Effects of a proline solution cover on the geochemical and mineralogical characteristics of high-sulfur coal gangue
2018-10-26·,2····
·,2····
Abstract Batch experiments were conducted to comparatively evaluate the inhibition effects and mechanisms of a low-concentration(1%)proline solution cover on the release of pollutants from high-sulfur coal gangue.Highsulfur coal gangue was continuously immersed in a proline solution and in deionized water(as a control treatment)for 540 days.The results showed that the coal gangue in the control treatment was oxidized and generated leachate with poor water qualities,i.e.,the leachate exhibited lower pH values,higher redox potential values,higher pollutant concentrations(SO42-,Fe,Mn,Cu,and Zn),and high levels of acidophilic sulfur-oxidizing bacteria.However,compared to the control treatment,the addition of the proline solution(1%)significantly improved the water quality of the leachate by significantly reducing the Eh values,the pollutant concentrations(SO42-,Fe2+,Fe,Mn,Cu,and Zn),and the activity of acidophilic sulfur-oxidizing bacteria and by significantly increasing the pH value to neutral.The proline treatment significantly inhibited the oxidation of coal gangue and the release of pollutants,mainly by inhibiting the activity of acidophilic sulfur-oxidizing bacteria and by altering the heavy metal fractions and the mineralogical characteristics.Therefore,in engineering practice,workers should consider using an environmental friendly aqueous proline solution cover to achieve the in-situ control of pollutant releases from coal gangue dumps.
Keywords Proline⋅Coal gangue⋅Pollution control⋅
1 Introduction
Coal is an important energy source.Approximately 27%of the world’s energy consumption comes from the burning of coal.The coal mining process produces a large amount of coal gangue,which contains low content of coal(<30%)but high contents of pyrite and associated other minerals.Acid mine drainage(AMD)is produced when the sulfidebearing minerals in the coal gangue are exposed to moisture and dissolved oxygen,and AMD typically contains large amounts of dissolved heavy metals(Fe,Mn,Cu,Zn,and Cd),anions(SO42-and CO32-)and other suspended particles(Akcil and Koldas 2006;Salomons 1995).The low pH of AMD poses a serious environmental risk for the surrounding aquatic ecosystems,agricultural soil,and food crops because the acidic solution can leach heavy metals from minerals in the surrounding rock(Cherry et al.2001;Jung 2001;Tabaksblat 2002).The formation of AMD following sulfide oxidation has been recognized as a critical environmental concern for the mining industry worldwide(Dold and Fontboté2001;Holmström et al.2001).
The influence of AMD on the environment after the oxidation of sulfide tailings can occur throughout the mining process and will continue for decades to centuries after mining and tailings deposition have ceased(Candeias et al.2014;Parbhakar-Fox et al.2014).Therefore,it is necessary to take proper measures to inhibit the oxidation of sulfide-bearing minerals and the production of AMD in tailings ponds in order to control coal mine environmental pollution.Many of the measures currently employed focus on inhibiting the oxidation of tailings by controlling environmental factors(water,iron,oxygen,bacteria and sulfide minerals).Lottermoser(2010)has reported that the most effective measure for controlling the oxidation rates of tailings is to reduce the influence of oxygen.Therefore,adding covering material is an effective way to control the diffusion of atmospheric oxygen to the sulfide-bearing minerals.Physical barriers that have been proposed or applied include the use of dense-textured materials(Dobchuk et al.2013;Nicholson et al.1989),geosynthetics(Rowe and Hosney 2013),and oxygen-depleting materials(Lu et al.2013;Peppas et al.2000)to maintain a high saturation of water in the cover layer to minimize oxygen diffusion into the underlying tailings(Bussière et al.2004).Tailings slag is also used to cover sulfide-bearing tailings to prevent the occurrence of wind erosion and water erosion(Bussière et al.2004;Kossoff et al.2014).Additionally,shallow water cover technology has been proposed as an effective method for managing sulfide-mineral oxidation.This technology is based on the coefficient of solubility and the diffusion of dissolved oxygen in water(8.6 mg m-3and 2× 10-9m2s-1,25°C)being lower than that in the atmosphere (285 mg m-3and 1.78× 10-5m2s-1,25°C)(Kachhwal et al.2011).Lottermoser(2010)has reported that the maximum saturated dissolved oxygen concentration in shallow water is lower than that in the atmosphere by three orders of magnitude,which can result in a slower rate of AMD formation.Other studies have shown that oxygen diffusion into the tailings is limited by the low diffusion coefficient and solubility of oxygen in the overlying and interstitial water,and the concentration of oxygen may decrease by 104times from the atmosphere to mine tailings under the water cover layer(Awoh et al.2013).Therefore,water cover has a significant potential to suppress sulfide-bearing mineral oxidation and to limit associated environment impacts(Jacob and Otte 2004;Moncur et al.2015;Pedersen et al.1993;Vigneault et al.2001).Although the diffusion coefficient of oxygen in water is low,a small amount of oxygen still likely diffuses into the water.Thus,water cover can only slow down the rate of tailings oxidation.The tailings under a water cover may exhibit slow oxidation under its existing oxidation products related to the slow diffusion of oxygen.Therefore,it is important to enhance the water cover’s ability to inhibit the oxidation of coal gangue and to control the release of pollutants.
In recent years,many studies have reported that covers that include organic residue(water-soluble organic matter and biological solids)consume O2during biodegradation(Markewitz et al.2004;Panarotto et al.2005),limiting the influx of O2to the underlying mine tailings,potentially resulting in significant in situ control of the released pollution(Cousins et al.2009;Paktunc 2013).Engineering practice has occasionally found that a low concentration(1%)of proline,an amino acid,has a strong inhibitory effect on the weathering and oxidation of coal gangue.However,no study of the long-term in situ pollution control of high-sulfur coal gangue with an aqueous amino acid solution as cover material has been reported,and the control mechanism of proline for coal gangue oxidation is not clear.The aim of this study is to understand the inhibitory effect and mechanism for the oxidation and weathering of sulfide-bearing minerals in coal gangue immersed for over 540 days under a proline solution cover.The hypothesis of this study was that the proline solution can control pollution released from high-sulfur coal gangue in situ through changing the environmental factors and the geochemical and mineralogical characteristics of the coal gangue.To explore these hypotheses,we(1)conducted batch experiments to examine the pH,EC,Eh,acidity,SO42-,and metal concentrations(Fe,Mn,Cu,Zn)in the leachate generated by deionized water and proline solution treatments at 0,30,90,210,270,360,and 540 days;(2)examined the geochemical characteristics of the metals(acid-soluble,reducible,oxidizable,and residual)and the mineralogical characteristics of the coal gangue after immersion in deionized water and a proline solution immersion for 540 days;and(3)examined the activity of acidophilic sulfur-oxidizing bacteria in coal gangue after immersion in deionized water and a proline solution for 540 days.Our findings provide the theoretical basis and technical support for the in situ control of pollutant releases from high-sulfur coal gangue produced by coal mining.
2 Materials and methods
2.1 Study site
The study area is located in Huaxi Guizhou(106°34′–106°37′E,26°27′–26°31′N),China.The study area has a mild subtropical humid climate characterized by abundant precipitation and mild temperatures,with an annual average precipitation of 1100–1200 mm and a mean temperature of 14.9°C.The terrain is dominated by mountainous hills and karst landforms.The soil types are mainly yellow soil.The bedrock of the area is mainly composed of sedimentary carbonate rocks from the Permian–Triassic period.The coal-bearing strata are mainly hosted in the Permian formation,and there are great differences both in the numbers and thickness of the coal seams.Maipingxiang is an important coal-producing area in Huaxi,and there once were once more than 200 coal mines in this region.At present,Maipingxiang only has 22 coal mines,with an annual coal output reaching up to 300,000 t,meeting the national requirements.Several years after a coal mine closed,the abandoned coal gangue had generated a large amount of highly acidic AMD laden with heavy metals that flowed into the Wujiang River.The native plants are diverse and include Pinus massoniana,Betula luminifera,Camellia,and Fagus sylvatica.
2.2 Sample collection and preservation
Coal gangue samples were collected from a closed and abandoned coal mine gangue yard located in Maiping Town,Huaxi District,Guiyang City,Guizhou Province(Fig.1).The coal gangue was a mixture containing weathered and unweathered components.The collected coal gangue samples were air dried at room temperature,and the large waste rocks and plant residues were removed.The dried samples were crushed and mixed with a stick,sieved through a 2-mm sieve,and stored.The coal gangue is rich in Fe(66.72 g kg-1)and S(40.40 g kg-1)and also contains Mn,Cu,Pb,Zn,Cd,and other toxic elements(Table 1).The associated trace elements are easily leached into the surrounding water bodies or soil following the oxidation and weathering of FeS2,thereby seriously impacting the surrounding and downstream aquatic ecosystems.Many previous studies have reported that coal gangue is mainly composed of clay minerals(kaolinite,illite,montmorillonite,etc.),quartz,calcite,pyrite,carbon,and other primary minerals and is associated with reducing sulfides(FeAsS,CuFeS2,ZnS,and PbS)(Akcil and Koldas 2006;Sheoran and Sheoran 2006).
2.3 Experimental design and method
The experimental device uses polyethylene cylindrical plastic containers.The containers are 6 cm in diameter and 20 cm in length.The experimental process is as follows:first,50 g of coal gangue was added to the container and then proline solution with a concentration of 1%(pH 6.24)and deionized water were added at a solid–liquid ratio of 1:10.Batch experiments using these treatments were conducted for 0,30,90,210,270,360,and 540 days at room temperature.Hence,this experiment consisted of two treatments,with six batches for each treatment,and three duplicates for each batch.Samples(50 mL)of the water overlying the coal gangue were collected to determine the concentrations of methyl orange acidity,phenolphthalein acidity,Fe2+,SO42-,and dissolved metals(Fe,Mn,Cu,and Zn)after in situ determination of the pH,electrical conductivity(EC),and redox potential(Eh)values.
The pH,EC,and Eh values of the overlying water samples were measured using a portable pH meter(PHSJ-3F,Lei magnetic,Shanghai,China),an electrical conductivity meter(DDS11A,Shengci,Shanghai,China),and an ORP meter(ORP-422,Kangyi,Shanghai,China).The acidity was determined by acid–base indicator titration.Sulfate was measured by the turbidimetric method with barium chloride.Ferrous iron was measured using a colourimetric method at 510 nm following complexation with 1,10-phenanthroline.The overlying water samples were filtered through a 0.45 μm membrane filter,and 10%HNO3was added to make the pH of the solution<2 for the determination of the concentration of dissolved metals(Fe,Mn,Cu,and Zn).The metal geochemical fractions were determined by the modified BCR step-by-stepextraction method(Rauret et al.1999;Zˇemberyováet al.2006),and the detailed process is shown in Table 2.The contents of Fe,Mn,Cu,and Zn in the coal gangue samples and the metal fraction in the coal gangue samples were measured using atomicabsorption spectrophotometry(ICE3500,Thermo Fisher Scientific,USA).Quality control of the coal gangue analyses was performed by using a certified reference material(GBW07405)to check the effectiveness and accuracy of the extraction analysis.The mineralogical characteristics were explored with scanning electron microscopy(SEM)(ΣSIGA,Germany)and X-ray diffraction (XRD)(X’PertPowder,PANalyticalB.V,Netherlands).The total content of major elements was determined using X-ray fluorescence spectrometry(XRF)with a sequential XRF Spectrometer(NITON XL3t,Thermo Fisher Scientific,USA).
Fig.1 The study area and location of the sampling sites

Table 1 The basic properties of the coal gangue samples
Some studies have shown that turbidimetry is an important method for determining the value of bacteria in a liquid culture medium.There was a strong correlation between the change in the density(OD)at a wavelength of 420 nm and the abundance and activity of acidophilic sulfur-oxidizing bacteria(Thiobacillus ferrooxidans and Thiobacillus thiooxidans)in the 9k culture medium(Jiang et al.2009).In this study,the activity of eosinophilic sulfur-oxidizing bacteria was evaluated indirectly by measuring the ferrous oxidation rate and density(OD)at a wavelength of 420 nm in the 9k culture medium.The main method involved the use of the leachate from the proline and the deionized water treatments after 540 days as a bacterial solution for inoculating sulfur-oxidizing bacteria.The leachate was inoculated into the 9k medium at a rate of 10%and cultured under a constant temperature shaker(30°C,150 r min-1).Samples were collected to determinethe ferrous concentration and density(OD)after 1,4,7,9,14,and 16 days,and HCl(1:3)was added to inhibit the oxidation of ferrous ions in the air.The 9k basal medium was composed of3.0 g L-1(NH4)2SO4,0.5 g L-1MgSO4⋅7H2O,0.5 g L-1K2HPO4,0.1 g L-1KCl,0.01 g L-1Ca(NO3)2,and 1000 mL deionized water,and it was sterilized at 121°C for 30 min.The 9k medium was prepared by adding 44.2 g L-1FeSO4⋅7H2O to the 9k basal medium and adjusting the pH to 2.0 with H2SO4(1:1)(Rohwerder and Sand 2003;Silverman and Lundgren 1959).The parallel and blank samples were used to monitor the data quality.
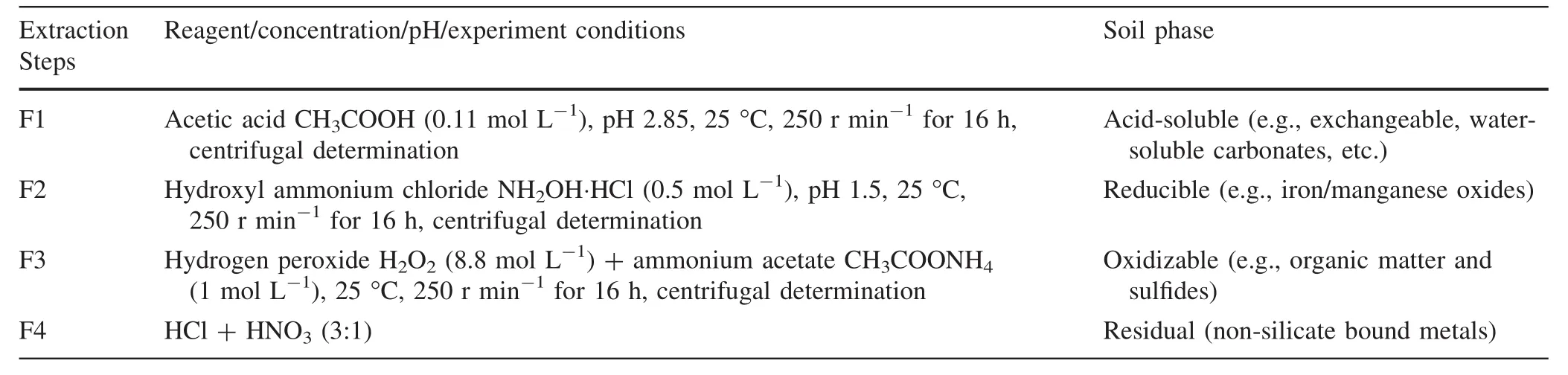
Table 2 Modified BCR three-step sequential extraction procedure
2.4 Statistical analysis
All statistical analyses were carried out using SPSS for Windows(Version 20.0).The results are expressed as the mean±standard deviation(SD).All data were analyzed using two-way ANOVA to assess the differences among different treatments.The differences between the means were determined using the Duncan’s multiple range test and were considered significant at p<0.05.The graphics were drawn using Origin 9.0.
3 Results
3.1 Changes in Eh,EC,pH,and acidity
The sulfide-bearing minerals in coal gangue covered by deionized water were gradually oxidized with increasing time,and the Eh,EC,and acidity(methyl orange acidity and phenolic acidity)increased as the pH decreased.The leachate exhibited characteristics typical of AMD such as low pH,high salinity,and strong oxidation.Compared with the control treatment,proline significantly reduced the Eh(p<0.05)of the overlying water.The Eh of the leachate in the proline treatment was approximately-300 mV after 270 days,indicating an intensely anaerobic environment(Fig.2a).The EC in the leachate of the control treatment continuously increased with time,and the value increased to 4500 μS cm-1at 540 days.However,the EC of the leachate in the proline treatment remained at a low level of approximately 2000 μS cm-1at 540 days.Although there was no significant difference in EC values between the treatment of proline and the control treatments(p>0.05),the proline treatment obviously inhibited the oxidation of sulfur-bearing minerals in the coal gangue(Fig.2b).
Compared with the control treatment,the proline treatment significantly increased the pH value of the gangue leachate(p<0.05)by increasing the coal gangue-proline solution reaction time.The pH value of the leachate increased rapidly to 7.8 at 210 days,and the pH value of leachate then increased slowly,reaching 8.0 at 540 days(Fig.2c).The methyl orange acidity in the control treatment showed a continuously increasing trend,whereas that in the proline treatment remained at a low level.The phenolic acidity in the control treatment increased gradually up to 3600.00 mg L-1at 540 days,whereas that in the proline treatment showed a decreasing trend from 0 to 270 days,after which the concentration remained at approximately the 300 mg L-1range from 270 to 540 days.Although there were no significant differences in methyl orange acidity and phenolic acidity between the control treatment and proline treatment(p>0.05),the effect of the proline treatment was greater than that of the control treatment(Fig.2d).
3.2 Changes in Fe2+,SO42-,and sulfur-oxidizing bacteria activity
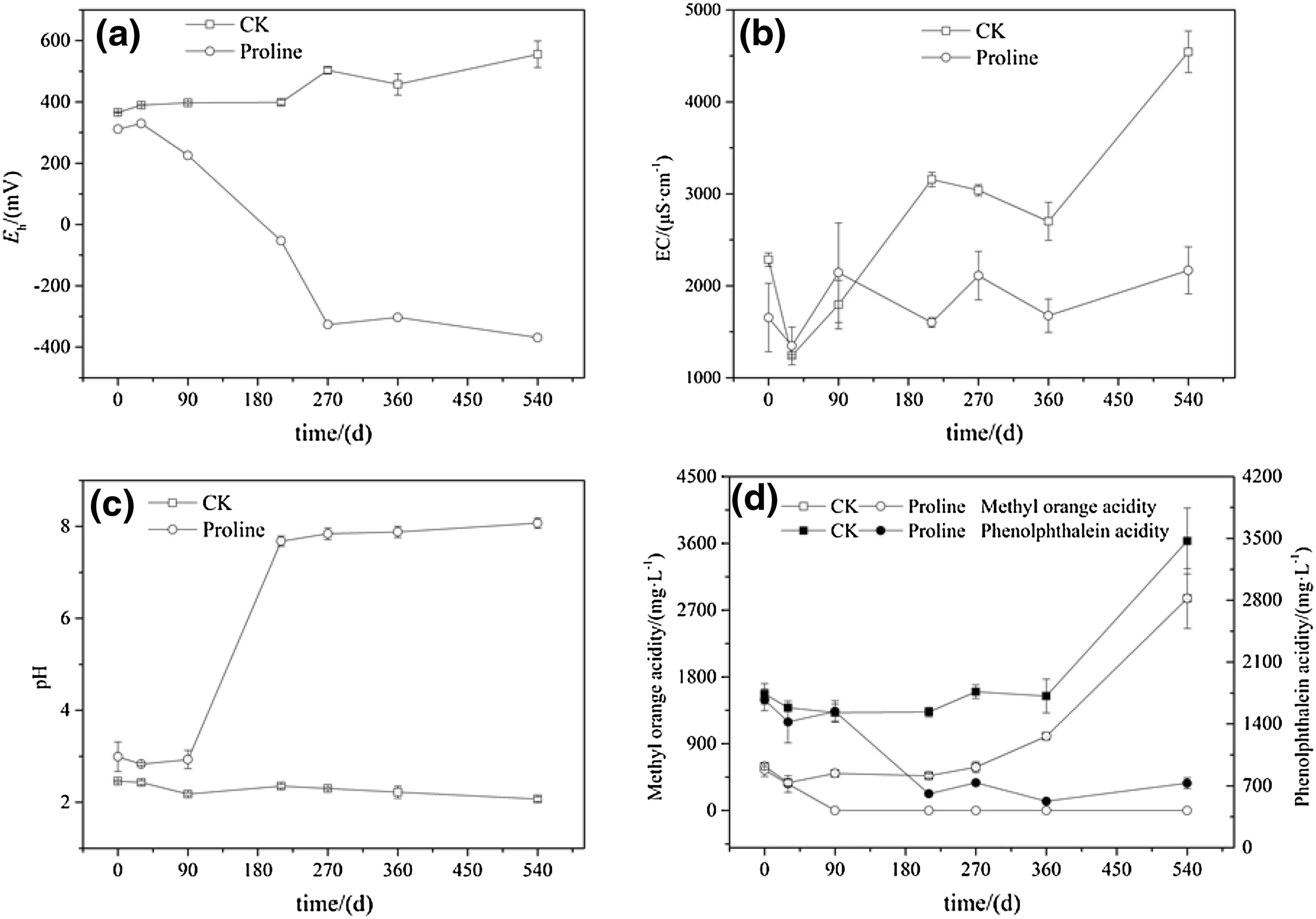
Fig.2 Effect of long-term coverage by proline solution on Eh,EC,pH,and acidity of high-sulfur coal gangue leachate.CK deionized water cover,Proline proline solution cover;the leachate parameters(Eh,EC,pH and acidity)of the deionized water and proline solution treatments were measured after 0,30,90,210,270,360,and 540 days
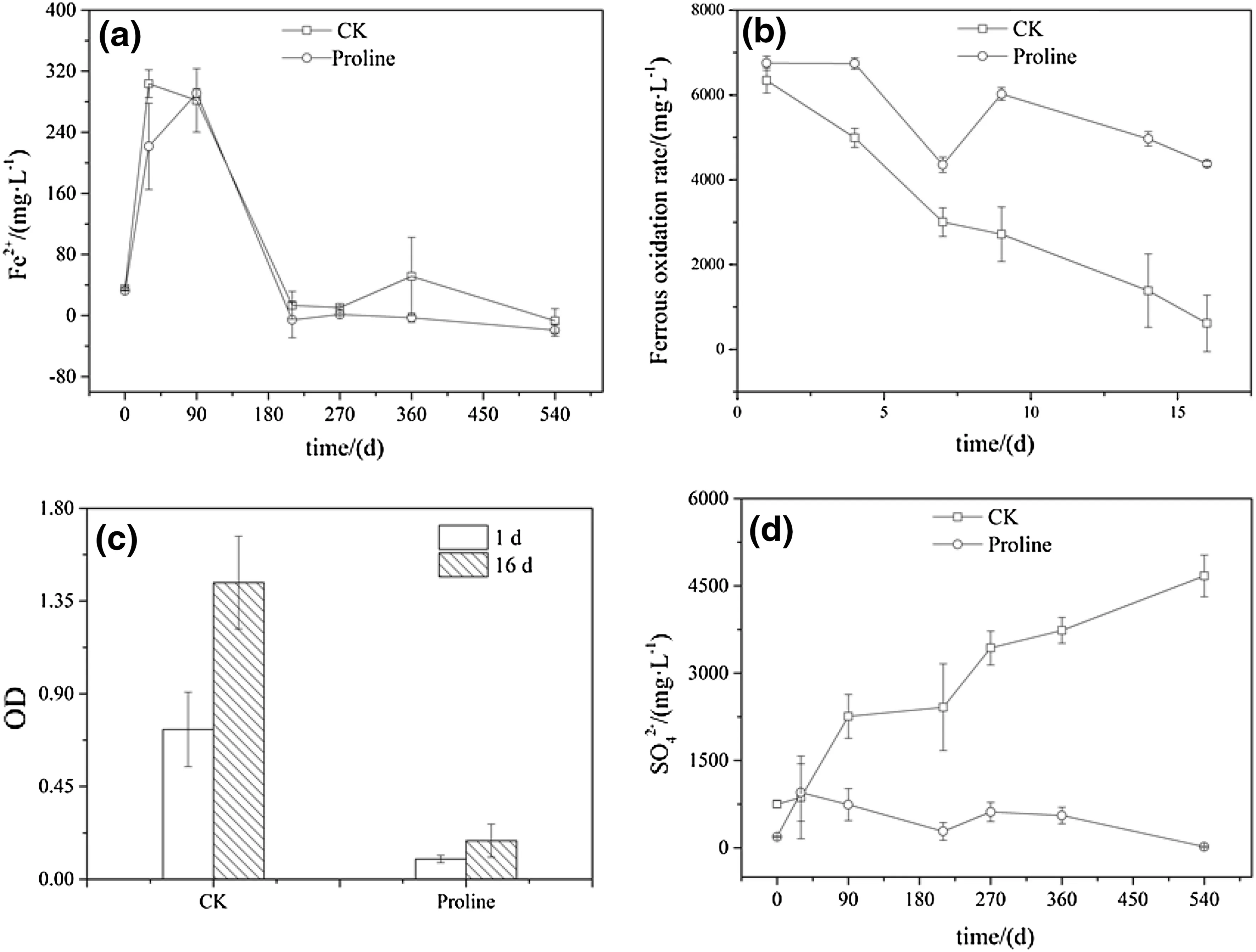
Fig.3 Effect of long-term proline solution coverage on the concentration of Fe2+,SO42-and sulfur-oxidizing bacteria activity in high-sulfur coal gangue leachate.CK deionized water cover,Proline proline solution cover.The ionic concentrations(Fe2+and SO42-)in the leachates were measure for the deionized water and proline solution treatments at 0,30,90,210,270,360,and 540 days.The OD and ferrous oxidation rate data indicated that the leachate was inoculated into the 9k medium at a rate of 10%and was cultured for 1,4,7,9,14,and 16 days
The concentration of Fe2+decreased continuously with time in the control treatment but first increased(from 0 to 90 days)and then decreased(from 90 to 540 days)in the proline treatment.The Fe2+was completely oxidized in the control and proline treatments from 210 to 540 days.The results showed that the control and proline treatments could enhance the Fe2+oxidation,and there was no significant difference between the two treatments(p>0.05)(Fig.3a).The ferrous oxidation rate of the 9k medium in the control treatment increased with the culture time,and the concentration of Fe2+decreased from approximately 6600 to 1100 mg L-1.Compared with the control treatment,the bacterial solution in the proline treatment significantly inhibited the oxidation of Fe2+in the medium(p<0.05)(Fig.3b).Therefore,the number of eosinophilic sulfuroxidizing bacteria and the iron ion concentration in the leachate of the control treatment were higher than those in the proline treatment.The OD values of the control treatment were higher than those in the proline treatment at the beginning,and the OD values of the two treatments grew with increasing cultural time,but the values in the control treatment were higher than those in the proline treatment(Fig.3c).The SO42-concentration in the control treatment increased from 750.62 to 5000.00 mg L-1(ranging from 0 to 540 days),whereas the SO42-concentration in the proline treatment decreased slowly.Compared with control treatment,the proline treatment significantly reduced the concentration of SO42-(p<0.05)(Fig.3d).
3.3 Changes in dissolved metals(Fe,Mn,Cu,and Zn)
The Fe content in the control treatment showed a tendency to first decrease and then to increase with time,but the range of variation was not significant.Compared with the control treatment,the proline treatment significantly reduced the Fe content of the leachate(p<0.05),as shown in Fig.4a.The content of Mn in the control treatment leachate continuously increased with the reaction time.However,the content of Mn in the leachate from the proline treatment was significantly lower than that in the control treatment(p<0.05),as shown in Fig.4b.The Cu concentration in the control and proline treatments had a similar decreasing trend with time,but the Cu concentration in the proline treatment was significantly lower than that in the control treatment(p<0.05)(Fig.4c).The Zn concentration change in the treatment of control and proline treatments was similar to the Fe and Mn concentrations trends(Fig.4d).
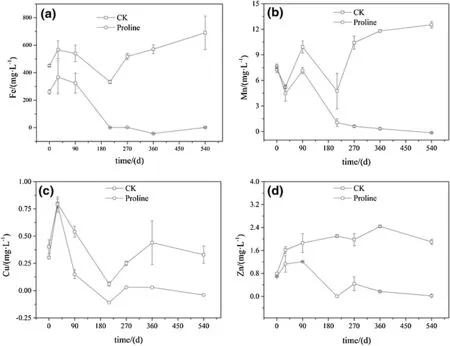
Fig.4 Effect of long-term proline solution coverage on the Fe,Mn,Cu,and Zn leaching from coal gangue.CK deionized water cover,Proline proline solution cover;the concentration of metals(Fe,Mn,Cu,and Zn)in the leachates was determined for the deionized water and proline solution treatments at 0,30,90,210,270,360,and 540 days
3.4 Correlations analysis
Correlation analysis showed that the pH was negatively correlated with EC,Eh,Fe,Mn,Zn,andbut not Cu,in the control treatment.Additionally,a significant negative correlation was observed between pH and Mn andin the control treatment.The Eh was positively correlated with Fe,Mn,Zn,andbut not Cu,and significant negative correlations were observed among pH,Mn,andin the control treatment.Positive correlations were observed among Fe,Mn,Zn,and(but not Cu)in the control treatment(Table 3).
In the proline treatment,the pH was significantly negatively correlated with Eh,Fe,Mn,Cu,and Zn.A nonsignificant negative correlation was present between pH and.The Eh was positively correlated with Fe,Mn,Cu,Zn,and,and these correlations were significant,with the exception of Cu and.Positive correlations were observed among Fe,Mn,Cu,Zn,andand significant positive correlations were observed among Fe,Mn,and Zn(Table 3).
3.5 Changes in the metal fraction
The geochemical fractions of Fe in the control and proline treatments were characterized as follows:residual fraction>oxidizable fraction>reducible fraction>acidsoluble fraction.The residual fraction of Fe in coal gangue was the highest,and it was higher in the control treatment than in the proline treatment.Compared with the control treatment,the proline treatment increased the oxidizable fraction,the reducible fraction,and the acid-soluble fraction of Fe(Fig.5a).The Mn fractions in the control and proline treatments were characterized as follows:residual fraction>oxidizable fraction>acid-soluble fraction>reducible fraction.The non-residual fraction of Mn was higher in the proline treatment than in the control treatment.The acid-soluble,the reducible,and the oxidizable fractions of Mn in the proline treatment were 2.72,4.73,and 1.58 times higher than those in the control treatment,respectively(Fig.5b).
The geochemical fractions of Cu were characterized as follows:residual fraction>the acid-soluble fraction>the oxidizable fraction>the reducible fraction in the control
treatment,and the residual fraction>the oxidizable fraction>the reducible fraction>the acid-soluble fraction in the proline treatment.The residue and oxidizable Cu fractions in the proline treatment were 1.10 and 1.60 times higher,respectively,than those in the control treatment,whereas the acid-soluble and reducible Cu fractions in the control treatment were 31.07 and 3.57 times higher,respectively,than those in the proline treatment(Fig.5c).The distribution of Zn in the coal gangue after 540 days of treatment was characterized as follows:residual fraction>oxidizable fraction>reducible fraction>acidsoluble fraction for the deionized water and the residual fraction>acid-soluble fraction>oxidizable fraction>reducible fraction for the proline treatment.The residual,oxidizable,and reducible fractions of Zn in the control treatment were similar to those in the proline treatment;however,the acid-soluble Zn fraction in the proline treatment was 2.72 times higher than that in the control treatment(Fig.5d).
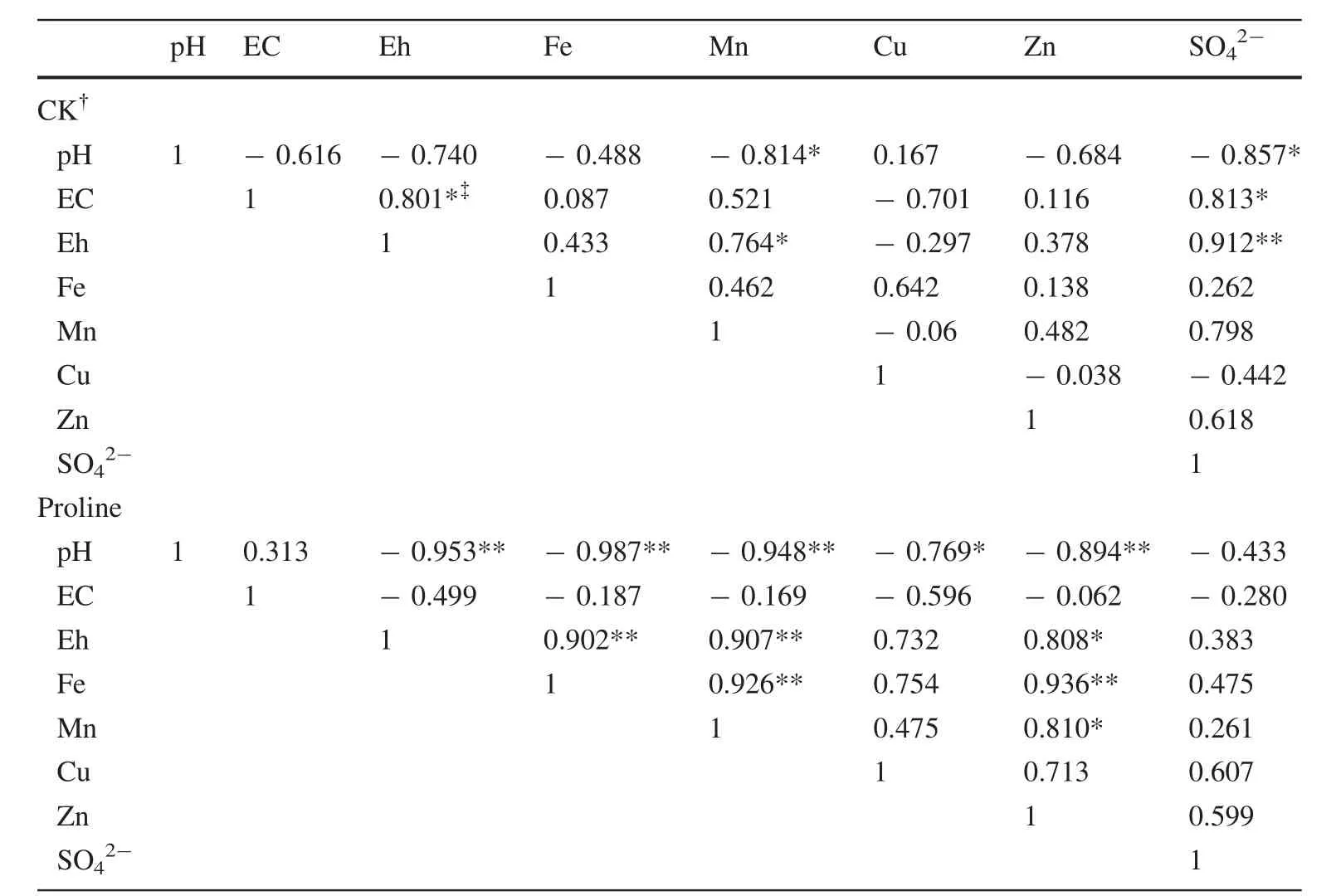
Table 3 Correlations between physiochemical properties of coal gangue leachate
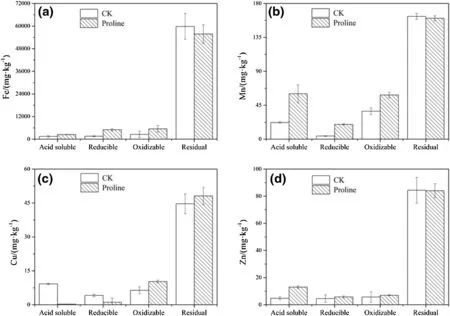
Fig.5 Effect of long-term proline solution coverage on different fractions of heavy metals in the coal gangue.CK deionized water cover,Proline proline solution cover;the metal fraction data are from coal gangue that had been covered by deionized water and proline for 540 days
3.6 Changes in mineralogical characteristics
The surface of the coal gangue treated with deionized water was loose and porous,and all of gangue fragments were lamellar.Thus,the coal gangue experienced a serious weathering process,resulting in the release of a large amount of contaminants.However,the surface of the gangue treated with proline solution for a long period of time was still dense,and only a small part of the coal gangue was slightly weathered.The SEM energy spectra showed that the peak value of S in the control treatment coal gangue was low,indicating that a large amount of reducible sulfide had been oxidized to sulfate and then released into the overlying water.This then lead to the release of heavy metals,such as Fe,Mn and Cu combined,along with the sulfur,into the environment.In contrast,the peak value of S in the coal gangue in the proline treatment was higher than that in the control treatment.This indicates that a large amount of reducible sulfide in the proline treatment had not oxidized,with only a small amount of S having oxidized,leading to a much smaller release of Fe,Mn and Cu.Additionally,the release of other heavy metals with close sulfur adhesion was significantly inhibited(Fig.6).
The mineral composition analysis of the coal gangue covered with deionized water and the proline solution after 540 days showed that the main mineral composition of the coal gangue in the two treatments was quartz(SiO2),pyrite(FeS2), albite [Na0.986(Al1.005Si2.995O8)], and illite[KAl2(Si3Al)O10(OH)2].The FeS2in the control treatment was oxidized and formed some secondary minerals such as(H3O)Fe3(SO4)2(OH)6and Cu4(OH)6(SO4)(H2O)2.However,the coal gangue covered by the proline solution only experienced weak weathering and oxidation effects,and no secondary minerals formed(Fig.7).
4 Discussion
Sulfur-bearing tailings,e.g.,coal gangue,exposed to oxygen,moisture,and the catalytic oxidation effect of sulfuroxidizing bacteria easily form acid mine drainage(AMD).The high acidity and the associated dissolution of toxic and harmful heavy metals can lead to serious acidification and pollution of surrounding waters and pose potential ecological risks to surrounding and downstream ecosystems.Many studies have shown that in situ pollutant control is an effective way to prevent the release of contaminants into the environment.Further,reducing the influence of oxygen is one of the most effective measures for controlling the oxidation rate of sulfur tailings(Lottermoser 2010).This study showed that the addition of a low-concentration of proline solution can significantly reduce the leachate Eh(Fig.2a).This phenomenon may occur because the proline solution,as an organic solution,is rich in water-soluble organic matter with oxygen-depleting properties and can therefore effectively limit the in filtration of dissolved oxygen(Hulshof et al.2006).With the participation of microorganisms,the decomposition of the organic solution in the proline solution system,which requires the consumption of oxygen,could significantly reduce the Eh of the whole reaction system and form an anaerobic environment,providing favorable conditions for in situ control of pollutant release from the coal gangue(Andrés and Francisco 2008;Peppas et al.2000).The present study showed that low concentrations(1%)of a proline solution can significantly reduce the Eh(Fig.2a)and in fact increased the pH of the leachate(Fig.2c).The pH increase in the earliest stage may be attributed to the presence of higher contents of alkali-producing substances(calcite,with a main composition of CaCO3),which can neutralize a portion of the acid produced by the coal gangue oxidation process and increase the pH value.However,the sulfide weathering and oxidation process is relatively slow,and the acid neutralizing ability of calcite is very limited;thus,the proline reaction system showed a slight increase in pH from 30 to 90 days(Fig.2c).The Eh of the proline treatment was approximately 200 mV at 90 days and then decreased rapidly to-300 mV(Fig.2a).The change in the Eh value,accompanied by a strong smell of rotten eggs,indicated sulfate reduction to hydrogen sulfide,which can be explained by the fact that the strong anaerobic environment in the system was beneficial to the growth of sulfate-reducing bacteria.These bacteria can use the proline solution as an organic carbon source and the electron donor to consume the acidity generated from the coal gangue oxidation process,resulting in an increase in the HCO3-concentration along with the increases in pH.Simultaneously,SO42-is reduced to H2S,S2-and other substances(Hulshof et al.2006;Neculita et al.2007),as shown in Eq.(1).The reduction of SO42-described in reaction(1)is consistent with the trends of increasing pH and decreasing Eh observed in the coal gangue leachate(Fig.2a,c).Table 3 also shows that a significant negative correlation was observed between pH and Eh.
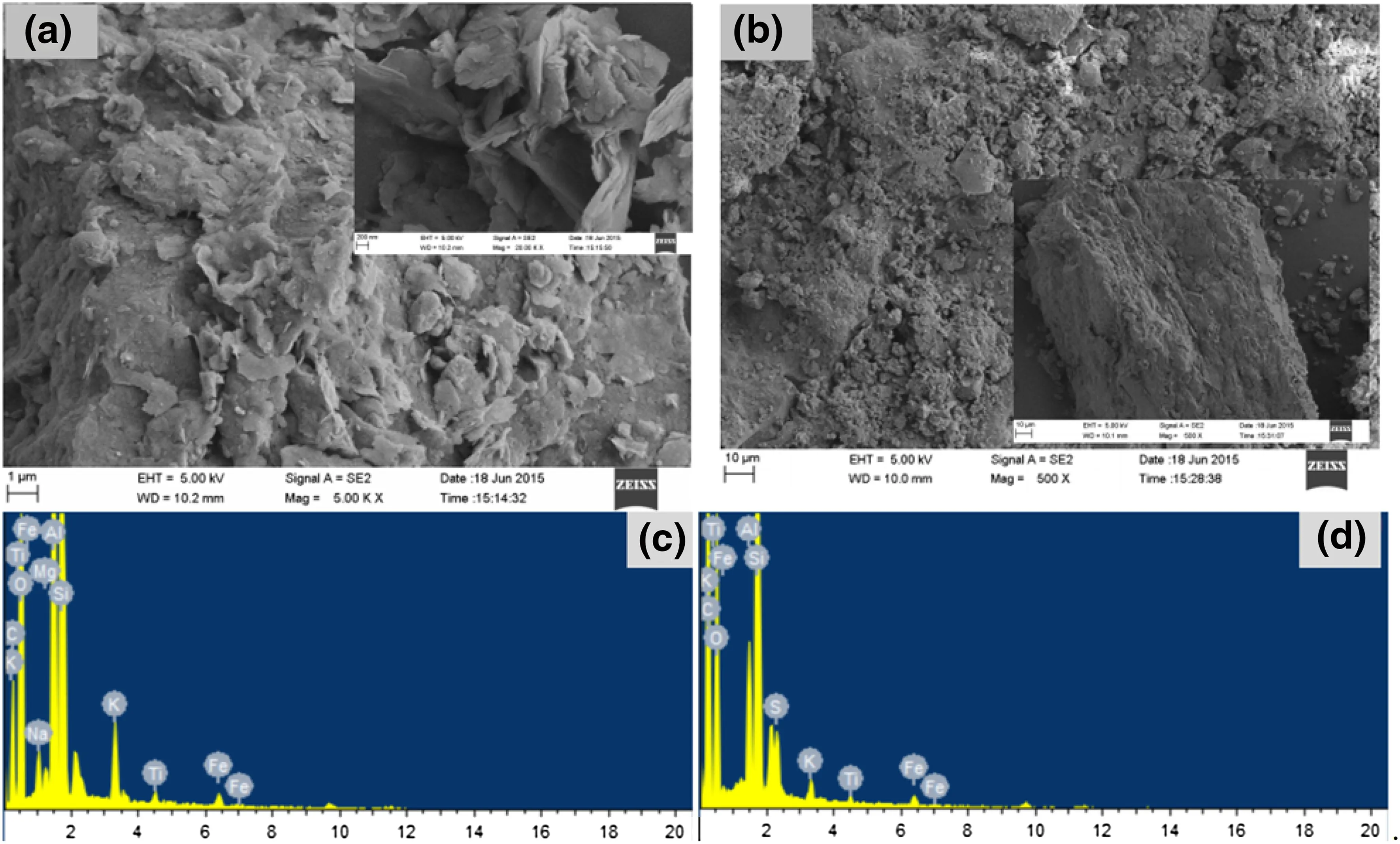
Fig.6 SEM–EDX figure of the coal gangue that was submerged for a long period of time in deionized water and a proline solution.a,c represent the control treatment;b,d represent the proline treatment
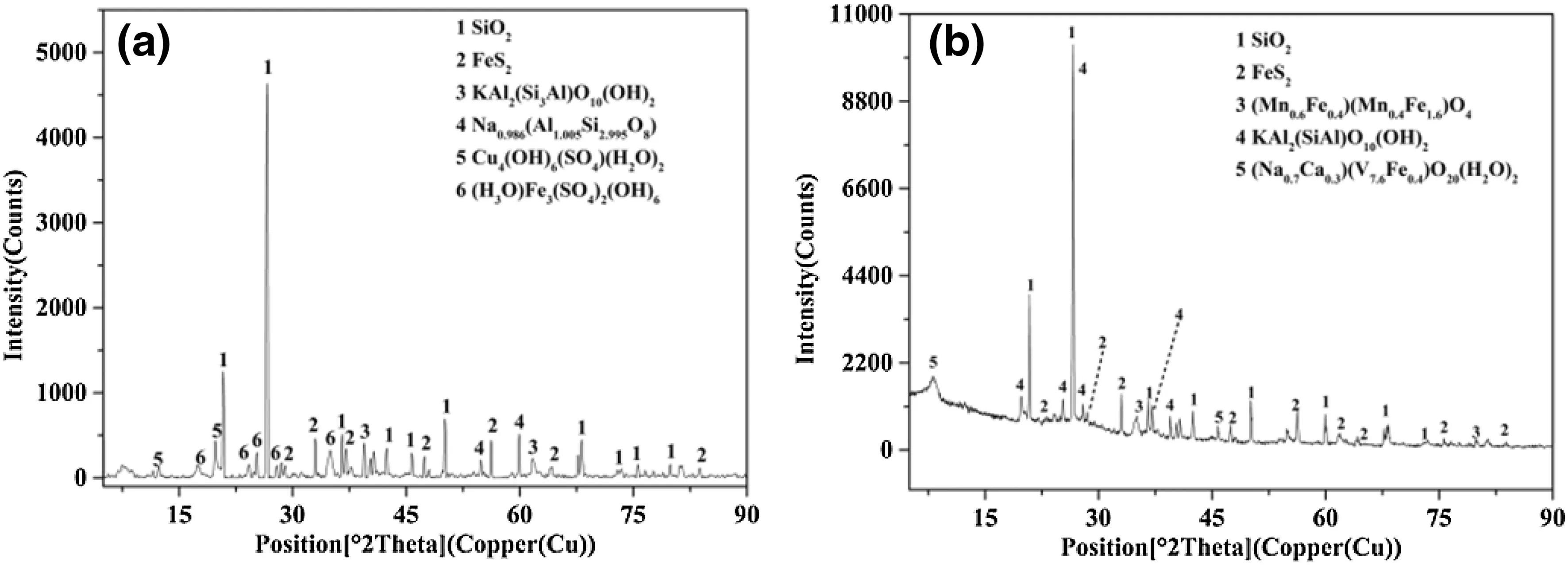
Fig.7 The XRD spectra for the coal gangue that was submerged for a long period of time in deionized water and a proline solution.a,b Represent the control and proline treatments,respectively
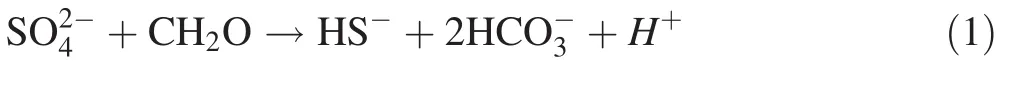
The content of Fe2+in the leachate of the control treatment decreased with the reaction time and remained at a low level after 210 days(Fig.3a),whereas the Fe content decreased continuously from 30 to 210 days and continuously increased after 210 days(Fig.4a).This pattern was due to the FeS2in the coal gangue in the control treatment dissolving to form Fe2+under the combined influence of water,oxygen,and sulfur-oxidizing bacteria;then,the Fe2+was further oxidized to Fe3+,promoting the oxidation and dissolution of the sulfur-bearing minerals under the environmental conditions characterized by a low pH and a high Fe3+ion concentration(Dold 2014).However,the changes in the Fe2+and Fe ion contents in the proline treatment were similar(Figs.3a,4a).That is,the Fe2+and Fe ions in the leachate increased first from 0 to 90 days,then decreased from 90 to 540 days,and finally remained at a low level.This pattern can be explained by the fact that the pH change of the leachate was small(pH 3)but that the Eh decreased markedly from 320 to 200 mV in the earliest stage(from 0 to 90 days)(Fig.2a,c),resulting in weakly oxidizing acidic conditions.The coal gangue in the proline treatment was mainly affected by the dissolved effect of acid,and the content of Fe2+increased slowly.The pH and Eh for the leachate changed significantly from 90 to 540 days.The pH values increased from 3 to 8,and the Eh values decreased from 200 to-300 mV.Although the strong pH and anaerobic environmental conditions in the proline treatment had an inhibitory effect on the growth of Thiobacillus ferrooxidans and Thiobacillus thiooxidans,there was a lower ferrous oxidation rate and OD in the 9k medium treated with proline solution(Fig.3b,c).However,in a hypoxic environment,ferrous iron can be oxidized by the catalytic action of anaerobic photosynthetic iron oxide bacteria(purple sulfur bacteria,purple nonsulfur bacteria and green bacteria,etc.)under low-oxygen conditions(Croal et al.2004;Kappler and Straub 2005),as shown in Eq.(2).Additionally,proline is an amino acid,and its decomposition products may contain nitrates in the initial oxidation environment and in the late reduction of the environment;later,nitrate-reducing bacteria may use the nitrates as electron acceptors to oxidize the ferrous iron in a neutral anaerobic environment(Hafenbradl et al.1996;Straub et al.1996;Wang et al.2014),as shown in Eq.(3).
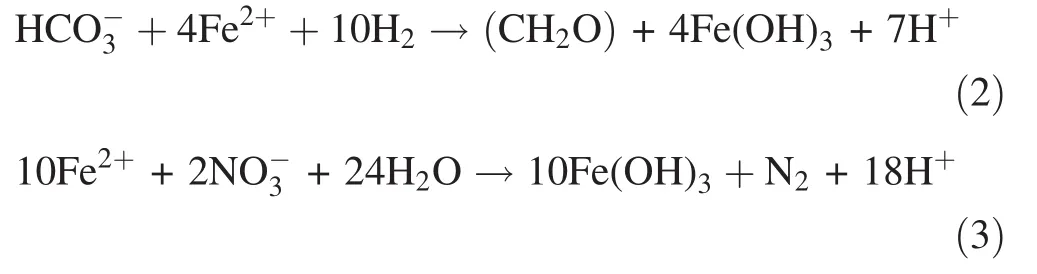
The Fe2+dissolved from coal gangue and its oxidation products(Fe3+)easily precipitate as ferric hydroxide or ferrous hydroxide under the environmental conditions with a high pH,low Eh,and low activity of eosinophilic sulfuroxidizing bacteria,and the proline component and Fe also easily form an organic chelate.Thus,the dissolved leachate Fe content was significantly reduced,and coal gangue oxidation was inhibited under environmental conditions with high pH and low Eh values.The specific mechanism may have the following three aspects:First,the Eh of the proline treatment is approximately-300 mV,which promotes sulfate-reducing bacteria growth.The sulfatereducing bacteria use the proline as an organic carbon source to reduce the sulfate to S2-,which reacts with Fe to form sulfide precipitates.Second,the pH of the proline treatment is alkaline,which inhibits the activity and abundance of Thiobacillus ferrooxidans and pyrite oxidation(Li et al.2016).Additionally,elemental Fe easily precipitates in the form of hydroxide colloids under high pH conditions,and this process has a certain adsorption or co-precipitation effect on the dissolved metals(España et al.2006),as shown in Eqs.(4)–(6).The present study also showed that the pH was significantly negatively correlated with Fe content and that the Fe content was significantly positively correlated with the Mn,Cu,and Zn contents in the proline treatment(Table 3).Third,the proline itself contains carboxyl,amino,and other functional groups that easily attach to the surface of coal gangue to form a protective film that can effectively prevent the oxidation of sulfide-bearing minerals and the dissolution of other pollutants,although the specific mechanism requires further study.
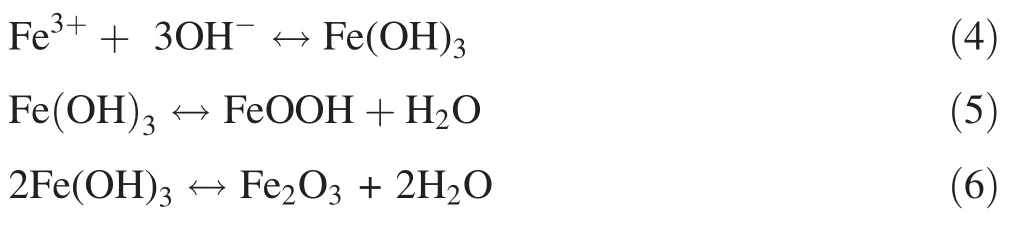
The contents of Mn,Cu,and Zn in the gangue leachate from the proline treatment were significantly lower than those in the control treatment(Fig.4).The concentrations of Mn,Cu,and Zn were near or below the analytical detection limits in the later stages.Figure 5 shows that the fraction of Mn was similar to Zn;that is,the oxidizable,reducible,and acid-soluble fractions of Mn and Zn were higher in the proline treatment than in the control treatment.For the Cu fraction,the acid-soluble and reducible fractions were higher in the control treatment than in the proline treatment.However,the oxidizable fraction in the proline treatment was higher than that in the control treatment.The proline treatment mainly restricts the dissolution of Mn,Cu,and Zn in the coal gangue by altering their fractions.It specifically converts the acid-soluble Mn and Zn into reducible and oxidizable forms,and it converts the acid-soluble and reducible Cu into oxidizable Cu,which is consistent with the findings of previous studies(Shuman 1999;Walker et al.2004).The decreases in the dissolved Mn,Cu,and Zn contents in the reaction system were not only affected by the formation of Fe-containing hydroxide or oxide precipitates but also by the formation of slightly soluble metal-sulfide precipitates due to the sulfate reduction process producing H2S and increasing the alkalinity or pH,thereby enhancing the precipitation of these dissolved metals under the strong reducing conditions,as shown in Eq.(7).Table 3 shows the significant negative correlations among pH,Eh,and metals(Fe,Mn,Cu,and Zn),indicating that the pH values were mainly controlled by the Eh values and that the metal contents were mainly controlled by the pH and Eh values.Additionally,the fact that the concentrations of metal elements(Mn,Cu,and Zn)associated with the oxidizable fraction were higher in the proline treatment than in the control treatment also demonstrates that the metals may have precipitated and formed from sulfide deposition(Fig.5).
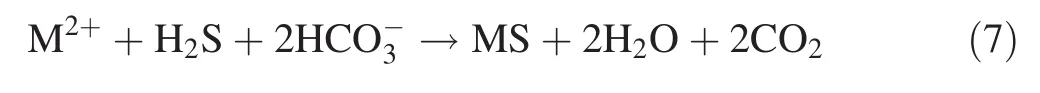
where Me2+represents a divalent metal such as Fe,Cu,Zn,Ni,Co,and Cd,and MeS represents the corresponding metal sulfide precipitate(Hulshof et al.2006;Neculita et al.2007).
Mineral composition and morphology analyses also showed that the surface of the coal gangue covered by deionized water suffered severe weathering and erosion.However,the coal gangue surface structure covered by the proline solution was flatter and smoother.The coal gangue in the control treatment was weathered and oxidized,resulting in the formation of secondary minerals,such as hydroniumjarosite[(H3O)Fe3(SO4)2(OH)6],whereas secondary minerals were not observed in the proline treatment.The secondary minerals therefore played a significant role in the removal of trace elements from solution by adsorption and co-precipitation(Webster et al.1998).The decrease in Cu concentration with reaction time in the control treatment(Fig.4c)may be explained by the fact that the precipitation of secondary minerals reduced the mobility of Cu.Metal sulfide precipitation,iron hydroxide and oxide precipitation,and organic complexes formation were not observed in the proline treatment in the present study,possibly because of the accuracy of the analytical instruments.Additional instrumental techniques,such as X-ray absorption near edge structure(XANES)analysis,FTIR spectrometry,and13C CP/MAS NMR,should be used to further explore the interactions between proline and coal gangue.The activity of acidophilic sulfur-oxidizing bacteria was a key parameter that influenced the oxidation process of coal gangue.However,the OD values only indirectly reflected the bacteria activity and the methods of 16S rRNA pyrosequencing required to investigate the community composition and function of acidophilic sulfuroxidizing bacteria(Chen et al.2013;Jones et al.2012).Fu et al.(2014)application of 30%of water-soluble organic materials(biogas slurry and livestock wastewater)covering coal gangue with a solid–liquid ratio of 1:10,showed that the technique also significantly inhibited the oxidation of coal gangue.Numerous studies suggested that the performance of the organic cover,in the respect of efficiency and life duration,depends on the maintenance of the favorable conditions with the organic mass,such as moisture and hydraulic permeability(Peppas et al.2000;Fu et al.2014).In contrast,the proline solution(1%)in the present study can significantly inhibit the oxidation of coal gangue;whether or not the addition of low content of proline at lower solid–liquid ratios has the same control effect requires further investigation.
5 Conclusion
The coal gangue covered by deionized water was severely weathered,and the leachate exhibited low pH values,high values of EC and Eh,high levels of acidity and SO42-,high activity of sulfur-oxidizing bacteria,and high concentrations of metals(Fe,Mn,Cu,and Zn).However,the addition of a low-concentration (1%) proline solution significantly inhibited the oxidation of coal gangue and the release of pollutants,mainly by decreasing the activity of sulfur-oxidizing bacteria and altering the metal fractions and mineralogical characteristics.Therefore,the use of an environmentally friendly aqueous proline solution cover enables in situ control of pollutant release from coal gangue dumps in engineering practice.
AcknowledgementsThe study was funded by a grant from the United Fund of Guizhou Province Government and National Natural Science Foundation of China(No.U1612442-3),the Project of the Education Department of Guizhou Province(Nos.KY 2016011,GZZ 201607,and ZDXK201611).
杂志排行
Acta Geochimica的其它文章
- Lithium elemental and isotopic disequilibrium in minerals from peridotite xenoliths from Shangzhi,NE China:products of recent melt/ fluid-peridotite interaction
- The influence of climate and topography on chemical weathering of granitic regoliths in the monsoon region of China
- Dynamics of soil organic carbon following land-use change:insights from stable C-isotope analysis in black soil of Northeast China
- The performance of the Noblesse multi-collector noble gas mass spectrometer for40Ar/39Ar geochronology
- Geochemistry of the Palaeo-Mesoproterozoic Tadpatri shales,Cuddapah basin,India:implications on provenance,paleoweathering and paleoredox conditions
- Iron isotopic analyses of geological reference materials on MCICP-MS with instrumental mass bias corrected by three independent methods
