells, leukemia cells, prostate cells, murine Ehrlich’s carcinoma, Hela cells, breast cancer and exhibited lower cytotoxic effect on noncancerous cells[22-25].
Apoptosis is a biological phenomenon that plays important role in the tissue homeostasis and embryogenesis[26]. Therefore selective induction of apoptosis of cancer cells has been considered the best option for the treatment and prevention of cancer[27]. Apoptotic cells possesses a lot of typical features such as condensation, shrinkages of cells, condensed and cleaved chromatin. These typical, distinctive morphological features in apoptotic cells are frequently used for the identification and quantification of apoptosis[28,29]. In the present study selected cancer treated with different concentrations of neem oil extract showed similar features under phase contrast microscope.This provides an evidence that neem oil extract induced apoptosis selectively in cancer cells.
Hoechst 33342 and propidium iodide double staining can be used to observe apoptotic features such as nuclear fragmentation and chromatin condensation using fluorescence microscopic analysis.It is possible to differentiate and quantify the live, apoptotic and necrotic cells populations by use of these dyes under fluorescence microscope[18,29]. In the present study, we also assessed and quantified apoptosis and necrosis using dual staining of propidium iodide and Hoechst 33342 under a fluorescence microscope.
The presence of necrotic cells along with early apoptotic cells suggested that these cells resulted from an apoptotic process rather than necrotic process[30] or it may be due to the incomplete process of apoptosis[31]. The presence of increased necrotic cell in the present study may result after the apoptotic process or incomplete process of apoptosis due to increased or decreased concentrations of extract or it may be due to the complex action of neem oil extract.It suggested that neem oil extract exerts an antiproliferative effect through induction of apoptosis.
Apoptosis is an irreversible process which is mainly conducted by a cascade of caspase proteins through two pathways i.e. intrinsic and extrinsic pathways[32]. These pathways are triggered by activation of initiator caspases 8, 9, 10 which result in activation of executioner caspase 3. The activation of caspase 3 causes the proteolytic degradation of many proteins like β-catenin and PARP, that leads to apoptosis[33]. Caspases help in early detection of apoptosis through assaying for cleaved caspases[34]. In this study, we confirmed that treatment of cancer cells with neem oil extract significantly increase the activity of caspase 3, 8 and 9 as compared with untreated cells.So, it is suggested that neem oil extract triggers the apoptosis in cancer cells through the activation of both initiators and effector caspases.
NF-κB is one of the most studied transcription factors which regulates the transcription of the genes involved in cell proliferation,migration, invasion, survival, inflammation, angiogenesis, and every cellular process. In most type of cancer cells increased activity or activation of NF-κB signaling is observed. Therefore, inhibition of NF-κB activity is a common anticancer mechanism of many therapeutic agents and natural compounds[35]. The migration and invasion of tumor cells is also connected with MMP-2 and MMP-9 expression through the control of transcription factors NF-κB, AP-1 and Sp-1[36,37]. The previous studies showed that neem components inhibits tumor cell migration, invasion, and angiogenesis by downregulating MMP-2 and MMP-9 expression via inhibiting ERK1/2 and reducing DNA binding activity of NF-κB in cancer cells[38,39].
In conclusion, the results of this study suggested that neem oil extract has a strong cytotoxic activity on the cancer cells and lower toxicity toward noncancerous cells. It induced apoptosis only in cancer cells and one of the apoptosis inducing mechanisms is through activation of caspase signaling pathway. Neem oil and its components could be envisaged as an antiproliferative agent. This study offers an avenue to exploit it further in clinical trials and in vivo models.
Conflict of interest statement
We declare that we have no conflict of interest.
Foundation project
This work was supported by the Global Research and Development Center (GRDC) Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2017K1A4A3014959).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Acute kidney injury in leptospirosis: Overview and perspectives
- Efficacy of voriconazole on leishmaniasis by Leishmania major: An in vitro and in vivo study
- Anti-schistosomal activities of Echinops kebericho Mesfin root and Hagenia abyssinica (Bruce) J.F Gmel flower part crude extracts in Swiss albino mice
- Calcium carbonate supplementation causes memory impairment in mice
- In vitro anticancer activity of polysaccharide extracted from red alga Jania rubens against breast and colon cancer cell lines
- Investigation of cryptic diversity and occurrence of echinostome metacercariae infection in Anentome helena (von dem Busch, 1847)
