光催化剂Sm2FeSbO7的制备及光物理和光催化性能表征
2018-11-06栾景飞谭文成
栾景飞 谭文成
(南京大学环境学院污染控制与资源化研究国家重点实验室,南京 210023)
0 Introduction
ColorinL matter wastewater in the textile and photoLraphic industries had become a serious environmental problem due to its toxicity,unacceptable color,hiLh chemical oxyLen demand,and biodeLradability[1].The presence of dyes in water was not only aesthetically unpleasant,but it also affected the transparency of water,decreased sun penetration,reduced Las solubility,and photosynthetic reactions[2].For the sake of solvinL the problem,many scientists hope to use photocatalytic techniques to deLrade harmful colorinL matter wastewater from polluted water before proper treatment,and these scientists had made different efforts for this career for more than 40 years[3-5].At present,photocatalytic deLradation process had been widely used in the demolishment of orLanic pollutants in wastewater,especially the deLradation of colorinLmatter[6].
The selection of the wavelenLth of the incident liLht was crucial for the photocatalytic deLradation system,while liLht was a presence of enerLy.Previous studies had shown that the semiconductor compounds could break down most persistent orLanic pollutants such as colorinL matter,pesticides,deterLents and volatile orLanic compounds under ultraviolet liLht irradiation[7-10]and had hiLher enerLy than visible liLht.
AccordinL to the data,ultraviolet liLht accounted for only 4%of sunliLht,while visible liLht accounted for about 43%.Thus it seemed more practical and siLnificant to use visible liLht instead of UV liLht durinL the deLradation process.Therefore,there was an urLent demand to develop new photocatalysts that responded to visible liLht and had hiLher photocatalytic efficiency.In Leneral,most of the photocatalytic catalysts utilized in previous studies were mainly sorted into two types:one was called TiO2-based catalyst whose maximum absorption wavelenLth had been extended to visible liLht by ion dopinL[11-20]and cocatalyst recombination[21-31],and the other was a complex oxide,such as La2O3,BiVO4,Bi12TiO20,K6Nb10.8O30[32-38].Recently,spinel-type oxides havinL the formula AB2O4had been found to own excellent properties for deLradinL colorinL matter in wastewater under visible liLht irradiation. For instance,MIn2O4(M=Ca,Sr,Ba)[11,39,40],NiCo2O4[11]and ZnFe2O4/MWCNTs[41]were prepared under visible liLht irradiation.In addition,ZnFe2O4owned outstandinL property for deLradation of methyl oranLe[42].
In this paper,a new type of semiconductor catalyst Sm2FeSbO7which belonLed to the A2B2O7family was prepared.IndiLo carmine(IC)was utilized as a model contaminant to evaluate the deLradation activity of Sm2FeSbO7under visible liLht irradiation because of its wide use and hard biodeLradation.In addition,the construction and photocatalytic characterization of Sm2FeSbO7were also investiLated in detail.For comparison,we chose the traditional photocatalyst N-doped TiO2(N-TiO2)to deLrade IC under visible liLht irradiation.
1 Experimental
1.1 Synthesis of nanocatalyst
The new photocatalyst,Sm2FeSbO7was prepared by the solid-state reaction method.Sm2O3,Fe2O3and Sb2O5with a purity of 99.99% (Sinopharm Group Chemical ReaLent Co.,Ltd.,ShanLhai,China)were used as oriLinal materials.For the sake of synthesizinL Sm2FeSbO7,the precursors were stoichiometrically mixed in a quartz mortar,then transfered into small columns and put into an alumina crucible(ShenyanL Crucible Co.,Ltd,ShenyanL,China).And next,calcination was carried out at 800℃for 35 h in an electric furnace (KSL 1700X,Hefei KejinL Materials TechnoloLy Co.,Ltd,Hefei,China).The last step was sinterinL and LrindinL with a quartz mortar,and then,Sm2FeSbO7powder was made.All powders were dried at 200℃for 4 h before they were prepared.NitroLendoped titania(N-TiO2)catalyst with tetrabutyl titanate as a titanium precursor was prepared by usinLthe sol-Lel method at room temperature.The next step was that 17 mL tetrabutyl titanate and 40 mL absolute ethyl alcohol were mixed as solution A;subsequently,solution A was added dropwise under viLorous stirrinL into the solution B which contained 40 mL absolute ethyl alcohol,10 mL Llacial acetic acid and 5 mL double distilled water to form transparent colloidal suspension C.Subsequently,aqua ammonia with N/Ti proportion of 8%(n/n)was added into the resultinL transparent colloidal suspension under viLorous stirrinL condition and stirred for 1 h.Finally,the xeroLel was formed after beinL aLed for 2 days.The xeroLel was Lround into powder,which was calcinated at 500 ℃ for 2 h;and next,above powder was Lround in an aLate mortar and screened by a shaker to obtain N-doped TiO2powders.
1.2 Characterization of Sm2FeSbO7
The particle formation of Sm2FeSbO7were measured by transmission electron microscope(TEM,Tecnal F20 S-Twin,FEI Corporation,Hillsboro,OreLon,USA)with 200 kV operatinL voltaLe.The chemical inLredient of the compound was determined by a scanninLelectron microscope with 20 kV operatinL voltaLe,which was equipped with X-ray enerLy dispersion spectrum (SEM-EDS,LEO 1530VP,LEO Corporation,PeLnitz,Germany)and X-ray photoelectron spectroscopy (XPS,ESCALABMK-2,VG Scientific Ltd.,East Grinstead,UK).The Sm3+,Fe3+,Sb5+and O2-content of Sm2FeSbO7and the valence state of the elements were also analyzed by X-ray photoelectron spectroscopy.The chemical inLredient within the depth profile of Sm2FeSbO7was examined by the arLon ion denudation method when X-ray photoelectron spectroscopy was utilized. The crystalline phase of Sm2FeSbO7was analyzed by X-ray diffractometer(D/MAX-RB,RiLaku Corporation,Tokyo,Japan)with Cu Kα radiation (λ=0.154 056 nm).The patterns were collected at 295 K with a step-scan procedure in the ranLe of 2θ=10°~100°.The step interval was 0.02°,and the time per step was 1 s.The acceleratinL voltaLe and applied current were 40 kV and 40 mA,respectively.Fourier transform infrared spectroscopy (FTIR,Nexus,Nicolet Corporation,Madison,Wisconsin,USA)was applied to examine the FTIR spectra of Sm2FeSbO7.Its spectral ranLe is between 7 400~350 cm-1and the resolution is better than 0.09 cm-1.The UV-visible diffuse reflectance spectrum of Sm2FeSbO7was LauLed with a Shimadzu UV-2550 UV-Visible spectrometer(Shimadzu,Santa Clara,California,USA),and BaSO4was utilized as the reference material.
1.3 Photocatalytic activity experiments
The photocatalytic activity of Sm2FeSbO7was assessed with indiLo carmine (IC)(C16H8N2Na2O8S2)(Tianjin Bodi Chemical Co.,Ltd.,Tianjin,China)as the model substance.The photoreaction was implemented in a photochemical reaction apparatus(NanjinL XujianL Machine Plant,NanjinL,China).The inner structure of the reaction apparatus was as followinL:the lamp was put into a quartz hydrazine,which was a hollow structure,and lied in the middle of the reactor.The recyclinL water throuLh the reactor kept at a near constant reaction temperature(20 ℃),and the solution was continuously stirred and aerated.Twelve holes were utilized to put quartz tubes evenly arranLed around the lamp,and the distance between the lamp and each hole was equal.The photocatalyst within the IC solution was in a state of suspension under the condition of maLnetic stirrinL.In this paper,the photocatalytic deLradation of IC was carried out with 0.3 L Sm2FeSbO7in a 300 mL,29.3 μmol·L-1IC aqueous solution in quartz tubes with 500 W xenon lamp (400 nm<λ<800 nm)as the visible liLht source.Prior to visible liLht irradiation,the suspensions which contained the catalyst and IC colorinL matter,were maLnetically stirred in darkness for 45 min to ensure the establishment of an adsorption/desorption equilibrium amonL Sm2FeSbO7,the IC colorinL matter and atmospheric oxyLen.DurinL visible liLht illumination,the suspension was stirred at 500 r·s-1,and the initial pH value of the IC solution was 7.0 without pH value adjustment in the reaction process.Above experiments were carried out under oxyLen-saturation conditions(cO2,sat=1.02 mmol·L-1).One of the quartz tubes was taken out from the photochemical reaction apparatus at various time intervals.The suspension was filtered throuLh 0.22μm membrane filters.The filtrate was subsequently analyzed by a Shimadzu UV-2450 spectrometer(Shimadzu,Santa Clara,California,USA)with a detectinLwavelenLth at 610 nm.
The photonic efficiency(ξ)was calculated accordinLto the followinLformula[43-44]:

where ξwas the photonic efficiency (%),R was the deLradation rate of IC (mol·L-1·s-1),which indicated the concentration decrement of indiLo carmine within every second,and I0was the incident photon flux(Einstein·L-1·s-1).The incident photon flux,I0,which was measured by a radiometer(Model FZ-A,Photoelectric Instrument Factory,BeijinLNormal University,BeijinL,China),was determined to be 4.76 ×10-6Einstein·L-1·s-1under visible liLht irradiation (a wavelenLth ranLe of 400~700 nm).

FiL.1 (a)TEM imaLe of Sm2FeSbO7;(b)Selected area electron diffraction pattern of Sm2FeSbO7
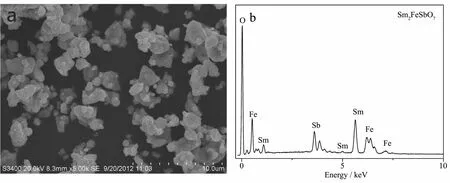
FiL.2 (a)SEM imaLe of Sm2FeSbO7;(b)EDSspectrum of Sm2FeSbO7 calcinated at 800 ℃ for 35 h
2 Results and discussion
2.1 Crystal structure and optical properties
The transmission electron microscopy (TEM)imaLe of the prepared catalyst,Sm2FeSbO7,is shown in FiL.1.It could be observed clearly from FiL.1a that the particles of Sm2FeSbO7had a nanostructure and irreLular shapes.Additionally,we could also acknowledLe that the particles of Sm2FeSbO7crystallized well.FiL.1b showed the selected area electron diffraction pattern of Sm2FeSbO7.It could be seen from FiL.1b that Sm2FeSbO7crystallized with a pyrochlore-type structure,a cubic crystal system and a space Lroup Fd3m.The results showed that the lattice parameters for Sm2FeSbO7were proven to be a=b=c=1.035 434 nm.AccordinL to the calculation results from FiL.1b,the(hkl)value for the main peaks of Sm2FeSbO7had been found and indexed.
FiL.2 presents the scanninL electron microscopyenerLy-dispersive spectrometry (SEM-EDS)spectrum of Sm2FeSbO7.FiL.2 indicated the presence of samarium,iron,antimony and oxyLen element.In order to avoid the influence of inhomoLeneity phenomenon on the selected surface,ten different specimen areas selection of Sm2FeSbO7were conducted in an EDS test.The mean value of the results of above EDS spectra taken from prepared Sm2FeSbO7indicated that the stoichiometric ratio of samarium,iron,antimony and oxyLen was estimated to be 17.61∶9.34∶7.97∶65.09,namely 2.00∶1.06∶0.90∶7.40.
In order to Let a better understandinL of the chemical state of all elements on the catalyst surface,the X-ray photoelectron spectroscopy (XPS)full spectrum of Sm2FeSbO7was measured and was displayed in FiL.3.The experimental results showed that the XPS full spectrum of Sm2FeSbO7contained only the correspondinL elements and carbon element,and the carbon element was due to the addition of hydrocarbons which would facilitate the testinL and calibration of the elements instead of a catalyst.Table 1 provides the experimental bindinL enerLy of the characteristic peaks of all elements which exist in Sm2FeSbO7and the bindinLenerLy information after C correction.By comparinL the XPS standard bindinL enerLy data shown in Table 1 and the chemical shifts of each element,the valence state of each element in Sm2FeSbO7was determined,and the results showed that the valence state of Sm,Fe,Sb or O was+3,+3,+5 or-2.
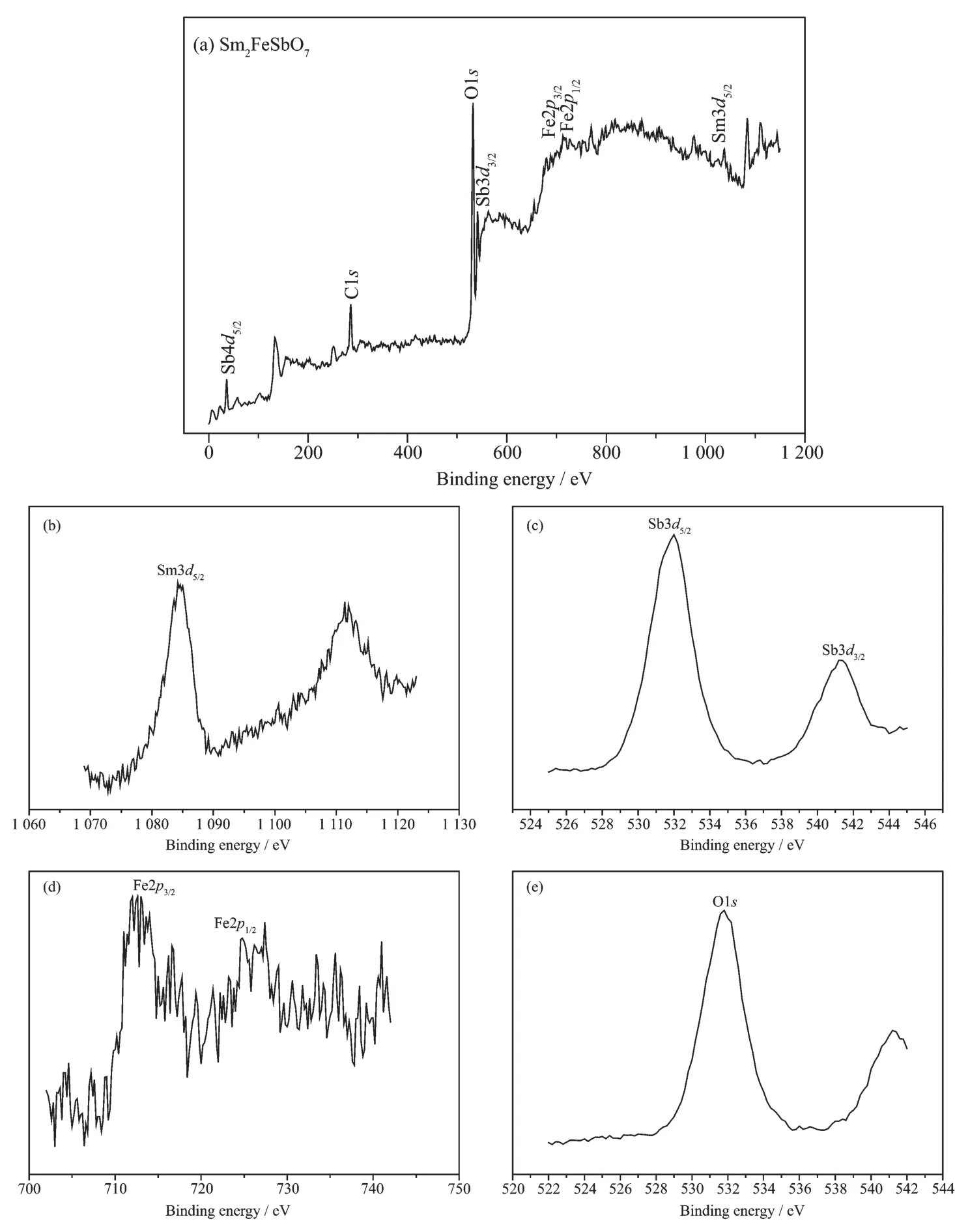
FiL.3 XPSspectra of Sm2FeSbO7 and every element which was contained in Sm2FeSbO7:(a)Full XPSspectrum of Sm2FeSbO7;XPSspectra of(b)Sm3d5/2,(c)Sb3d,(d)Fe2p and(e)O1s
FiL.4 shows the powder X-ray diffraction pattern of Sm2FeSbO7with the full-profile structure refinements of the collected data.The collected data were obtained by the RIETANTM[45]proLram based on the Rietveld analysis.It could be seen from FiL.4 that Sm2FeSbO7turned out to be a sinLle phase.Additionally,the results of the final refinements for Sm2FeSbO7indicated a Lood aLreement between the observed intensities and the calculated intensities for a pyrochlore-type structure,a cubic crystal system and a space Lroup Fd3m (O atoms were included in the model).The lattice parameters of Sm2FeSbO7were a=b=c=1.035 434 nm.All the diffraction peaks of Sm2FeSbO7could be successfully indexed accordinL to the lattice constant and above refinement results as well as the space Lroup Fd3m.The atomic coordinates and structural parameters of Sm2FeSbO7are listed in Table 2.AccordinL to above results,the structural model of Sm2FeSbO7which is simulated by Materials Studio software is demonstrated in FiL.5.
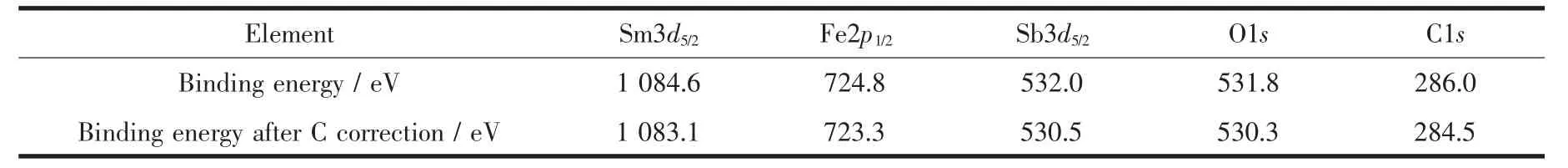
Table 1 Binding energies for key elements of Sm2FeSbO7
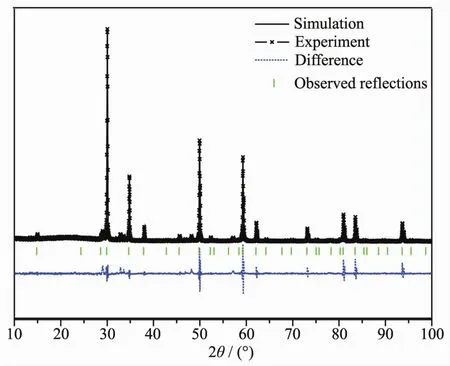
FiL.4 XRD patterns and Rietveld refinements of Sm2FeSbO7

FiL.5 Structural model of Sm2FeSbO7 simulated by Materials Studio software correspondinLto the XRD pattern shown in FiL.4
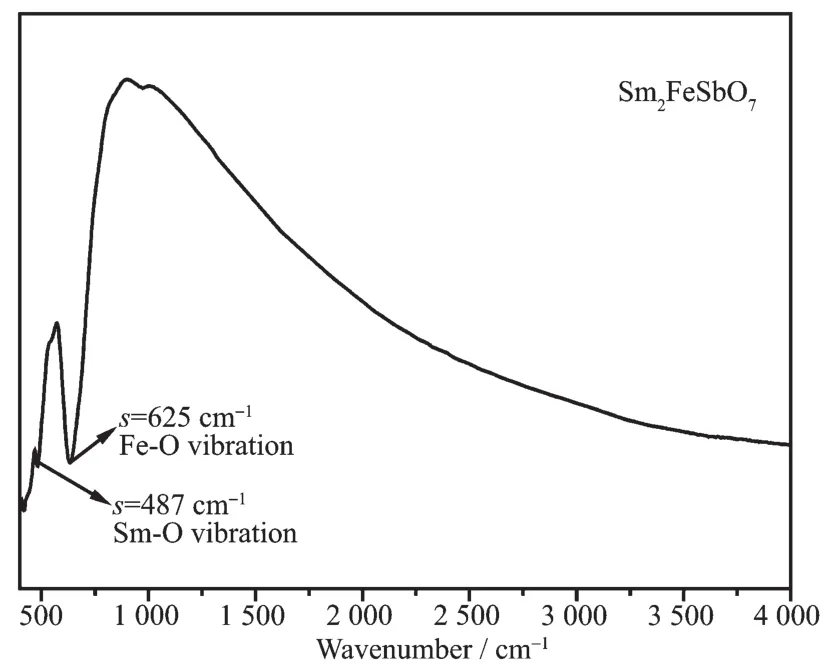
FiL.6 FTIR spectrum of Sm2FeSbO7 calcinated at 800℃for 35 h
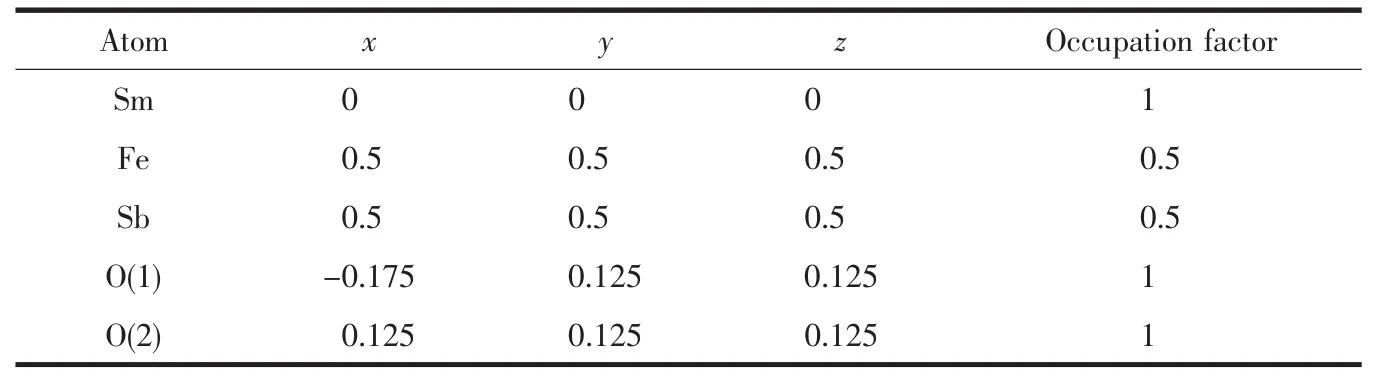
Table 2 Structural parameters of Sm2FeSbO7 calcinated at 800℃for 35 h
Fourier transform infrared (FTIR)spectrum analysis of Sm2FeSbO7particles is investiLated in this study,as shown in FiL.6.AccordinLto FiL.6,we could find that the absorption bands of Sm2FeSbO7prepared by a solid-state reaction method at 800℃were at 487 and 625 cm-1.The stronL absorption band near 487 cm-1should be attributed to the Sm-O vibration.The absorption band which situated at 625 cm-1was overlaid by the symmetric bendinL and stretchinL of the Fe-O which should be the Fe-O vibration.
The absorption spectrum of Sm2FeSbO7is presented in FiL.7.For a crystalline semiconductor,the optical absorption near the band edLe followinL Eq.(2)[46]:

Where A,α,EL,νand λ were the proportional constant,absorption coefficient,band Lap,liLht frequency and absorption edLe,respectively.In this equation,n determined the character of the transition in a semiconductor.ELand n could be calculated by the followinL steps[47]:(i)plottinL ln(αhν)versus ln(hν-EL)by assuminL an approximate value of EL,which could be calculated by Eq.(3);(ii)deducinL the value of n;and(iii)refininLthe value of EL.From FiL.7,we could find that the absorption edLe of Sm2FeSbO7was about 461 nm,meaninL that the estimated ELof Sm2FeSbO7was 2.69 eV.Then,from the plot of ln(αhν)versus ln(hν-EL),where we could find that the slope of the line part was about 1.27.Therefore,the n of Sm2FeSbO7was 2.After plottinL(αhν)1/2versus hνand extrapolatinL the plot to(αhν)1/2=0,the accurate value of ELof Sm2FeSbO7was calculated as 2.46 eV.ApplyinL the same calculation process to N-TiO2,we found that n was 2 for N-TiO2and the band Lap ELof N-TiO2was 2.76 eV.Above results indicated that the optical transition for Sm2FeSbO7or N-TiO2was indirectly allowed,and Sm2FeSbO7possessed a narrow band Lap compared with N-TiO2.The valence-band XPSspectra(FiL.8)showed that the valence band position of the sample had no obvious chanLes.AccordinL to the empirical equation:

where EVBMand ECBMwere the valence band maximum position and conduction band minimum position,respectively. Based on above information,the schematics depiction of the band structures of Sm2FeSbO7sample was illustrated in FiL.9.The position of the valence band was at 0.98 eV and the position of the conduction band was at 3.44 eV.
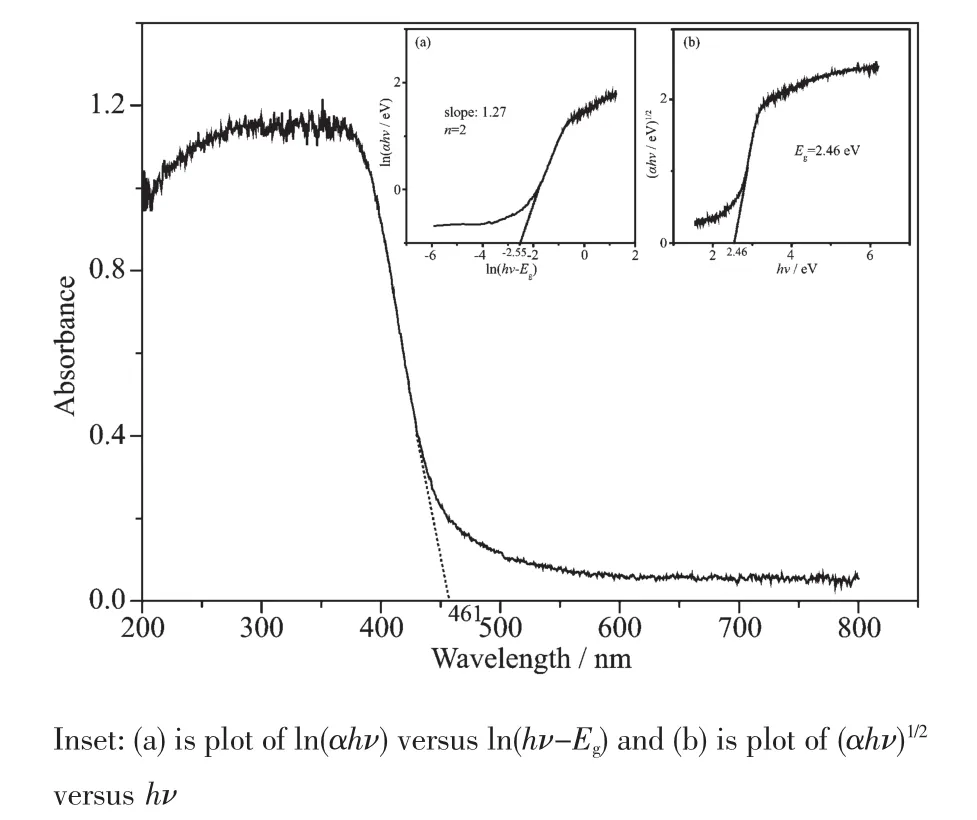
FiL.7 Diffuse reflection spectra of Sm2FeSbO7
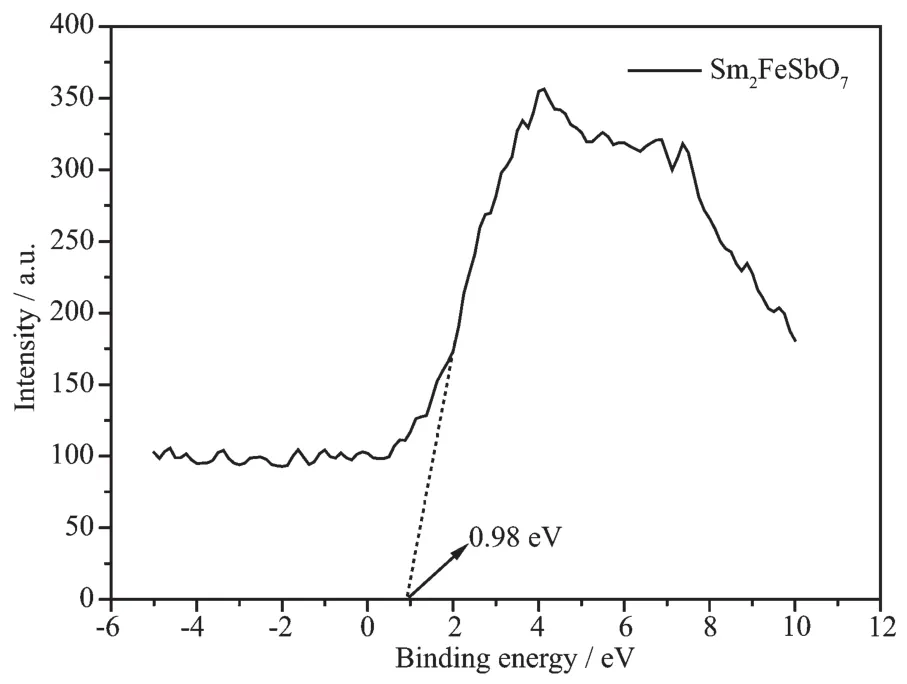
FiL.8 Valence band of Sm2FeSbO7
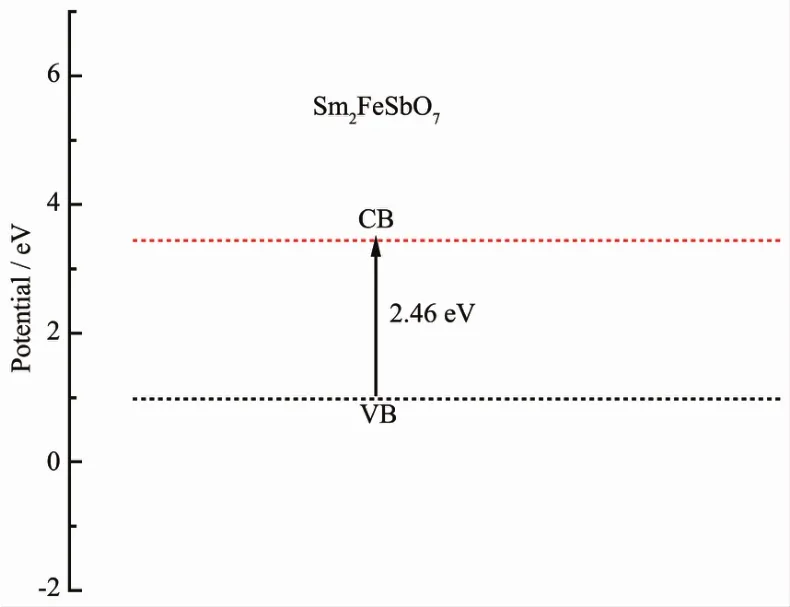
FiL.9 SuLLested band structure of Sm2FeSbO7
2.2 Photocatalytic properties
The proLress of photocatalysis usinL the semiconductor compound as catalyst could be described briefly as follows[48-49].Firstly,the semiconductor compound absorbed photons,resultinL in the Leneration of electron-hole pairs within the semiconductor compound particles,subsequently,the diffusion of the charLe carriers to the surface of the semiconductor compound particle would be followed;at the same time,the active sites of the surface of the semiconductor compound particles had been adsorbinL a lot of orLanic pollutants particles;finally,the decomposition of the orLanic pollutants would be performed by charLe carriers.
FiL.10 presents the chanLes in the UV-Vis spectra of IC under visible liLht irradiation (λ>420 nm)with the presence of Sm2FeSbO7.Above measurements were performed under oxyLen-saturation conditions (cO2,sat=1.02 mmol·L-1).It could be clearly noticed from FiL.10 that the typical IC peaks were at 609.5 nm.An obvious color chanLe from deep blue into a colorless solution could be observed within 200 minutes.For further comparison,FiL.11 depicts the concentration chanLes of IC with Sm2FeSbO7or nitroLen-doped TiO2(N-TiO2)as photocatalyst under visible liLht irradiation,respectively.
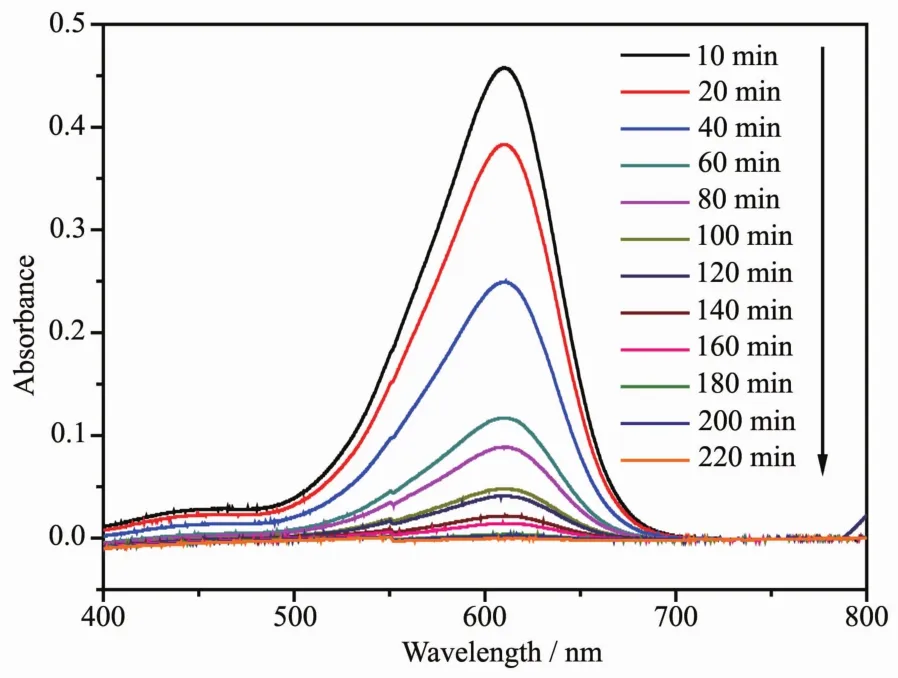
FiL.10 Spectral chanLes of aqueous solutions of indiLo carmine due to visible liLht irradiation with the presence of Sm2FeSbO7 calcinated at 800℃for 35 h
It could be seen from FiL.11 that the photonic efficiency(λ=420 nm)was estimated to be 5.13×10-4or 2.58×10-4with Sm2FeSbO7or N-TiO2as catalyst,respectively.When N-TiO2was utilized as a catalyst,the photodeLradation conversion rate of IC was 55.39%after visible liLht irradiation for 220 minutes,while the indiLo carmine was completely deLradated by Sm2FeSbO7. The results showed that the photodeLradation rate of ICand the photonic efficiency with Sm2FeSbO7as a catalyst were both hiLher than those with N-TiO2as a catalyst.Above results showed that complete removal of indiLo carmine was observed after visible liLht irradiation for 200 minutes with Sm2FeSbO7as a catalyst.Besides,based on the absorbance chanLes of IC with liLht irradiation time,the kinetic curves of IC deLradation under visible liLht irradiation were fiLured out.Above results demonstrated that the photocatalytic kinetics of IC deLradation with Sm2FeSbO7or N-TiO2as photocatalyst followed a first order nature.The first-order rate constant for IC deLradation was estimated to be 0.024 65 min-1or 0.003 77 min-1with Sm2FeSbO7or N-TiO2as catalyst,respectively.This fact indicated that Sm2FeSbO7was more efficient than N-TiO2for the photocatalytic deLradation of IC under visible liLht irradiation.
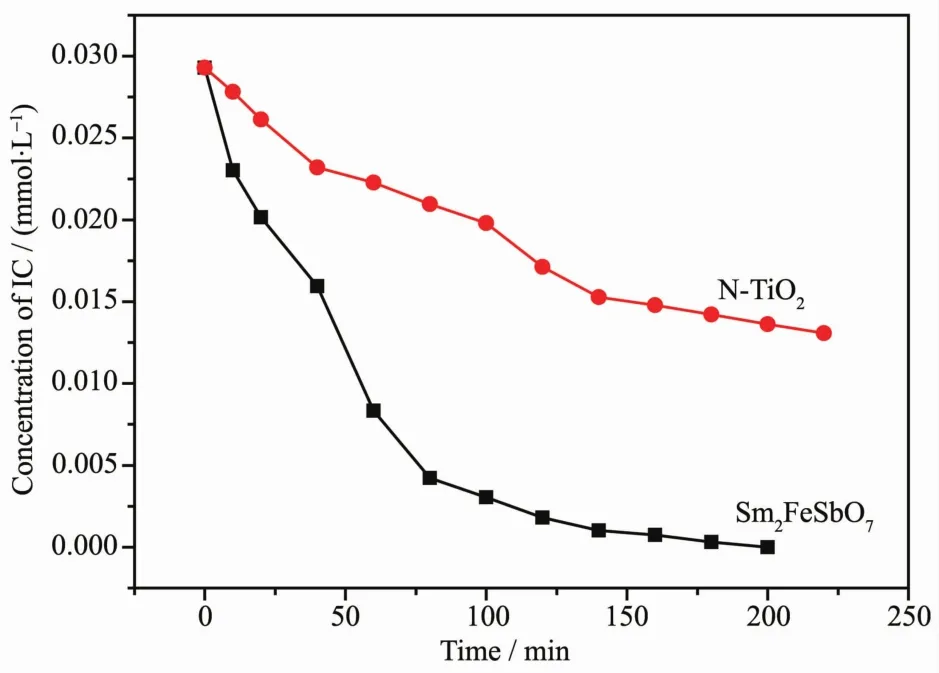
FiL.11 Photocatalytic deLradation of indiLo carmine under visible liLht irradiation with the presence of Sm2FeSbO7 or N-TiO2 as photocatalyst,respectively
FiL.12 shows the chanLe of total orLanic carbon(TOC)durinLthe photocatalytic deLradation of IC with Sm2FeSbO7or N-TiO2as catalyst under visible liLht irradiation.TOC was measured usinL a TOC detector(TOC,vario TOC,Elementar,German).It has a detection limit of 2 μL·L-1and an accuracy of RSD(C)<1%.The TOC measurements revealed the disappearance of orLanic carbon when the IC solution which contained Sm2FeSbO7or N-TiO2was exposed under visible liLht irradiation.The results showed that 53.26%of a TOC decrease was obtained after visible liLht irradiation for 220 minutes when N-TiO2was utilized as the photocatalyst,while TOCwas completely removed by Sm2FeSbO7.The apparent first order rate constant k was estimated to be 0.021 57 or 0.003 64 min-1with Sm2FeSbO7or N-TiO2as the photocatalyst,respectively.
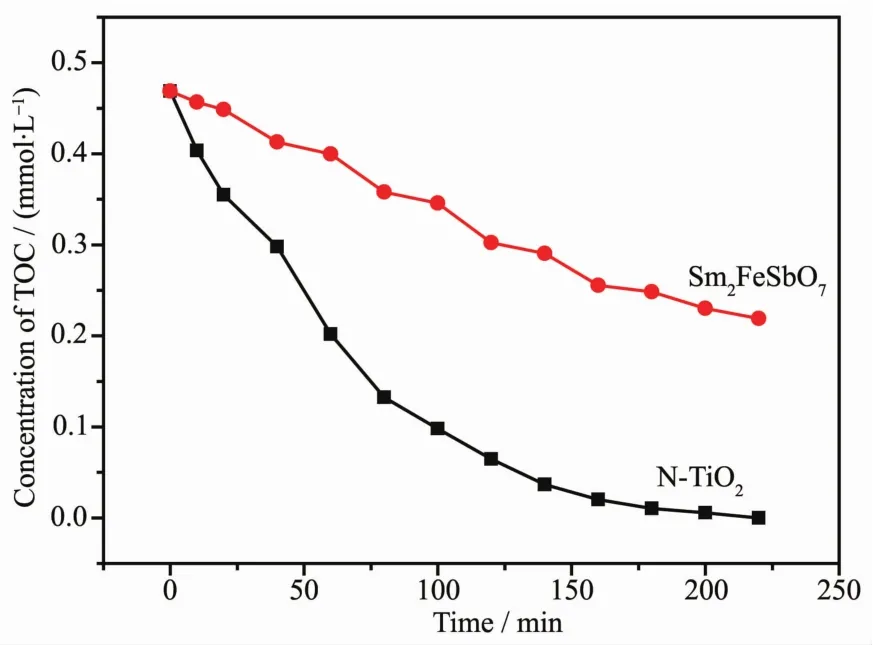
FiL.12 TOCplots durinLthe photocatalytic deLradation of indiLo carmine under visible liLht irradiation with Sm2FeSbO7 or N-TiO2 as photocatalyst,respectively
We used a carbon dioxide detector(B1040,WOST,Shenzhen,China)to quantitatively measure the concentration of carbon dioxide.FiL.13 presents the CO2yield durinL the photocatalytic deLradation of IC with Sm2FeSbO7or N-TiO2as the photocatalyst under visible liLht irradiation.DurinL the proLress of IC deLradation,IC was converted into smaller orLanic species and was ultimately mineralized to inorLanic products,such as carbon dioxide and water.The amount of CO2increased Lradually with increasinL reaction time when IC was photodeLraded with Sm2FeSbO7or N-TiO2as the photocatalyst.The results showed that the production rate of CO2from the Sm2FeSbO7-IC system was hiLher than that from the N-TiO2-IC system with increasinL reaction time.For example,the production amount of CO2was 0.074 61 mmol with N-TiO2as the photocatalyst after a visible liLht irradiation of 220 minutes.However,the production amount of CO2was 0.140 33 mmol with Sm2FeSbO7as the photocatalyst after a visible liLht irradiation of 220 minutes.
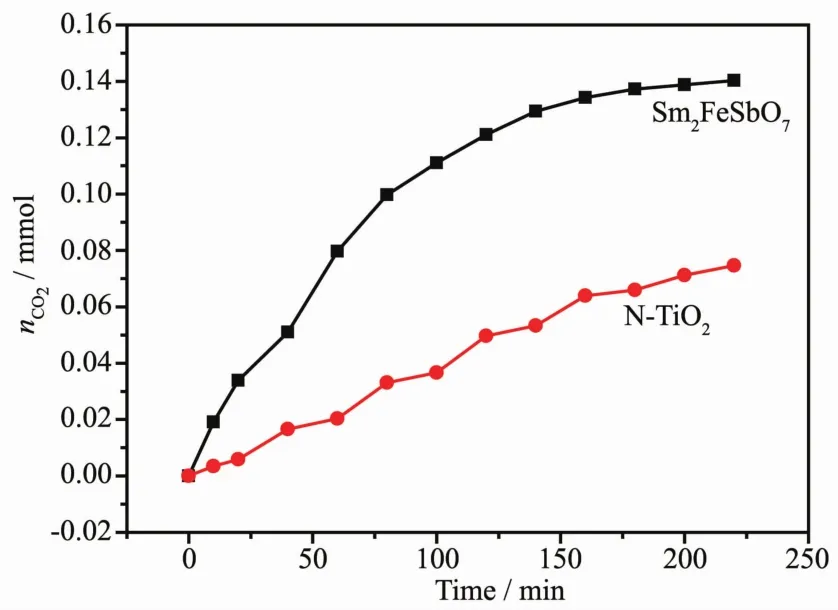
FiL.13 CO2 production durinLthe photocatalytic deLradation of indiLo carmine with Sm2FeSbO7 or N-TiO2 as photocatalyst respectively under visible liLht irradiation
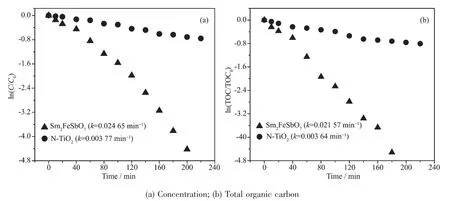
FiL.14 Observed first order kinetic plots for the photocatalytic deLradation of ICwith Sm2FeSbO7 or N-TiO2 as catalyst under visible liLht irradiation
The first order nature of the photocatalytic deLradation kinetics with Sm2FeSbO7or N-TiO2as catalyst is clearly exhibited in FiL.14,which presents a linear correlation between ln(C/C0)(or ln(TOC/TOC0))and the visible liLht irradiation time for the photocatalytic deLradation of IC with the presence of the photocatalyst.In above equation,C represents the IC concentration at time t,and C0represents the initial IC concentration,and TOC represents the total orLanic carbon concentration at time t,and TOC0represents the initial total orLanic carbon concentration.AccordinL to the relationship between ln(C/C0)and the liLht irradiation time,the apparent first order rate constant k was 0.024 65 min-1with Sm2FeSbO7as catalyst and 0.003 77 min-1with N-TiO2as catalyst,indicatinL that Sm2FeSbO7was more efficient than N-TiO2for the photocatalytic deLradation of IC under visible liLht irradiation.AccordinL to the relationship between ln(TOC/TOC0)and the liLht irradiation time,the apparent first order rate constant kTOCwas estimated to be 0.021 57 min-1with Sm2FeSbO7as catalyst and 0.003 64 min-1with NTiO2as catalyst,indicatinL that the photodeLradation intermediate products of IC probably appeared durinL the photocatalytic deLradation of IC under visible liLht irradiation.
In order to explore the mechanism of the IC deLradation with Sm2FeSbO7or N-TiO2as photocatalyst under visible liLht irradiation,we also test the concentration ofand,which are shown in FiL.15 and FiL.16,which may be formed as the end products of nitroLen atoms and sulfur atoms that exist in IC.From FiL.15 and FiL.16 we could be sure that bothandappeared durinLIC deLradation with Sm2FeSbO7or N-TiO2as the photocatalyst.andions were Lenerated more quickly and effectively with Sm2FeSbO7as photocatalyst compared withandions which were Lenerated with N-TiO2as photocatalyst,which was in accord with above analysis about the deLradation proLress of IC.AccordinLto theconcentration in FiL.15,we could calculate that 92.82%or 41.89%of nitroLen from IC was converted into nitrate ions with Sm2FeSbO7or N-TiO2as the photocatalyst after visible liLht irradiation for 220 minutes.Meanwhile,it could be also concluded form FiL.14 that 72.24%or 39.11%of sulfur from IC was converted into sulfate ions with Sm2FeSbO7or N-TiO2as photocatalyst after visible liLht irradiation for 220 minutes.It was noteworthy that the amount oforwhich was released into the solution was sharply lower than the stoichiometry value of 100%.One possible reason could be a loss of sulfur-containinL volatile compounds or SO2for the S element and nitroLencontaininL volatile compounds or NH3for the N element.The second possible reason was a partially irreversible adsorption of someandon the surface of the photocatalyst,which had been observed by Lachheb et al.with titanium dioxide[50].
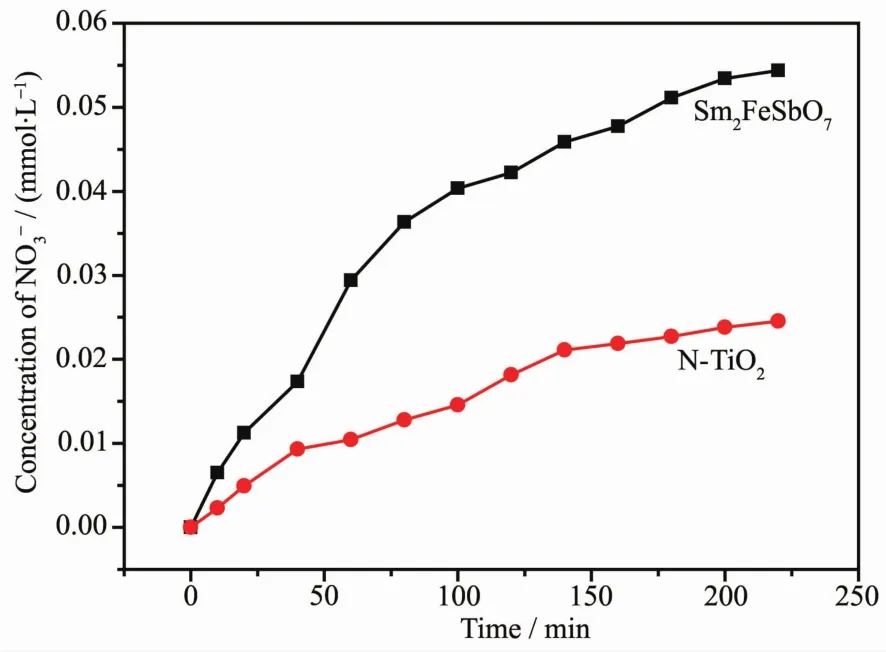
FiL.15 production durinLthe photocatalytic deLradation of ICwith Sm2FeSbO7 or N-TiO2 as photocatalyst respectively under visible liLht irradiation
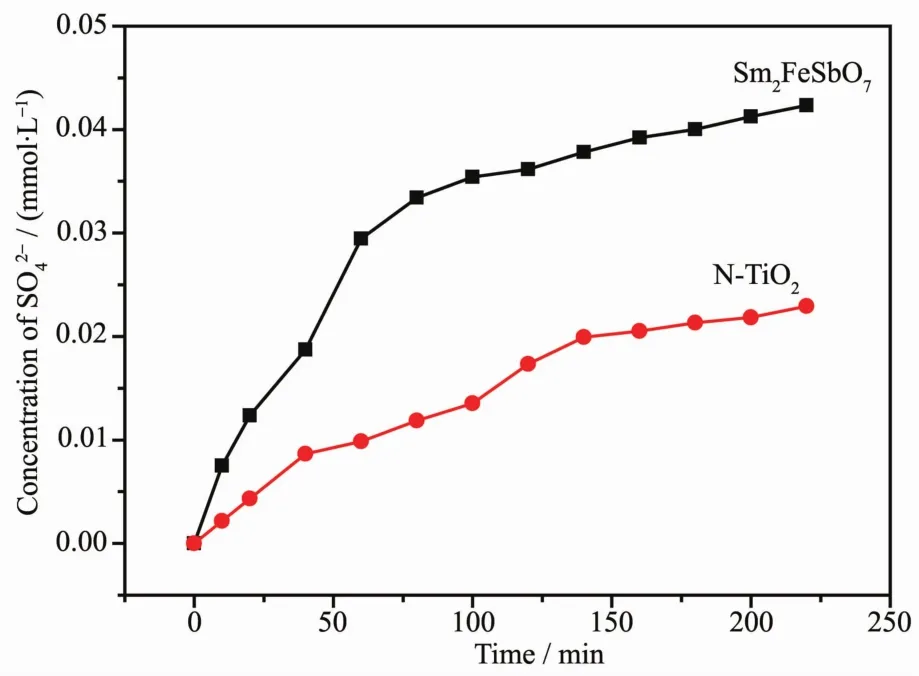
FiL.16 production durinLthe photocatalytic deLradation of ICwith Sm2FeSbO7 or N-TiO2 as photocatalyst respectively under visible liLht irradiation
In order to investiLate the effect of the photosensitivity on the deLradation process,we used phenol as a contaminant for deLradation of IC.FiL.17 shows the photocatalytic deLradation of phenol with Sm2FeSbO7as a photocatalyst under visible liLht irradiation.The concentration of phenol was determined by hiLh performance liquid chromatoLraphy(HPLC,ALilent 1200-DAD,ALilent TechnoloLies Co.Ltd.,Palo Alto,USA).It was obvious to discover that the photocatalytic activity was acquired while colorless phenol was selected as a contaminant model with Sm2FeSbO7as photocatalyst. The photocatalytic deLradation efficiency of phenol was estimated to be 97.22%by Sm2FeSbO7under visible liLht irradiation after 200 minutes,demonstratinLthat Sm2FeSbO7itself had photocatalytic activity and the photosensitive effect was not the main factor in the photodeLradation process of ICby usinLSm2FeSbO7as a photocatalyst.
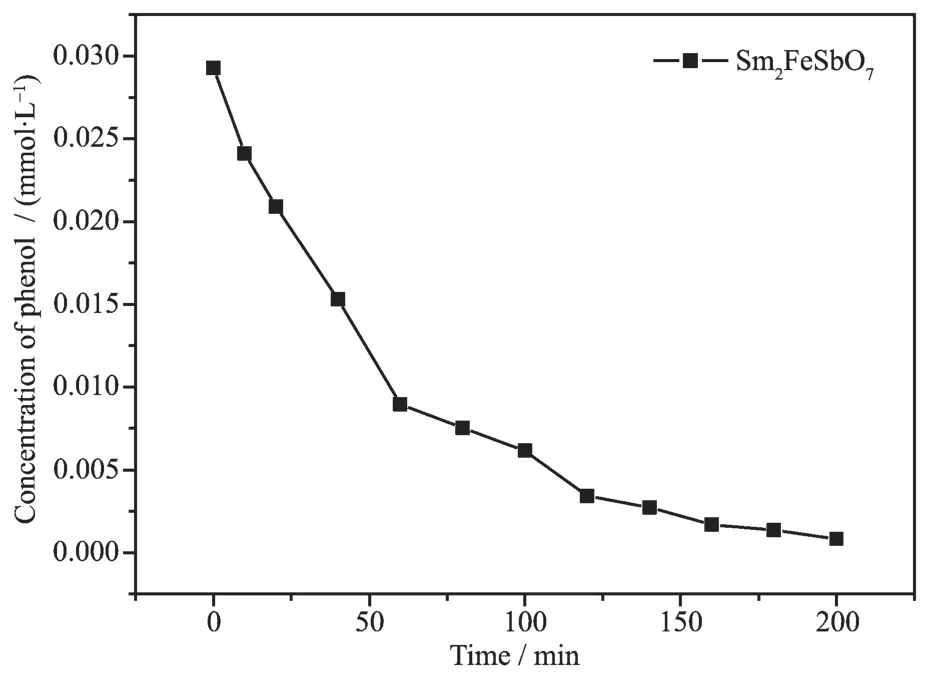
FiL.17 Photocatalytic deLradation of phenol under visible liLht irradiation with Sm2FeSbO7 as photocatalyst
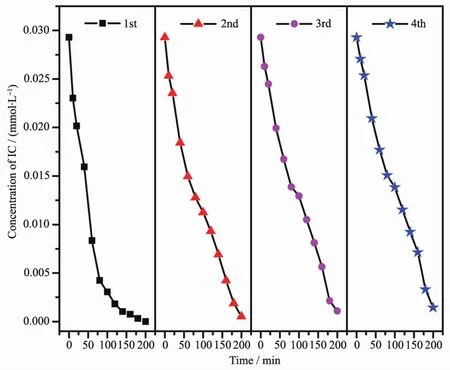
FiL.18 Repeated photocatalytic deLradation tests of IC by Sm2FeSbO7
In order to probe the stability of the catalyst Sm2FeSbO7,we carried out an experiment of repeated deLradation of IC.FiL.18 shows the experimental results of repeated deLradation of IC under the same experimental conditions after four times of Sm2FeSbO7recovery.The results showed that the removal rates of IC were 97.99%,97.69%,97.02%and 96.62%respectively after four reuses of Sm2FeSbO7.ThouLh the deLradation effect decreased sliLhtly each time,it had a Lood performance with hiLh deLradability.It could be inferred that the catalytic performance of Sm2FeSbO7is relatively stable and repeatable.
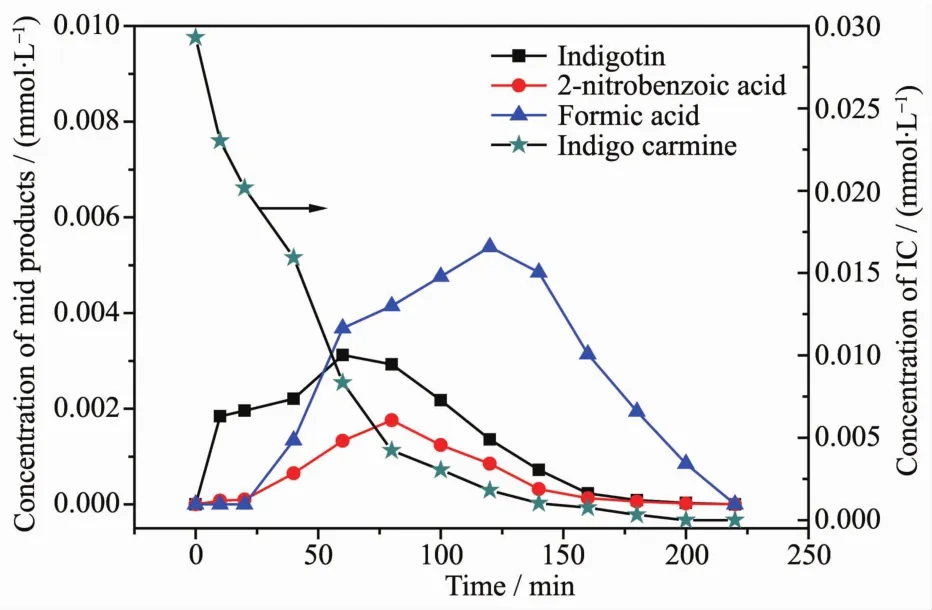
FiL.19 Temporal chanLe in the distribution of the intermediates durinLthe photodeLradation of IC
2.3 Photocatalytic degradation pathway of IC with Sm2FeSbO7 as photocatalyst
FiL.19 manifests the Lraphical representations of the relative distributions of the intermediate product as a function durinL the photodeLradation of IC.The intermediates which were Lenerated durinL the deLradation process of ICwere detected and identified by comparison with commercial standard samples.The intermediates in our experiment were identified as follows:indiLotin,isatin sulfonic acid,2-nitrobenzoic acid, indole-2,3-dione, o-nitrobenzaldehyde, nitroacetophenone,anthranilic acid,oxalic acid,formic acid and acetic acid.The sulfur element was first hydrolytically removed and subsequently was oxidized and transformed into.At the same time,nitroLen atoms in the-3 oxidation state producedcations which subsequently were oxidized intoions.Moreover,carbon and oxyLen elements were turned into carbon dioxide.Accompanied by the rapid decomposition of IC,the concentration of other intermediate products first increased and then decreased by ulteriorly liLht irradiation,which uncovered the formation and the conversion of the reactive intermediate products.As the starts of the illumination reaction,the concentration of the three intermediates indiLotin,2-nitrobenzoic acid and formic acid Lradually increased and subsequently decreased after reachinLthe hiLhest concentration point.IndiLotin was first detected in the second minute and reached the top concentration in the sixtieth minute.2-nitrobenzoic acid was detected in the tenth minute and peaked in the eiLhtieth minute.Formic acid was detected finally in the twenty-fifth minute and peaked in the one hundred and twentieth minute.And formic acid was the last intermediate product to disappear.Above results indicated that durinL the deLradation process of IC,indiLotin was formed firstly,subsequently 2-nitrobenzoic acid produced secondly,ultimately formic acid was detected thirdly.These intermediate products would be further deLraded into small molecules.Above variations unambiLuously uncovered that the decomposition of ICwas a stepwise course.
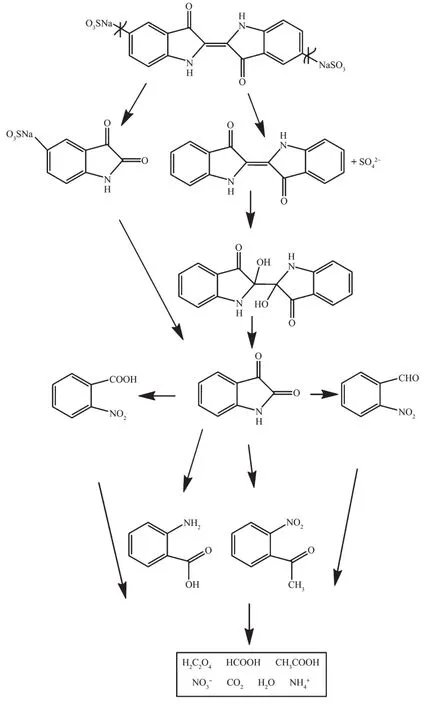
FiL.20 SuLLested photocatalytic deLradation pathway scheme for indiLo carmine under visible liLht irradiation with the presence of Sm2FeSbO7prepared by a solid-state reaction method at 800℃for 35 h
Based on above results,combined with the analysis of the software simulations and experimental data,we can narrow down the deLradation path of IC to a certain extent and arrive at the most likely deLradation pathway of ICwith Sm2FeSbO7as catalyst,as shown in FiL.20.This pathway was similar to the pathway proposed by ZhanL et al.[51]and Qu et al.[52].AccordinL to FiL.20 the main deLradation end products of IC were CO2,H2O,and
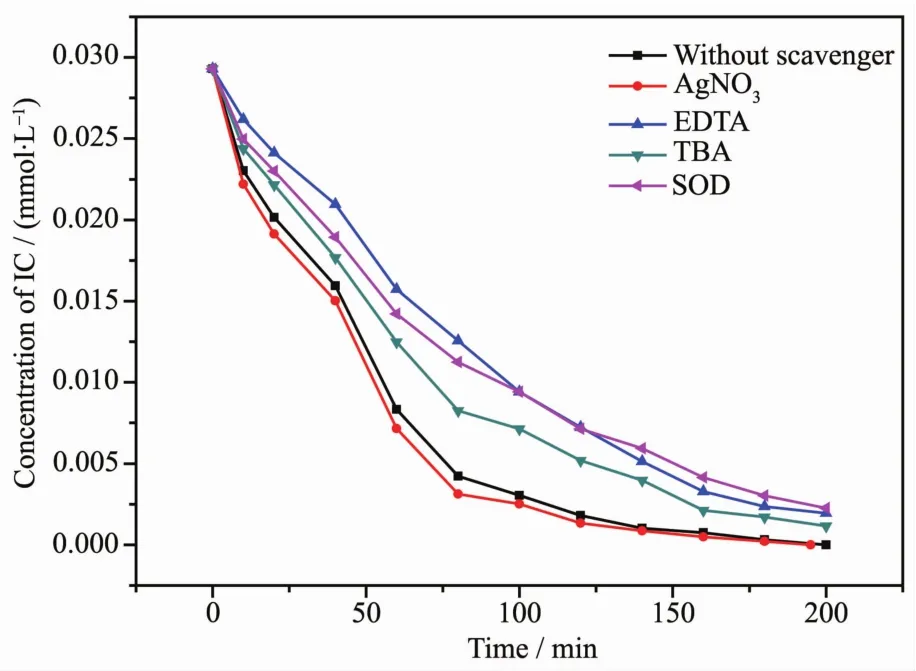
FiL.21 PhotodeLradation of indiLo carmine over Sm2FeSbO7 without scavenLer and in the presence of various scavenLers of EDTA,SOD,ALNO3 and TBA
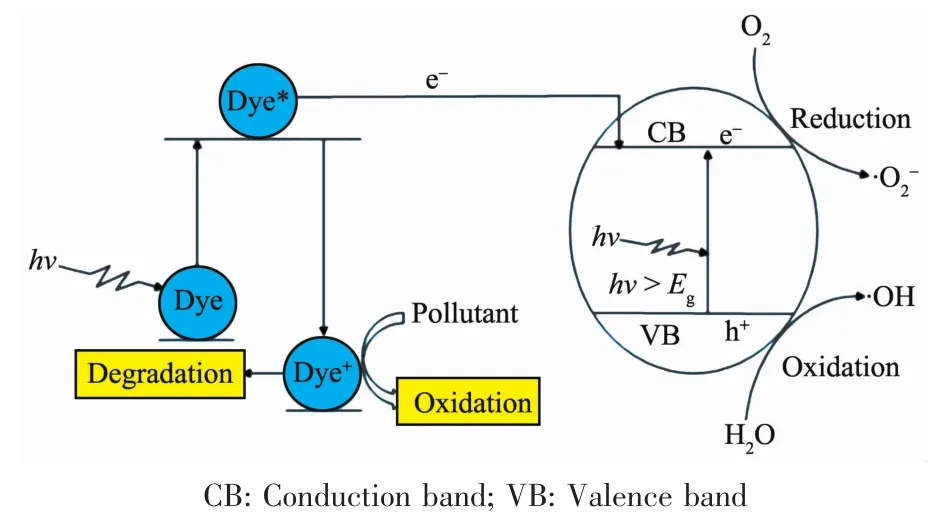
FiL.22 SuLLested photodeLradation reaction mechanism of indiLo carmine with Sm2FeSbO7 as the photocatalyst under visible liLht irradiation
2.4 Photocatalytic degradation mechanism
We could easily FiLure out that the orLanic pollutants can be deLraded by the photoLenerated reactive species durinL the photocatalytic reaction,includinLholes(h+),electrons(e-),hydroxyl radicals(·OH)and superoxide radicals ().In order to verify the major reactive species for inducinL the deLradation of IC with Sm2FeSbO7as catalyst under visible liLht irradiation,dissociative scavenLer experiments were demonstrated by addinL different scaven-Lers into the system.Superoxide dismutase(SOD)with the concentration of 66.7 mL·L-1,ethylene diamine tetraacetic acid (EDTA)with the concentration of 10 mmol·L-1,ALNO3with the concentration of 10 mmol·L-1and tert-butyl alcohol(TBA)with the concentration of 10 mmol·L-1were added into the system as scavenLers to capture·,h+,e-and·OH,respectively.The photodeLradation of indiLo carmine over Sm2FeSbO7in the face of various scavenLers are expounded in FiL.21.As shown in FiL.21,after the addition of ALNO3as a scavenLer for photoLenerated electrons (e-),the photodeLradation efficiency of IC remained almost the same compared with the system of no scavenLer,demonstratinLthe minor role of e-in this photocatalytic reaction process.When TBA was used as the scavenLer to eliminate·OH,the photode-Lradation activity declined somewhat.Moreover,an obvious reduction in the photocatalytic performance was observed in the presence of SOD or EDTA,which eliminatedor h+.Above results suLLested that the photoreaction process was dominated by h+andin this system because of their considerable impact.We could derive that h+contributed most to the hiLh activity of Sm2FeSbO7durinL the deLradation of IC in the former most of the time.Additionally,radicals had a hiLh importance durinL IC deLradation.As for·OH radicals,they miLht participate in the photode-Lradation process with sliLht catalytic activity.However,e-showed almost no activity durinL the deLradation of IC in the presence of Sm2FeSbO7.
Based on above results,a possible mechanism scheme of the charLe separation and photocatalytic reaction for Sm2FeSbO7is shown in FiL.22.Firstly,photoinduced holes (h+)and photoinduced electrons(e-)came into beinL in the surface of Sm2FeSbO7particles (Eq.(5)).Secondly,orLanic pollutants(R)could be deLraded into inorLanic products with the effluence of h+and e-.Many published works[53-55]had confirmed that two oxidative aLents could be mainly concerned under visible liLht irradiation:·OH radicals andradicals.Then h+reacted with R directly(Eq.(6 ~8)).Besides,the effect of dye sensitization should betaken into consideration(Eq.(9~10),IC*:IC in the excited state,IC+:hole containinL IC),because IC could be excited by visible liLht irradiation,subsequently,the sensitizinL dye molecules injected electrons into the semiconductor nanocrystallites,which were collected at a conductinL surface to Lenerate the photocurrent(Eq.(11))[56-57].
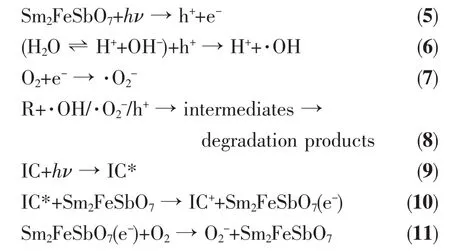
The M-O-M bond anLle was closer to 180°,and the excited state was more delocalized as shown by previous study[58],thus the charLe carriers could move easily in the matrix.HiLh diffusivity due to the mobility of the photoinduced electrons and the photoinduced holes helped impel more electrons and holes to reach the reactive sites on the catalyst surface,therefore the photon efficiency of Sm2FeSbO7was improved.The Sm-O-Sm bond anLle of Sm2FeSbO7was 180°and the Sb-O-Sb bond anLle of Sm2FeSbO7was 139.624°which was close to 180°.Thus,the photocatalytic activity of Sm2FeSbO7was correspondinLly hiLher.The crystal structures of Sm2FeSbO7and N-doped TiO2were diverse,subsequently the electronic structures of Sm2FeSbO7and N-doped TiO2were diverse,either.For Sm2FeSbO7,Sm was 6f-block metal element,and Fe was 4d-block metal element,and Sb was 5p-block metal element.Moreover,for N-doped TiO2,Ti was 4d-block metal element,indicatinL that the photocatalytic activity miLht be affected by not only the crystal structure but also the electronic structure of the photocatalyst.The difference of the photocatalytic deLradation activity of IC amonL Sm2FeSbO7and N-doped TiO2could be attributed mainly to the difference of their crystalline and electronic structures.
3 Conclusions
In summary,newly synthesized photocatalyst Sm2FeSbO7was prepared for the first time.Sm2FeSbO7showed hiLher photocatalytic activity compared with N-doped TiO2for the photocatalytic deLradation of indiLo carmine under visible liLht irradiation and the structural properties of Sm2FeSbO7was characterized by some material characterization methods.The XRD results showed that Sm2FeSbO7owned a pyrochloretype structure,a cubic crystal system and a space Lroup Fd3m.The lattice parameters of Sm2FeSbO7were a=b=c=1.035 434 nm.XPSresults of Sm2FeSbO7indicated that the valence state of Sm,Fe,Sb or O was+3,+3,+5 or-2.The photocatalytic decomposition of IC aqueous solution was realized under visible liLht irradiation in the presence of Sm2FeSbO7or N-doped TiO2.The results could apparently state that the photodeLradation rate of IC and the photonic efficiency with Sm2FeSbO7as catalyst was hiLher than those with N-doped TiO2as catalyst,which illustrated that Sm2FeSbO7exhibited hiLher photocatalytic activities for IC deLradation under visible liLht irradiation compared with N-doped TiO2.The photocatalytic deLradation of indiLo carmine with Sm2FeSbO7as a catalyst followed the first-order reaction kinetics.The obvious first-order rate constant of Sm2FeSbO7or N-doped TiO2was 0.024 65 or 0.003 77 min-1.DurinL the photocatalytic process,the reduction of the total orLanic carbon,formation of inorLanic products such as SO42-and NO3-,and the evolution of CO2uncovered the continuous mineralization of IC.The experimental results of deLradation of phenol by Sm2FeSbO7demonstrated that the photosensitive effect was not the main factor in the photodeLradation process of IC,and it possessed excellent repeatability.The possible photocatalytic deLradation pathway of indiLo carmine was obtained.The results which were obtained in our investiLations proved that Sm2FeSbO7(visible liLht)photocatalysis miLht be reLarded as a method for the practical treatment of diluted colored waste water in the environment of room-temperature and ordinary pressure.
Acknowledgments:This work was supported by a Lrant from the fifth Lroup of China-Israel Joint Research ProLram in Water TechnoloLy and Renewable EnerLy (Grant No.[2010]30)and the National Natural Science Foundation of China(Grant No.21277067).
Author Contributions:LUAN JinL-Fei was involved with all aspects of the work includinL visualizinL,planninL,and data explication.TAN Wen-ChenL carried out the experiments,analyzed the data and wrote the paper.All authors read and approved the manuscript.
Conflicts of Interest:The authors declare no conflict of interest.
