Lithium elemental and isotopic disequilibrium in minerals from peridotite xenoliths from Shangzhi,NE China:products of recent melt/ fluid-peridotite interaction
2018-10-26······
······
Abstract Lithium elemental and isotopic disequilibrium has frequently been observed in the continental and oceanic mantle xenoliths,but its origin remains controversial.Here,we present a combined elemental and Li isotopic study on variably metasomatised peridotite xenoliths entrained in the Cenozoic basalts from Shangzhi in Northeast(NE)China that provides insight into this issue.Li concentration(0.3–2.7 ppm)and δ7Li(mostly 2‰–6‰)in olivine from the Shangzhi peridotites are similar to the normal mantle values and show roughly negative correlations with the indices of melt extraction(such as modal olivine and whole rock MgO).These features are consistent with variable degrees of partial melting.In contrast,clinopyroxene from the Shangzhi xenoliths shows significant Li enrichment(0.9–6.1 ppm)and anomalously light δ7Li(-13.8‰ to 7.7‰)relative to normal mantle values.Such features can be explained by Li diffusion from silicate melts or Li-rich fluids occurring over a very short time(several minutes to several hours).Moreover,the light Li isotopic compositions preserved in some bulk samples also indicate that these percolated melts/ fluids have not had enough time to isotopically equilibrate with the bulk peridotite.We thus emphasize that Li isotopic fractionation in the Shangzhi mantle xenoliths is mainly related to Li diffusion from silicate melts or Li-rich fluids that took place shortly before or coincident with their entrainment into the host magmas.
Keywords Mantle peridotite⋅Li isotope⋅Mantle metasomatism⋅Northeastern China
1 Introduction
Lithium(Li)and its isotopes(6Li and7Li)were regarded as a very useful tools for tracking fluid-related processes and the recycling of surface materials to the mantle due to their moderate incompatibility,strong fluid mobility,large mass difference(~17%),and significant isotopic fractionation at low temperatures near the surface(e.g.,Tomascak et al.2002,2016;Tomascak 2004;Elliott et al.2004,2006;Tang et al.2007a,2014).
To date,studies of Li isotopes in mantle xenoliths reveal a great range of δ7Li in coexisting minerals(40‰)(Tang et al.2010;Su et al.2016;Penniston-Dorland et al.2017)that contrasts with the relatively uniform δ7Li of MORBs(+2‰ to+6‰),which samples the convecting mantle(Chan et al.1992;Tomascak et al.2008).The origin of such striking isotopic fractionation still remains elusive.Theoretical and case studies have proved that equilibrium fractionation of Li isotopes during high temperature processes,such as mantle partial melting and magma fractionation,is negligible(< 1.0‰)(e.g.,Tomascak et al.1999;Chan and Frey 2003)while Su et al.(2017a)and Xiao et al.(2017)argued that Li isotopic fractionation during magma differentiation and partial melting are more significant.In addition,the diffusion process typically produces striking isotopic fractionation due to the exceptionally high diffusivity of Li and faster diffusion of6Li than7Li(Richter et al.2003;Coogan et al.2005).The Li diffusion process in mantle xenoliths may happen during slow cooling of mantle xenoliths after the eruption of their host magmas(Ionov and Seitz 2008;Gao and Snow 2011).This process may also relate to the interaction of peridotites with percolating melts(and/or host magmas)with normal mantle-like δ7Li shortly before or coincident with their entrainment into the host magmas(Jeffcoate et al.2007;Rudnick and Ionov 2007;Magna et al.2008;Su et al.2014b)or with recycled melt with low δ7Li(Nishio et al.2004;Tang et al.2007b,2012,2014;Ackerman et al.2013;Su et al.2016).Metasomatism,by hypothetical isotopically light components derived from subducted oceanic slab,was proposed to explain high Li concentrations and low δ7Li found in bulk and individual minerals from mantle xenoliths in eastern Asia(e.g.,Nishio et al.2004;Tang et al.2007b,2011,2012;Zhang et al.2010;Su et al.2012).In contrast,Magna et al.(2008)argued that metasomatism by‘‘subduction-related’’ components was not likely to strongly affect Li isotopic compositions in the lithospheric mantle.Previous studies also found strong inter-mineral Liisotope disequilibria in most xenoliths suites.Some researchers proposed that Li-isotope disequilibria are related to the different behaviors of Li in silicate and in carbonatite metasomatism(Seitz and Woodland 2000;Su et al.2012,2014a,2017b).Other researchers emphasized that the disequilibria is not likely to be preserved in the mantle for a long time and is therefore attributed to diffusion-controlled interaction with Li-bearing melt/ fluid shortly before or during the transport of the xenolith to the surface(Jeffcoate et al.2007;Rudnick and Ionov 2007;Aulbach et al.2008;Aulbach and Rudnick 2009;Xu et al.2013a,b).More recently,Xiao et al.(2017)suggested that the Li isotopic heterogeneity in the mantle is related to variable degrees of partial melting.
Here,we reported Li concentrations and isotopic compositions of pure olivine and clinopyroxene(Cpx)separates from variably metasomatised peridotite xenoliths from Shangzhi,NE China.The Shangzhi samples show negative δ7Li values and abnormally high Li concentrations in Cpx and strong Li disequilibria between coexisting olivine and Cpx.We emphasize that Li isotopic fractionation in the Shangzhi mantle xenoliths is mainly related to Li diffusion from silicate melts or Li-rich fluids occurring shortly before or coincident with their entrainment into the host magmas.
2 Geological background and sample description
Northeast China(NE China)is located in the eastern part of the Central Asian Orogenic Belt(CAOB),which was considered as a composite fold belt formed by amalgamation of several minor blocks(e.g.,Xing’an,Songliao,Jamusi,etc.)during subduction and collision between the North China Craton(NCC)and the Siberian Craton(Xu et al.2013a,2013b;Liu et al.2016).Since the Late Mesozoic,NE China became an important part of the circum Pacific tectonic–magmatic belt.During the Cenozoic time,intraplate basaltic magmatism was widely distributed in the Songliao Basin and its surrounding areas(Fig.1a).In an area of about two million square kilometers,there are over 590 volcanos with basaltic rocks covering about 50,000 km2(Fig.1a;Liu 1999;Ma et al.2002).The basaltic rocks have been grouped into three successive episodes:(1)Late Cretaceous-Paleogene sub-alkaline and alkaline basalts;(2)Neogene alkali basalts with a minor amount of tholeiitic basalts,and(3)Quaternary alkaline basalts(Fan and Hooper 1989;Liu et al.1992).Mantle xenoliths,mainly spinel lherzolite and harzburgite,are mostly found in the Neogene and Quaternary alkaline basalts and are generally not found in the tholeiitic flows.Numerous studies have been conducted on these rocks(e.g.,Basu et al.1991;Tatsumoto et al.1992;Zhang et al.2000;Xu et al.1998,2003;Yu et al.2009,2010).
The peridotite xenoliths in this study were collected from the Shangzhi volcanic field in Heilongjiang Province(Fig.1).The Shangzhi volcanic field is located along northern edge of the Songliao basin and occurs within the Yilan-Shulan fault zone(Fig.1a).The volcanic rocks in Shangzhi are Neogene alkali basalts(~13 Ma)varying from nephelinite to olivine basanite(Liu et al.1992).Abundant fresh spinel peridotite xenoliths entrained by alkali basalts,5–25 cm in diameter,were discovered in a quarry at Guixiang village.Thirteen spinel lherzolites and eight spinel harzburgites were collected from this locality and used in this study.
All studied xenoliths are fresh anhydrous peridotites and belong to the Group I peridotites of Frey and Prinz(1978).They are spinel peridotitesranging from lherzolites(Cpx>5%)to harzburgites(Cpx<5%).Mineral assemblage for harzburgites is olivine (Ol,70%–86%),orthopyroxene(Opx,10%–25%),clinopyroxene(Cpx,2%–4%)and spinel(Sp,1%–3%)(Table 1).Among the harzburgites,samples with protogranular texture are the most abundant(Fig.2a).Mineral grains in these samples are characterized by a coarse grain size of 3–5 mm.olivine and Opx grains are equant and exhibit curvilinear grain boundaries.Large olivine grains(>4 mm)are kinked.Some of them contain smallinclusion of spinel(0.1–0.2 mm).The typical mineral assemblage for lherzolites is olivine(54%–75%),Cpx(6%–18%),Opx(14%–29%)and spinel(1%–5%)(Table 1).Lherzolites from this region are characterized by equigranular texture with medium grain size(1–1.5 mm in diameter)and occasional porphyroblasts of Opx (Fig.2b).They are partially recrystallized and show a nearly 120°C triple junction(Fig.2b).Most Cpx in lherzolites are generally green and clear,but some(e.g.,GX-14)display a spongy appearance(Fig.2c).Most spinel grains are anhedral and occur as an interstitial phase among silicate minerals.Some of them also display a spongy texture(Fig.2d).
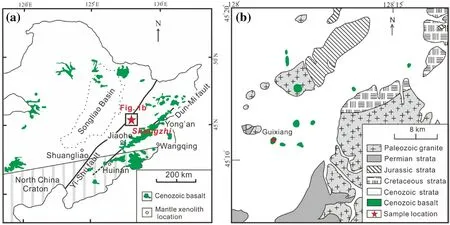
Fig.1 a Simplified tectonic scheme and distribution of Cenozoic basalts in northeastern China and location of the studied area.Samples used in this study were collected from Guixiang,Shangzhi City.Yi–Shu fault and Dun–Mi fault represent Yilan–Shulan fault zone and Dunhua–Mishan fault zone respectively;b enlarged sampling area in Shangzhi,Northeastern China
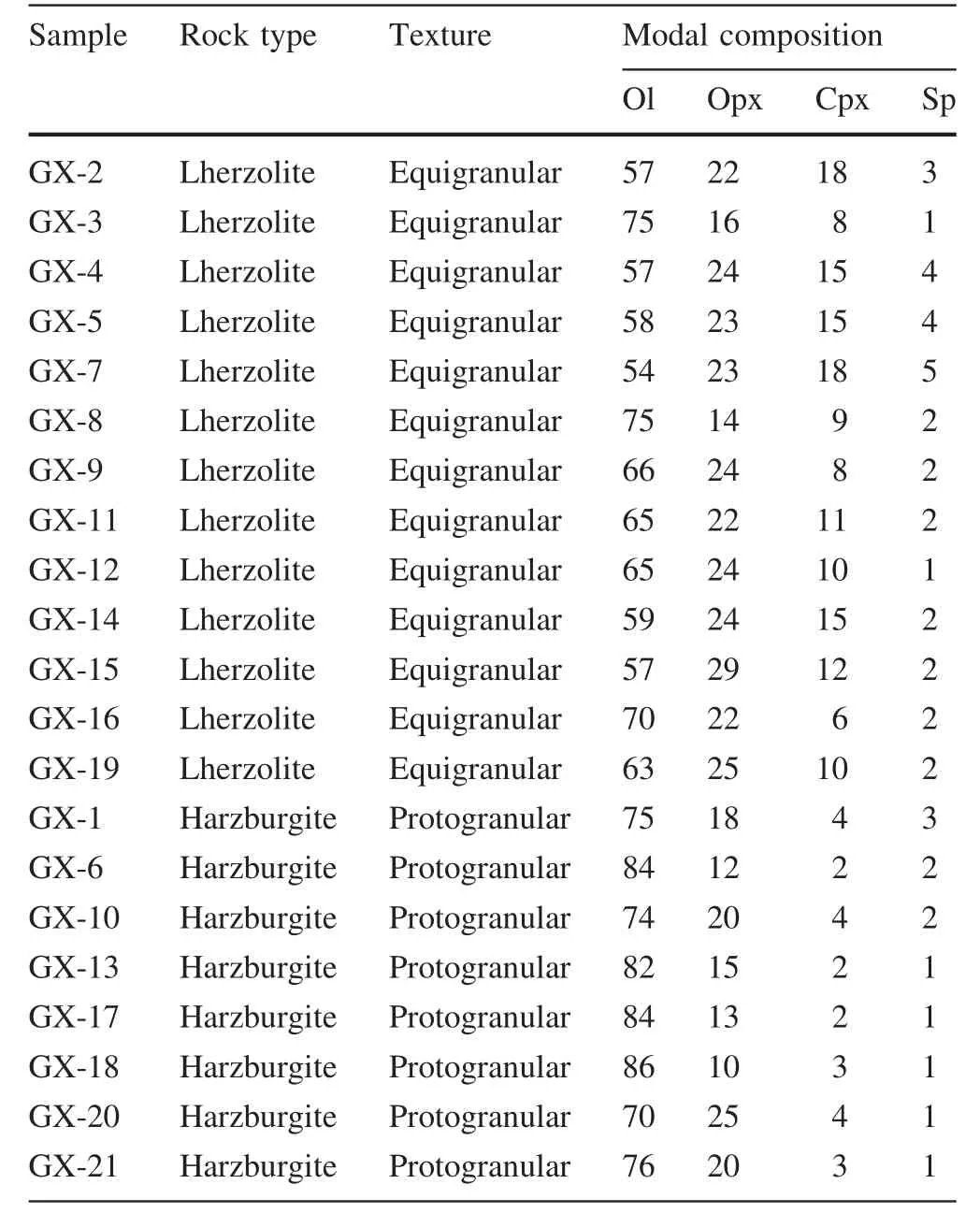
Table 1 Modal composition of Shangzhi peridotite xenoliths(vol%)
3 Analytical methods
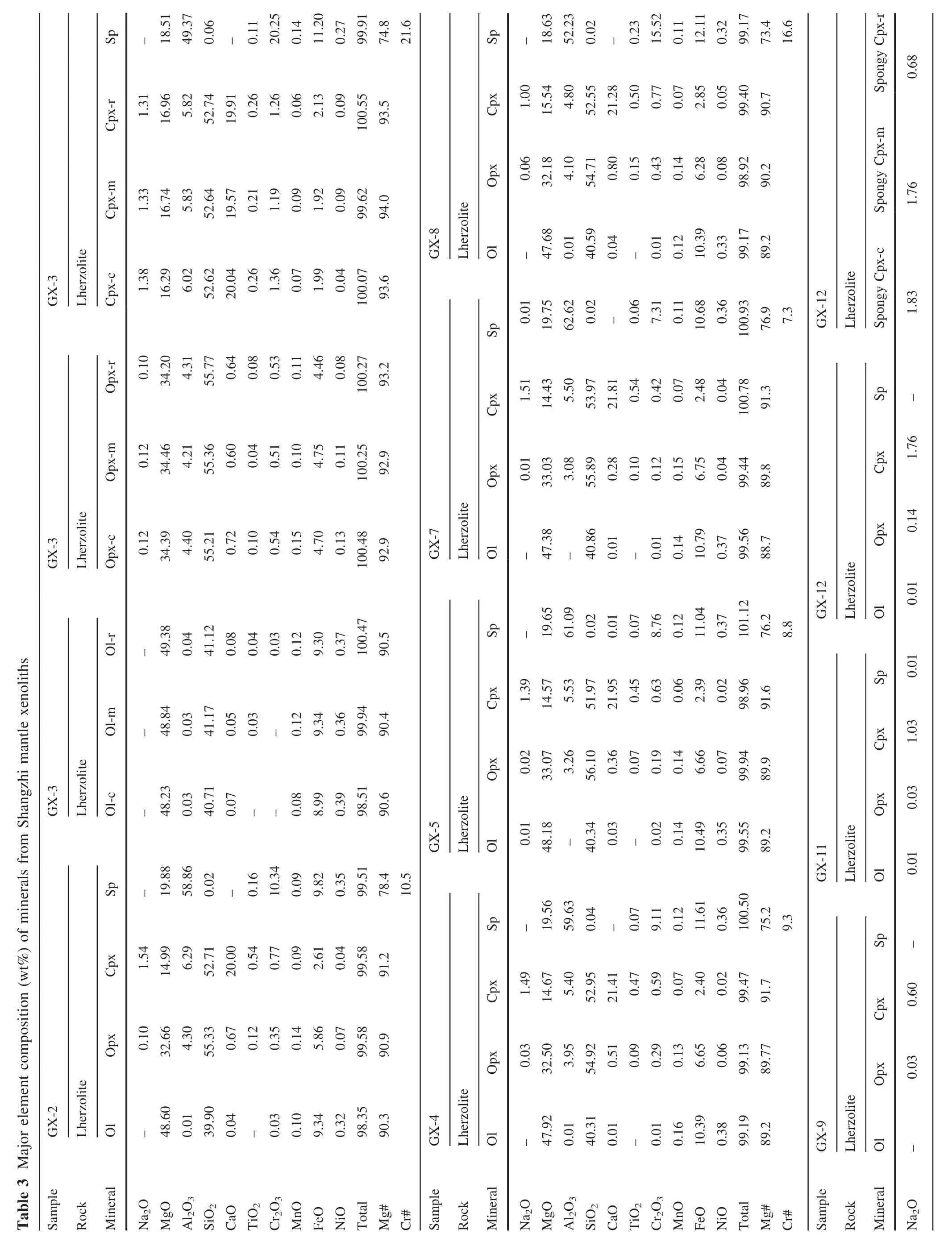
The xenoliths in this study were cut to remove exterior surfaces.Relatively fresh interiors without basaltic veins were used for bulk-rock analyses and for mineral separation.The rocks were crushed and sieved with stainless sieves.Olivine and Cpx were handpicked under a binocular microscope.Only the most pure and fresh mineral grains were selected for Li isotope analysis.These separates were then washed in an ultrasonic bath three times with Mill-Q ultra-pure water.The mineral separates and whole rock samples were then ground to 200 mesh powders using an agate mortar.The major elements were analyzed using a PANalytical Axios-advance X-ray fluorescence spectrometer(XRF)on fused glass beads in the laboratory of ALS Chemex Ltd in Guangzhou,China.The analytical errors were mostly<5%.Loss on ignition(LOI)was determined by heating a 1 g power sample to 1100°C for 1 h.The major element compositions of minerals were analyzed using the EMPA-1600 electron microprobe at the Institute of Geochemistry,Chinese Academy of Sciences(IGCAS).Analytical conditions for major elements were 25 kV accelerating voltage,10 nA beam current and 10 μm beam size.The detection limit for major elements under such conditions is 0.01 wt%.The analytical reproducibility was within 2%for major elements.The trace element compositions of Cpx separates were analyzed using anInductively Coupled Plasma-Mass Spectrometer(ICP-MS)at the Institute of Geochemistry,Chinese Academy of Science(IGCAS),following the analytical procedures described by Qi et al.(2000).The powdered samples(50 mg)were dissolved in a Hf+HNO3solution in high pressure Te flon bombs that were heated at~ 190°C for 12 h.Rh was used as an internal standard to monitor signal shift during analysis.The USGS standard BHVO-2 and BCR-2 were determined as unknowns.The overall analytical precision is better than 5%relative to the recommended values(Table 4).
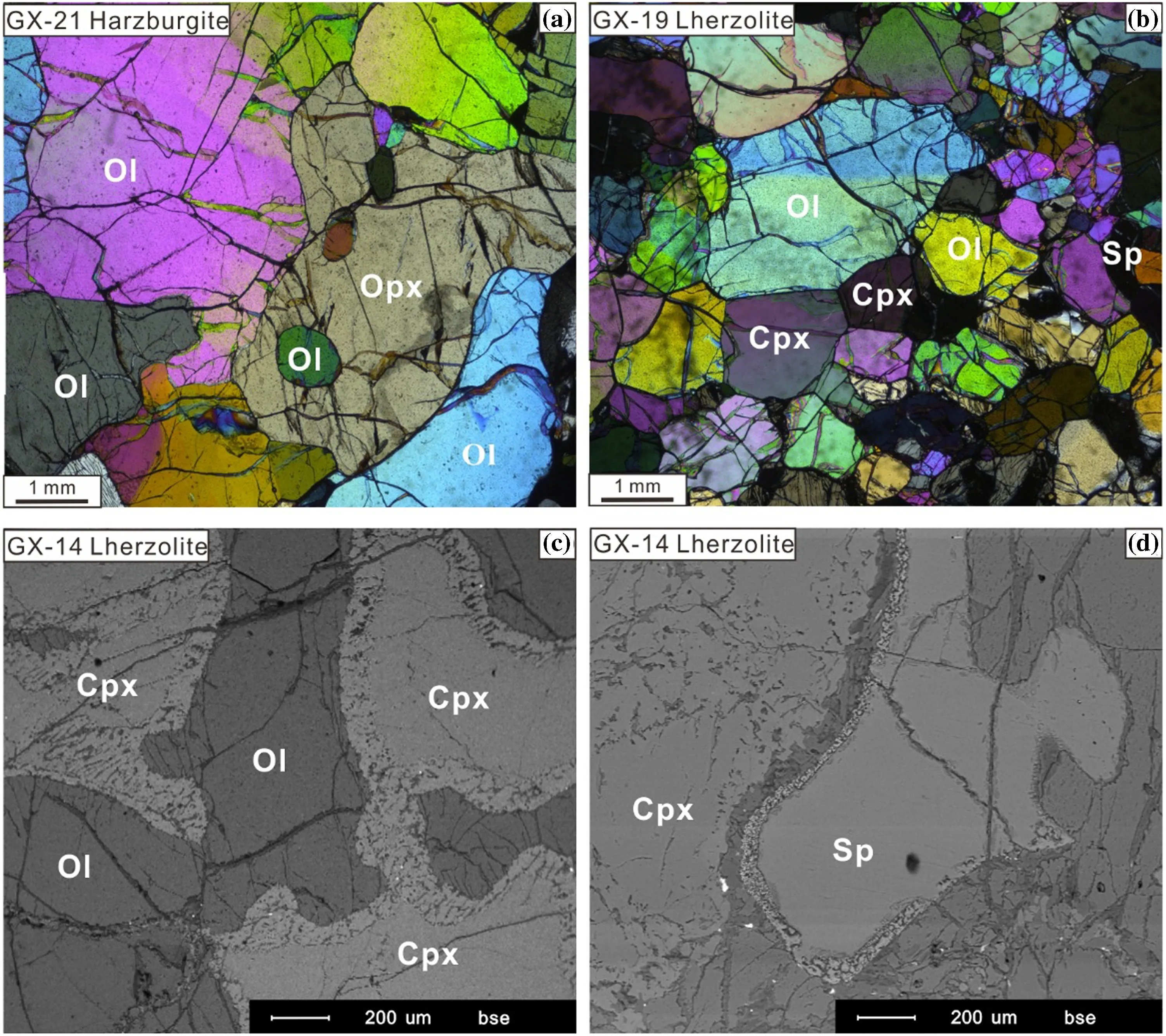
Fig.2 a Photomicrographs of representative textures of the Shangzhi peridotite xenoliths.Protogranular texture(crossed polarizer);b equigranular texture(crossed polarizer);c-d back-scattered secondary electron images displaying sieve-textured Cpx and spinel from the Shangzhi lherzolites;Ol olivine,Opx orthopyroxene,Cpx clinopyroxene,Sp spinel
Separation of Li for isotopic composition analysis was undertaken in a clean lab at the University of Science and Technology of China(USTC)following the procedure described by(Gao and Casey 2012;Sun et al.2016).Samples were initially dissolved in a 1:1 HNO3-HF mixture solution,evaporated to dryness and treated two times with 8 N HCl.Finally the samples were dissolved in 3 mL 0.2 N HCl and loaded on cation exchange columns.Prior to Li isotope analysis,Na/Li ratios of all separations were measured semi-quantitatively using ICP-MS at USTC to guarantee both high Li yield(>99.8%recovery)and low Na/Li ratio(<0.5).The final concentration of Li in the solutions utilized for MC-ICP-MS analyses was targeted to be about 50–200 ppb to ensure the best precision and accuracy.
Li isotopic compositions were analyzed at IGCAS using a Neptune Plus MC-ICP-MS(Thermo Fisher Scientific)and a standard-sample bracketing method.IRMM016 was utilized as the isotopic standard during analysis.All samples and reference solutions were introduced using a microflow nebulizer attached to a double-Scott spray chamber and ran in a block of 30 cycles of measurement with an integration time of 2 s.Typical sensitivity achieved was 50–60 V/ppm for7Li at a flow rate of~ 50 μL⋅min-1.In house standard L-SVEC and QC were measured every 3 samples to monitor the quality of measurement.The Li isotope ratios were calculated relative to the average of two bracketing IRMM-016 runs and reported as δ7Li(‰)=[(7Li/6Li)sample/(7Li/6Li)standard-1]× 1000. The external precision is less than 0.3‰(2SD)based on longterm analysis of rock solutions and Li standard solutions over 2-years.The obtained δ7Li values for international standards BHVO-2 and GSP-2 were 4.04‰ ± 0.04‰ and-0.89‰ ± 0.04‰ (2SD)respectively,which were consistent with the previously published results(e.g.,Simons et al.2010;Vlastélic et al.2011;Krienitz et al.2012;Lin et al.2016).The Li concentration in rock samples was measured by voltage comparisons with those obtained for the L-SVEC reference material(of known concentration)and then adjusted for sample mass.The uncertainty in Li concentration determined by this method was less than±10%(Teng et al.2007).
4 Results
4.1 Whole-rock major element compositions
Whole-rock major element compositions of Shangzhi peridotites are listed in Table 2 and plotted in Fig.3.The Shangzhi peridotites have very low(i.e.,<1%)loss on ignition(LOI)and display a considerable spread in major element composition,ranging from fertile lherzolites to refractory harzburgites with up to 46.6 wt%MgO.There are negative correlations between MgO and Al2O3and CaO for Shangzhi peridotite xenoliths.Most samples lie along the partial melting trends in Fig.3a and are interpreted as residues of variable degrees of partial melting(Niu 1997).In contrast,most harzburgite samples and some lherzolite samples display significant enrichment of Na2O compared with the partial melting trend(Fig.3b).
4.2 Mineral chemistry
The minerals(olivine,Cpx and Opx)in Shangzhi peridotites are homogeneous in major oxides based on analyses by EPMA from core to rim.The average compositions are given in Table 3.The studied xenoliths display a wide range in mineral compositions,which matches the petrographic variation.Mg#ol[100×Mg/(Mg+Fe)at]and Cr#sp[100×Cr/(Cr+Al)at]in Shangzhi peridotites range from 88.8 to 91.1 and from 7.3 to 48.3,respectively.In the co-variation diagram ofMg#olversus Cr#sp(Fig.3c),most samples lie along the partial melting trend.In contrast,Cpx in most samples shows fairly high Na2O contents (0.2 wt%–1.8 wt%),which are significantly higher than expected from the partial melting trend(Fig.3d).The Cpx grains with spongy texture shows compositional variations from the core to the spongy rim.The spongy rim is typically enriched in CaO,TiO2and depleted in Na2O,Al2O3compared with the core(Table 3).These observations are consistent with the interpretation that spongy-textured Cpx in peridotite xenoliths developed from a decompression-induced partial melting event(Su et al.2011).
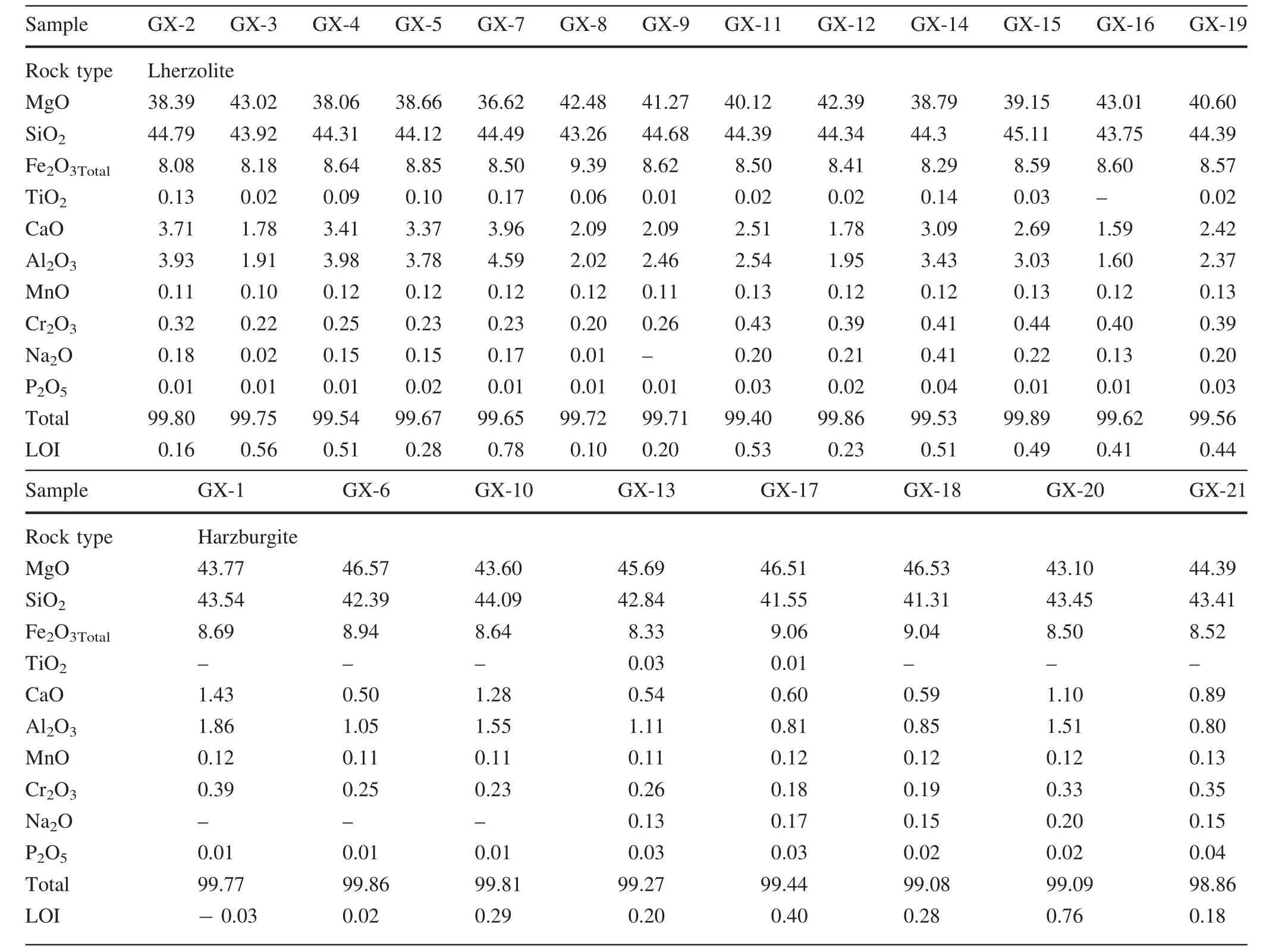
Table 2 Whole rock major element compositions of Shangzhi peridotite xenoliths
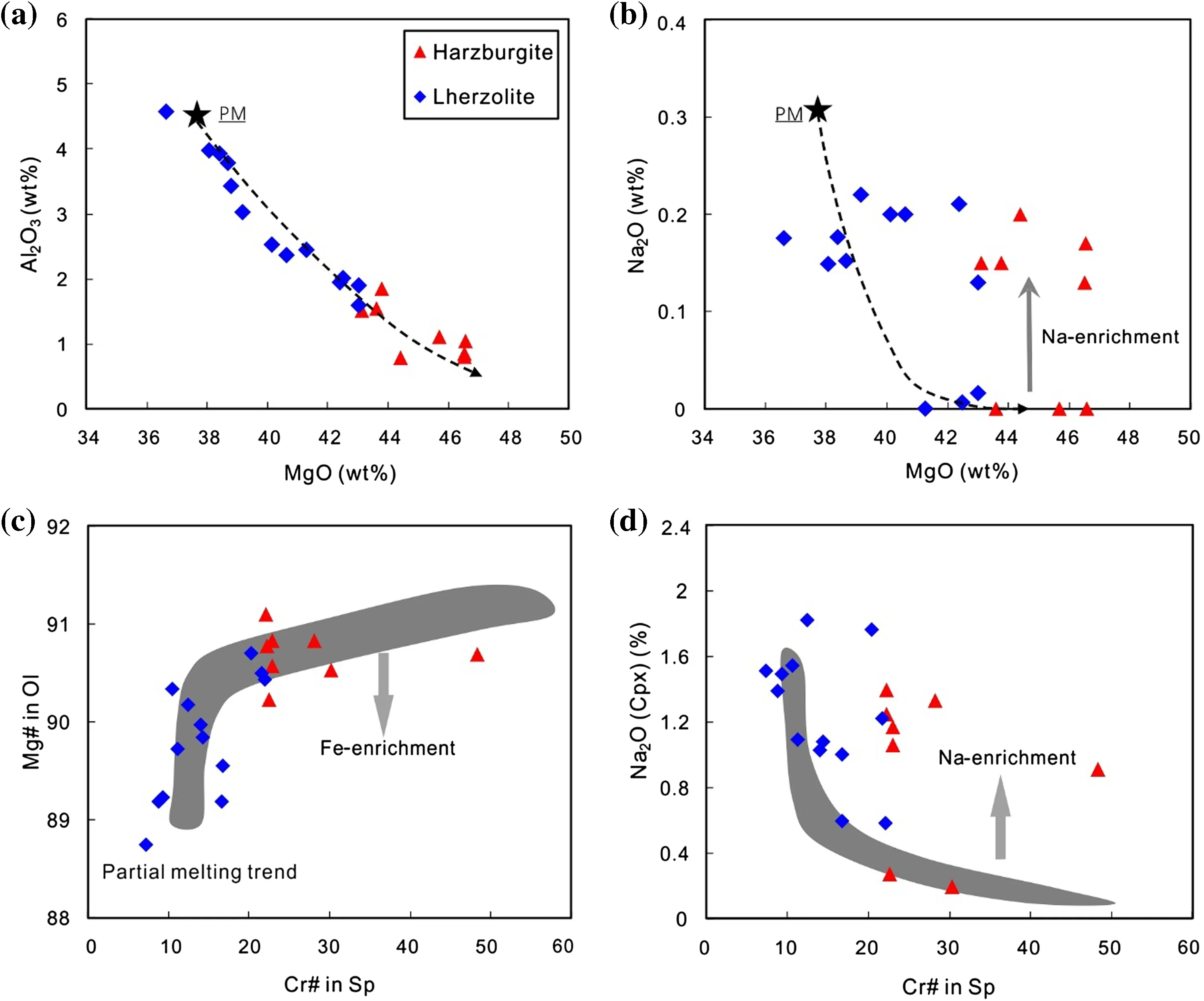
Fig.3 a–b Plots of whole-rock MgO against Al2O3and Na2O;c correlation of Cr#(Cr/(Cr+Al)atomic)in spinel versus Mg#of olivine;d plot of Cr#in spinel versus Na2O in Cpx;The black lines are near-fractional melting model trend of a starting source of primitive spinel peridotite using the model of Niu(1997).Primitive mantle compositions(PM)are after Mcdonough and Sun(1995).The partial melting trend is modeled using the fractional partial melting model of Johnson et al.(1990)
Cpx trace element compositions are listed in Table 4 and plotted in Fig.4.Cpx in harzburgite shows enriched LREE[(La/Yb)N=0.75–39.79], flat HREE[(Gd/Yb)N-=0.65–2.37]and variable enrichment of Th,U,Nb,Ta(Fig.4a,b).Cpx in lherzolites shows both LREE-enriched[(La/Yb)N=0.93–3.75]and LREE-depleted[(La/Yb)N-=0.1–0.54]patterns.In specific,Cpx in three lherzolite samples(GX-2,GX-3 and GX-4)displays both LREE-depleted patterns and weak positive anomalies of Th and U(Fig.4d).Cpx in other lherzolite samples shows variable LREE enrichment and strong positive anomalies of Th and U(Fig.4d).Two lherzolite samples(GX-12,GX-14)display strongest LREE enrichment and abnormally high contents of Nb and Ta in Cpx(Fig.4c,d).
4.3 Li concentrations and isotopic compositions of mineral separates
Li contents and isotopic compositions in the Shangzhi peridotite xenoliths are listed in Table 5.Li concentrations in olivine range from 0.3 to 2.7 ppm,and these values are significantly lower than those in Cpx(0.9–6.1 ppm)(Table 5;Fig.5a).This observation is consistent with previous studies of metasomatised peridotites in the North China Craton,where pyroxenes commonly show higher Li concentrations than olivine (Tang et al.2007b,2011,2012,2014;Zhang et al.2010;Su et al.2014a;Xiao et al.2015).Almost all Shangzhi peridotites plot out of the range of ‘‘equilibrated’’mantle peridotites(Fig.5a;Seitz and Woodland 2000).Li contents of olivine are similar to the normal mantle,but those in Cpx are strikingly higher than the normal mantle(Fig.5a;Ryan and Langmuir 1987;Jeffcoate et al.2007;Magna et al.2006;Seitz and Woodland 2000;Seitz et al.2004).In the diagram of the partition coefficient for Li between olivine and Cpx(ol/cpxDLi)versus the degree of isotopic equilibrium(Δ7Liol–cpx;Fig.5b),the Shangzhi peridotites display variable degrees of elemental and isotopic disequilibria.Such elemental and isotopic disequilibria were also observed in other metasomatically overprinted peridotite xenoliths(Seitz and Woodland 2000;Seitz et al.2004;Rudnick and Ionov 2007;Jeffcoate et al.2007;Su et al.2017b).The δ7Li values of olivine range from 0.3‰to 8.1‰and these values are much higher than the coexisting Cpx(-13.8‰ to+7.7‰)(Table 5;Fig.5d).Moreover,the Li contents of olivine tend to decrease with increasing modal olivine,which is consistent with the melt depletion trend(Fig.5e,Rudnick and Ionov 2007;Xiao et al.2017).The δ7Li values of olivine tend to decrease with increasing forsterite content(Fo)(Fig.5f).There are roughly negative correlations between whole rock MgO contents and Li concentrations(Fig.6a)and δ7Li values for olivine(Fig.6b).However,no correlation exists for Cpx in Shangzhi xenoliths(Fig.6a,b).Moreover,minerals in‘‘depleted’’samples[(La/Sm)nin Cpx < 1]show similar ranges of Li concentrations and δ7Li values compared with those in ‘‘metasomatised’’samples[(La/Sm)nin Cpx>1,Fig.6c,d].
?
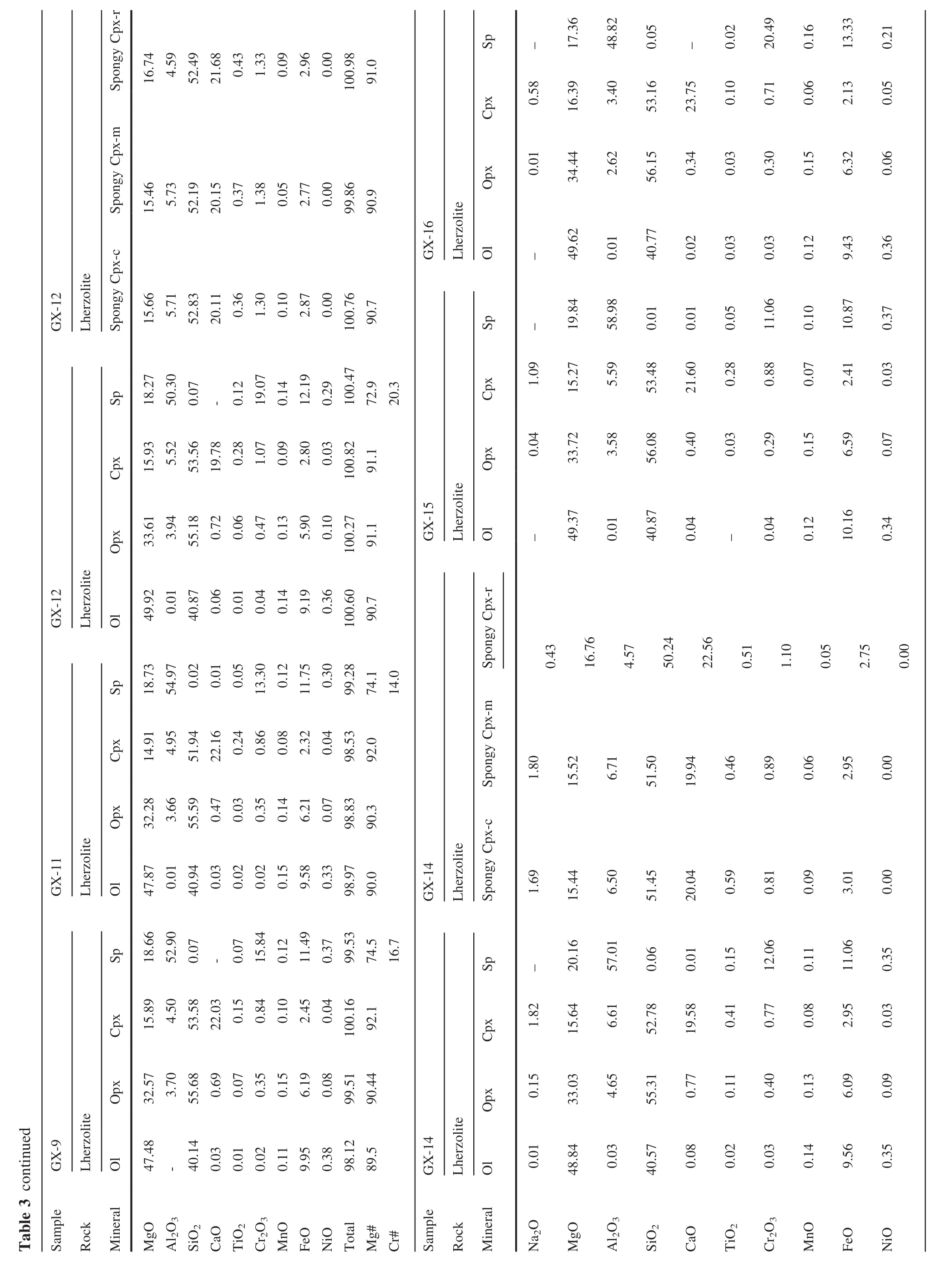
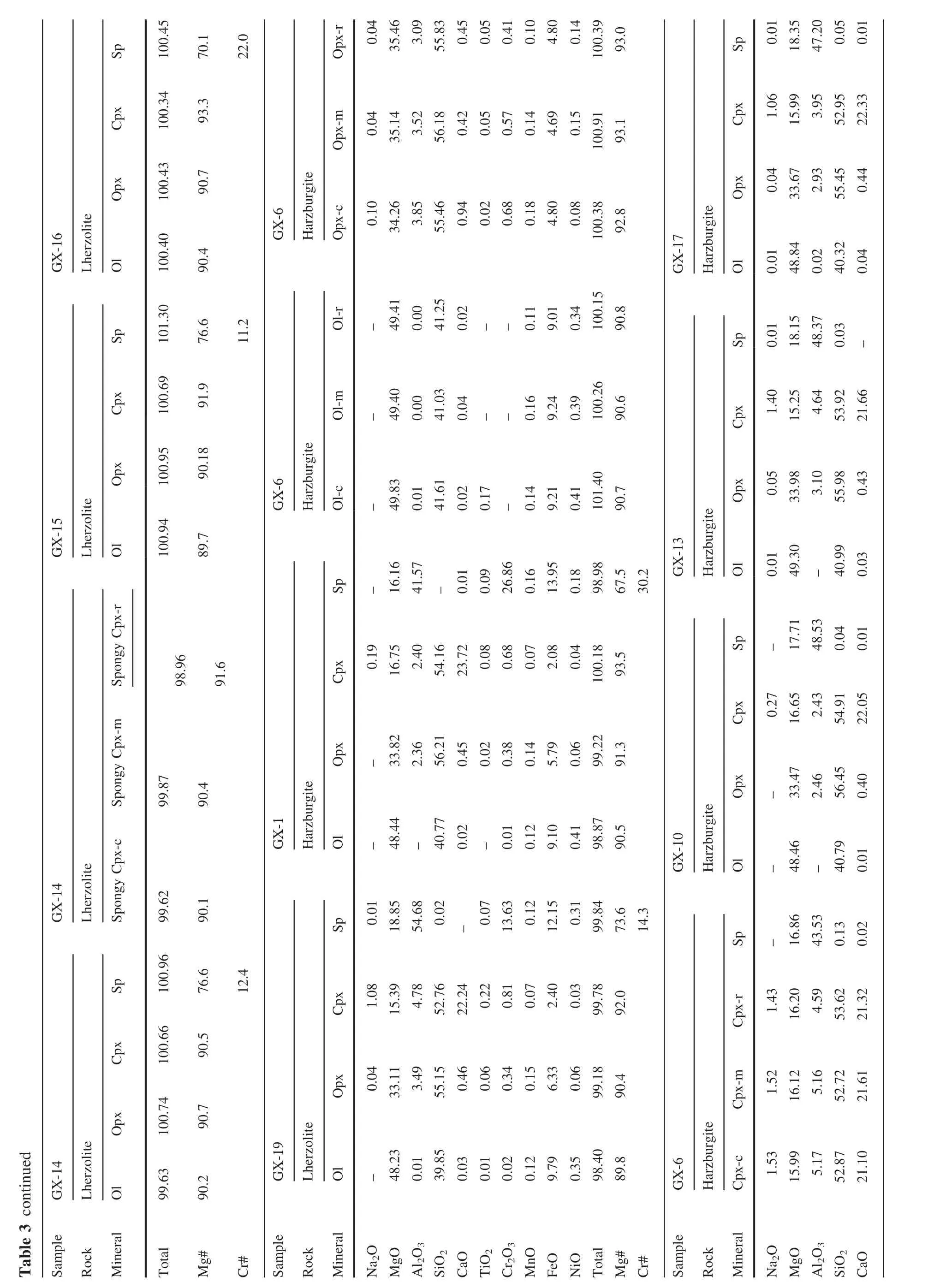
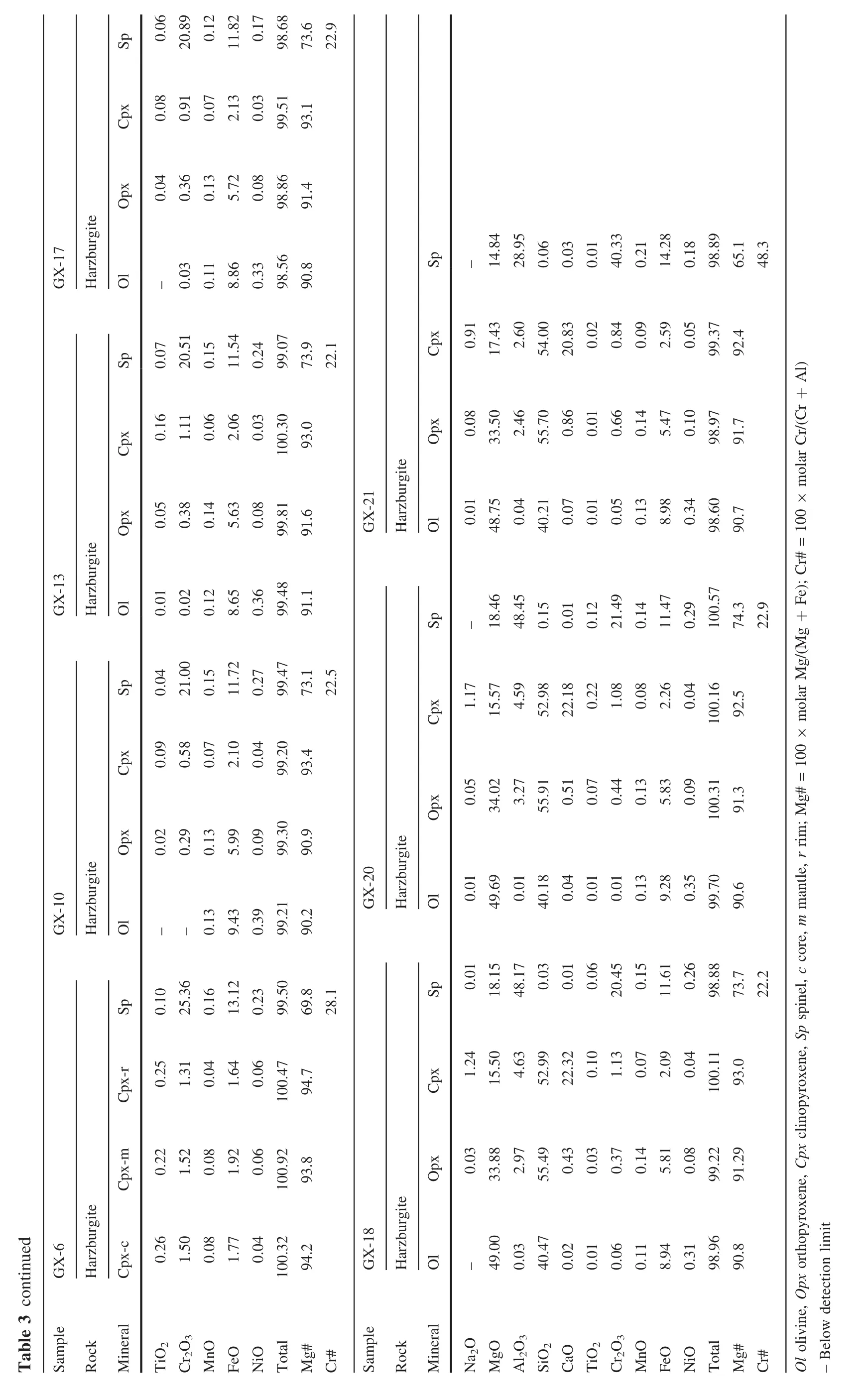
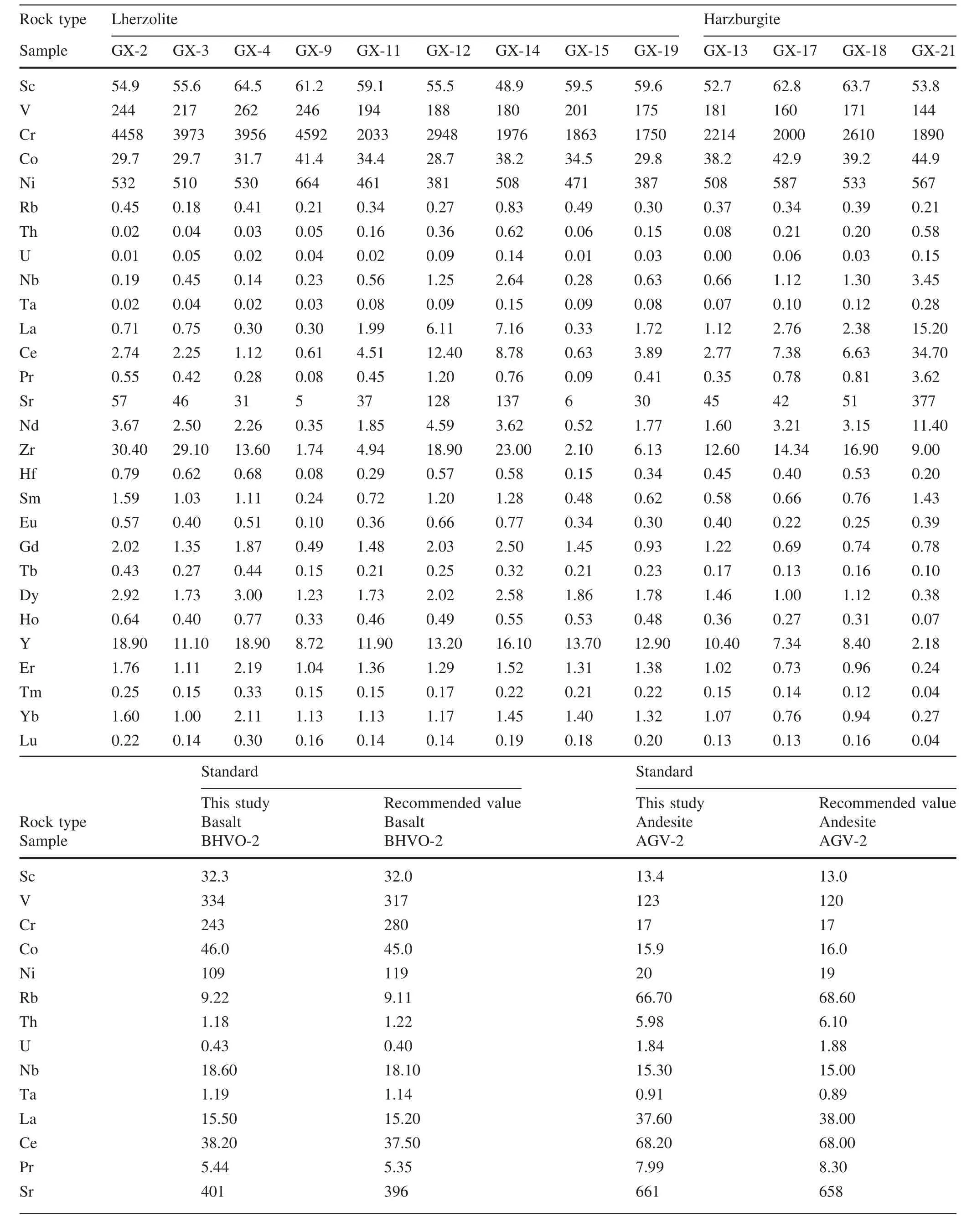
Table 4 Trace element abundances of clinopyroxene separetes from Shangzhi peridotite xenoliths
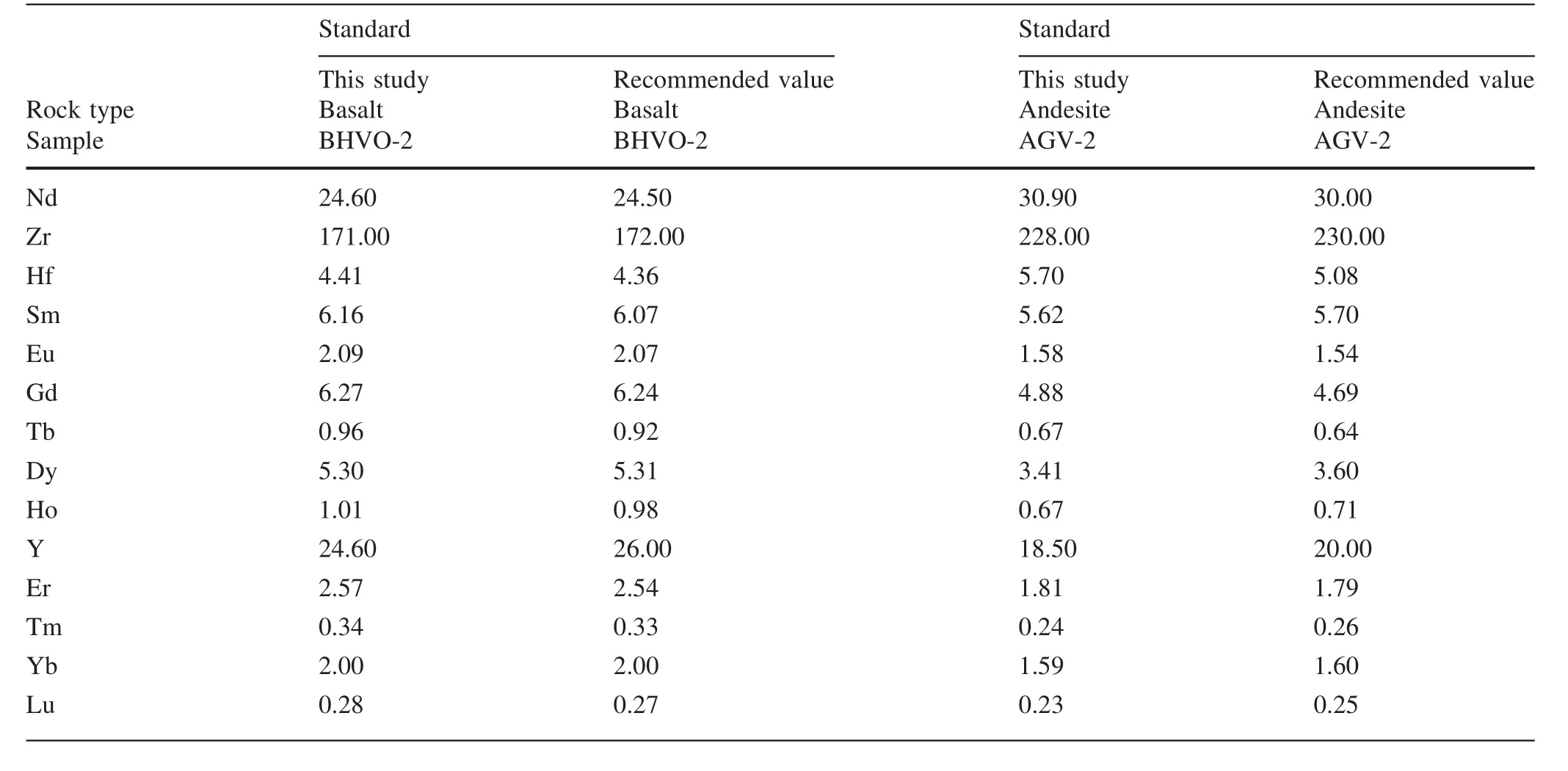
Table 4 continued
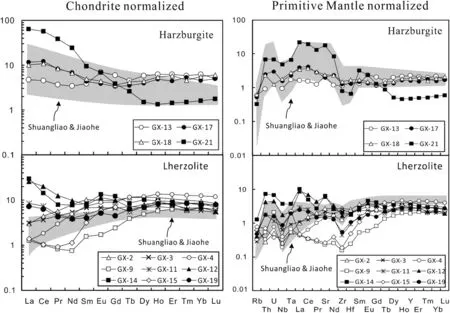
Fig.4 Chondrite-normalized REE patterns a,c and primitive mantle-normalized incompatible element patterns b,d for Cpx from the Shangzhi peridotite xenoliths.Chondrite normalization values are from Anders and Grevesse(1989).Primitive mantle normalization values are from McDonough and Sun(1995).Cpx in the Jiaohe and Shuangliao lherzolites and harzburgites reported by Yu et al.2009 are also shown for comparison
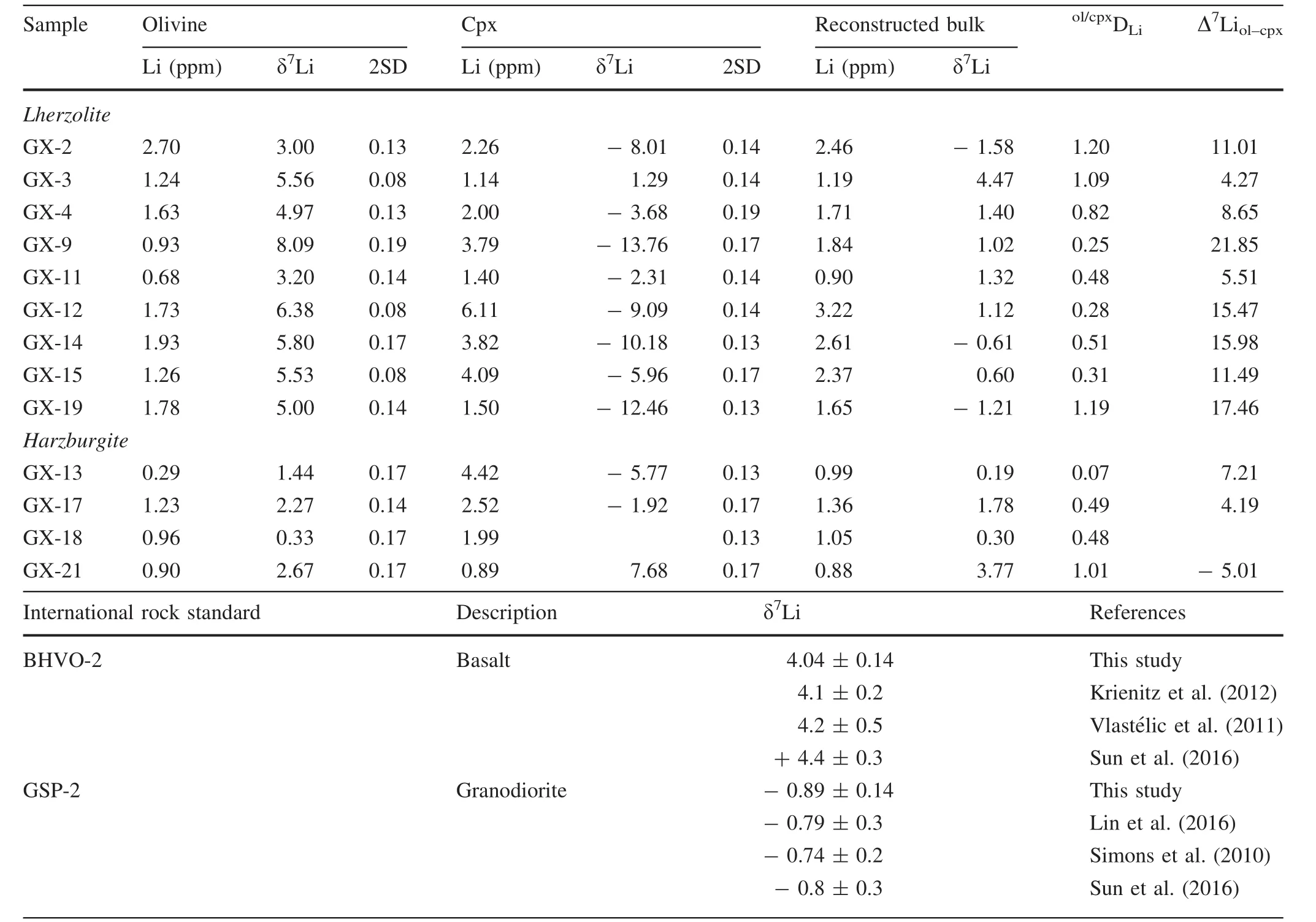
Table 5 Li concentration and isotopic compositions from Shangzhi peridotite xenoliths
5 Discussion
5.1 Partial melting and metasomatism in the Shangzhi peridotite xenoliths
The Shangzhi peridotites delineate a sequence from primitive lherzolite through Cpx-poor lherzolite to highly refractory harzburgite.These peridotites define a correlation between Mg#oland Cr#sp(Fig.3c),which is in accordance with experimental data(Jaques and Green 1980)and studies on natural samples(Cabanes and Mercier 1988).Specifically,Shangzhi lherzolites show low and restricted variation of Mg#ol(88.75–90.70)similar to the fertile normal-mantle peridotite,indicating low degrees of partial melting.Refractory harzburgites show high and restricted Mg#ol(90.23–91.10)corresponding to high degrees of partial melting.These observations are consistent with whole rock major element compositions(Fig.3a).For instance,in the diagram of MgO versus Al2O3,the Shangzhi peridotites follow the modeled partial melting trend of Niu(1997).Modeling the behavior of immobile elements during fractional melting within the spinel stability field(Johnson et al.1990;Norman 1998)suggests that the Shangzhi lherzolites are residues generated by relatively lower degrees of partial melting(<7%),whereas the Shangzhi harzburgites were formed through higher degrees of partial melting(8%–18%).
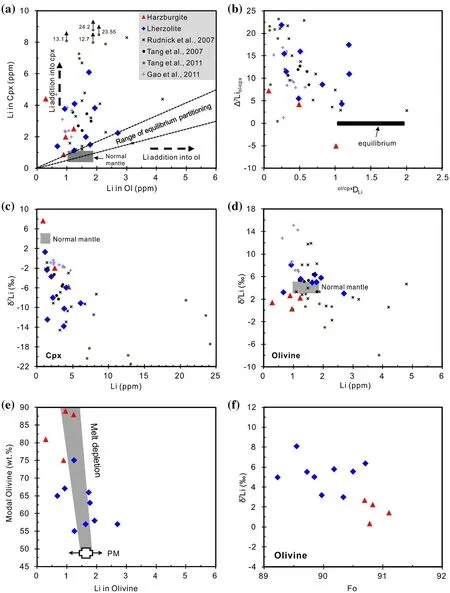
Fig.5 a Lithium abundance in Cpx versus coexisting olivine;b plot of partition coefficient for Li between olivine and Cpx(ol/cpxDLi)versus Li isotopic fractionation between olivine and Cpx(Δ7Liol–cpx).Gray horizontal bar shows equilibrium conditions from Rudnick and Ionov(2007)with equilibrium Δ7Liol–cpxassumed here to be zero(± 1‰)at mantle temperature;c-d plots of Li concentration against δ7Li for olivine and Cpx.Grey field for‘‘normal mantle’’in(a)is represented by fertile to moderately depleted peridotites(Seitz and Woodland 2000).The range of equilibrium partitioning of Li between olivine and Cpx are from Brenan et al.(1998),Eggins et al.(1998)and Seitz and Woodland(2000)(ol/cpxDLi=1.1–2.0).Grey fields in(c)and(d)are after Jeffcoate et al.(2007),Pogge von Strandmann et al.(2011)and Lai et al.(2015).Peridotitic samples from Rudnick and Ionov(2007),Tang et al.(2007b,2011)and Gao and Snow(2011)are shown for comparison;e plot of modal olivine against Li concentration in olivine.Melt depletion trend is after Rudnick and Ionov(2007).Primitive mantle(PM)is after Sun and Mcdonough(1989);f plot of Fo against δ7Li(‰)in olivine
Most Shangzhi peridotites show signs of mantle metasomatism,such as enrichment in Na2O(Fig.3b,d)and highly incompatible elements(e.g.,Th,U,Nb,LREE;Fig.4).Cpx in two lherzolites,i.e.GX-9 and GX-15,displays a spoon-shaped REE pattern,resembling the Cpx with analogous shapes and enrichment in mantle peridotites from Shuangliao and Jiaohe in NE China(Fig.4;Yu et al.2009),which can be explained by the chromatographic metasomatism of melt in filtration(Navon and Stolper,1987).Cpx in harzburgites overall displaysenriched-LREE patterns.It should be pointed out that Cpx in harzburgite GX-21 has remarkably higher LREE and LILE enrichment than other peridotites(Fig.4),which suggests that this sample has experienced stronger mantle metasomatism compared with other samples.The absence of modal metasomatic minerals(i.e.,mica,amphibole or apatite)suggests that the metasomatism that was responsible for the enrichment of incompatible elements in the Shangzhi peridotites is largely cryptic,i.e.,only introducing incompatible elements without any petrographic modification(Frey and Green 1974).It remains to assess whether the enrichment of incompatible elements in the Shangzhi peridotites was related to chromatographic metasomatism with a single agent,or multiple metasomatism involving different metasomatic agents.It has been suggested that mantle xenoliths metasomatized by silicate melt would be enriched in incompatible trace elements including Th,U,Nb,and LREE(e.g.,Wittig et al.2007;Neumann and Wulff-pedersen 1997).In contrast,percolating of the other kinds of melts,such as volatile-rich or carbonatite melts may only result in LILE enrichment,while their HFSE(e.g.,Ti and Zr)concentrations were little changed(e.g.,Ionov et al.1996;Yaxley et al.1991;O’Reilly and Griffin 1988).Cpx in the Shangzhi peridotite xenoliths have low(La/Yb)nbut highly variable Ti/Eu ratios,plotting within the field of silicate metasomatism(Fig.7).This suggests that the Shangzhi peridotites have been subjected to silicate metasomatism.
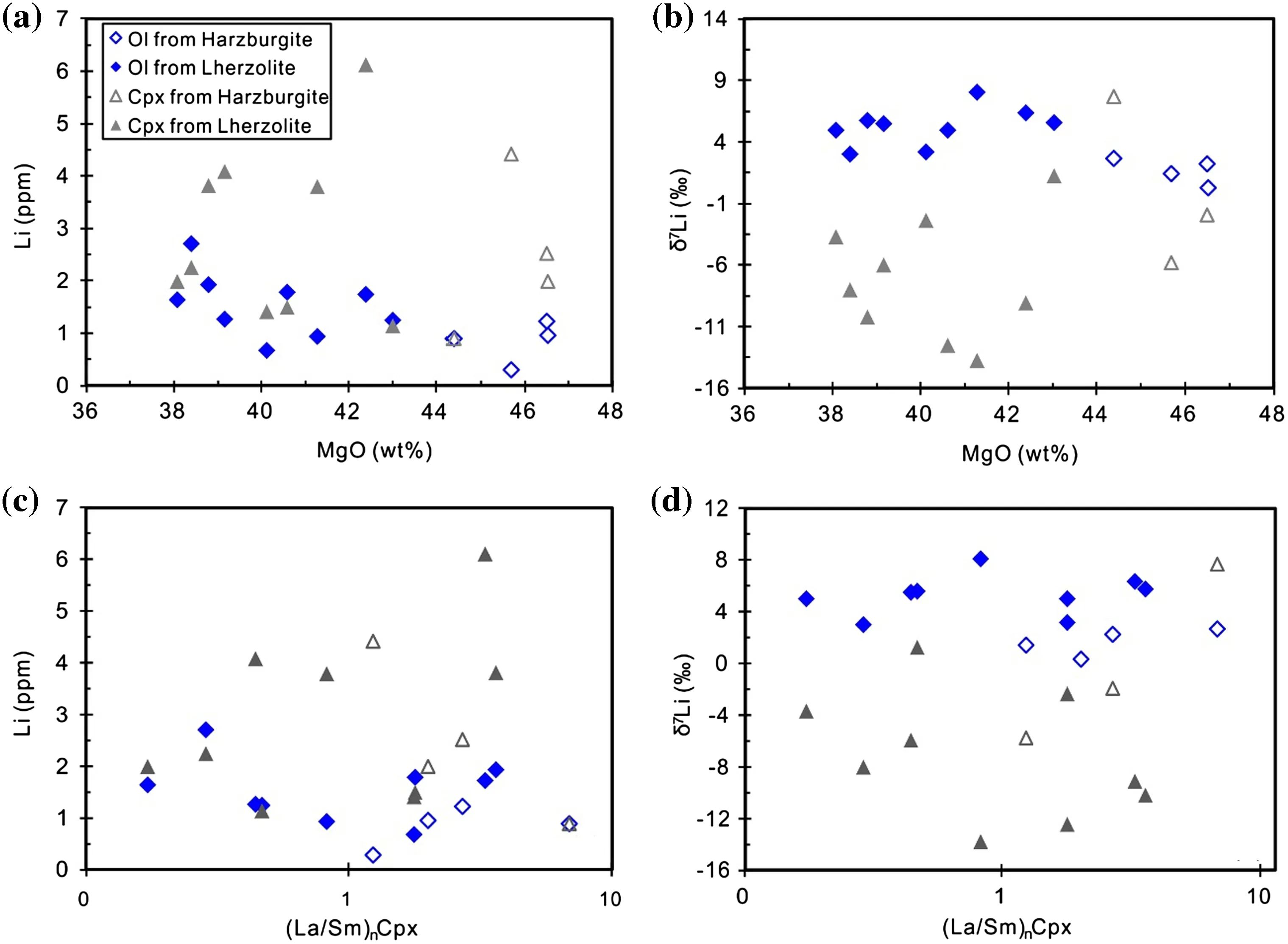
Fig.6 a–b Plots of whole-rock MgO against Li concentration and δ7Li in olivine and Cpx;cd plots of primitive mantle normalized La/Sm ratios in Cpx versus Li concentration and δ7Li in olivine and Cpx
5.2 Origin of Li isotopic fractionation in the Shangzhi peridotite xenoliths
5.2.1 Equilibrium fractionation during partial melting
Liconcentration (0.3–2.7 ppm)ofolivine from the Shangzhi peridotites are similar to the normal mantle(1–1.8 ppm;Seitz and Woodland 2000;Fig.5a)and show roughly negative correlations with the indices of melt extraction(such as modal olivine and whole rock MgO)(Figs.5e,6a).These features are consistent with variable degrees of partial melting because Li is a moderately incompatible element whose partition coefficient remains constant as long as equilibrium is maintained.Partial melting of mantle peridotite can lead to Li depletion of<1 ppm in olivine(Xiao et al.2017).On the other hand,δ7Li values for olivine(mostly ranging from 2‰ to 6‰)in the Shangzhi peridotites are comparable to the estimated isotopic composition of the normal mantle(3‰ to 5‰)(Magna et al.2008;Pogge von Strandmann et al.2011;Lai et al.2015).δ7Li values for olivine are also negatively correlated with Fo(Fig.5f)and whole rock MgO(Fig.6b).This feature can also be attributed to variable degrees of partial melting(Xiao et al.2017).
Coexisting Cpx displays variable degrees of Li enrichment(0.9–6.1 ppm)and extremely low δ7Livalues(-13.8‰ to+7.7‰)compared with the normal mantle(Fig.5c).Moreover,in the diagram of the partition coefficient for Li between olivine and Cpx(ol/cpxDLi)versus the degree of isotopic equilibrium(Δ7Liol/cpx,Fig.5b),the Shangzhi peridotites display strong element and isotopic disequilibria.Such characteristics clearly cannotbe explained by equilibrium fractionation during partial melting and may reflect later Li addition to Cpx after the partial melting events.
5.2.2 The effect of diffusive fractionation on minerals in the Shangzhi peridotite xenoliths
Diffusion is an important mechanism for the Li isotopic variation in mantle peridotites(Tomascak et al.2016).Various diffusion processes have been proposed to account for Li isotopic variations in mantle xenoliths,including Liredistribution between minerals during slow cooling of xenoliths after the eruption of their host magmas(Ionov and Seitz 2008;Gallagher and Elliott 2009;Gao and Snow 2011),lithium addition from recycled melt with low δ7Li(Nishio et al.2004;Tang et al.2007b,2012,2014;Su et al.2016)and Li diffusion from host magma/percolating melt(Rudnick and Ionov 2007;Jeffcoate et al.2007;Aulbach et al.2008,2013;Aulbach and Rudnick 2009;Lai et al.2015).These possibilities are evaluated below.
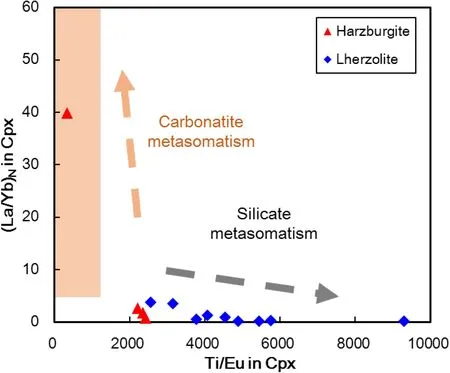
Fig.7 Plots of Ti/Eu against(La/Yb)Nin Cpx from the Shangzhi peridotites.Field for ‘‘silicate’’and ‘‘carbonatitic’’metasomatism are after Coltorti et al.(1999)
Gallagher and Elliott(2009)proposed a model that describes diffusion of Li from olivine into Cpx related to slow cooling of xenoliths after the eruption of their host magmas.In this model,δ7Li for Cpx tends to be lower,while δ7Li for olivine tends to be higher than the normal mantle due to faster diffusion of6Li than7Li from olivine to Cpx during slow cooling of the magmatic system(Fig.5c,d;Ionov and Seitz 2008;Gao and Snow 2011).Meanwhile,Li concentrations of Cpx tend to be higher,while Li concentrations of olivine tend to be lower than the normal mantle during cooling.However,olivine in the Shangzhi peridotites shows generally normal mantle-like δ7Li and Li concentrations(Fig.5d)(Magna et al.2008;Pogge von Strandmann et al.2011;Lai et al.2015),which is inconsistent with this model.Moreover,Li isotopic fractionation between minerals during slow cooling of a magmatic system typically displays increasing Δ7Liol–cpx(δ7Liol–δ7Licpx)values with decreasing temperature(Gao and Snow 2011).However,such a phenomenon was not observed in the Shangzhi peridotites.More direct evidence is from the reconstructed bulk δ7Li and Li concentrations of the Shangzhi peridotites(Table 5)which show higher Li concentration(0.9–3.2 ppm)and lower δ7Li(-1.6‰ to+4.5‰)than the normalmantle (1–1.5 ppm and 3‰-5‰)(Seitz and Woodland 2000;Magna et al.2008;Pogge von Strandmann et al.2011;Lai et al.2015).This clearly cannot be explained by redistribution of Li during slow cooling of a magmatic system because such closedsystem Li-redistribution would only cause transitory fractionations of Li isotopes between coexisting minerals.The bulk Li isotopic compositions should remain unchanged.Thus,all these features are inconsistent with the model of Li isotopic fractionation during slow cooling.
Low δ7Li melt/ fluid related to subducted slab has been invoked to explain the negative δ7Li values observed in Cpx separated from mantle xenoliths(Nishio et al.2004;Tang et al.2007b,2012,2014;Su et al.2012,2014a).High Li concentrations and negative δ7Li values for Cpx from the Shangzhi peridotites apparently can be attributed to subduction-related melt in filtration.Moreover,low bulk δ7Li(-1.6‰ to+4.5‰)and high bulk Li concentrations(0.9–3.2 ppm)of the Shangzhi peridotites are also consistent with low-δ7Li melt/ fluid addition.The possible role of low-δ7Li melt addition in the genesis of the Shangzhi peridotites can be evaluated by mixing calculations using Li concentration and δ7Li values(Fig.8).We use Cpx from normal mantle with δ7Li of 3.5‰ and Li concentration of 1 ppm to represent the composition of Cpx from uncontaminated mantle peridotite(Seitz and Woodland 2000;Magna et al.2008;Pogge von Strandmann et al.2011;Lai et al.2015).The δ7Li values for assumed in filtrated melt/ fluid range from-20‰ to-10‰,which is similar to the values of subduction-related eclogites(Zack et al.2003;Marschall et al.2007;Halama et al.2011).The Liconcentration of subduction-related melt/ fluid is assumed to be 50 ppm.The high Li concentrations of these melts/ fluids were generated by the variable degrees of partial melting which fall within the range of Li concentrations of arc lavas(7–53 ppm)(Chan et al.2002;Agostini et al.2008;Tang et al.2014).The results show that variations of Li concentrations and δ7Li values for Cpx from the Shangzhi peridotites require addition of up to 50%–60%of melt/ fluid derived from subduction oceanic crust(Fig.8).Such large quantities of melt addition are unrealistic and would not retain a peridotitic composition.Moreover,the δ7Li for olivine should be also low if the low δ7Li for Cpx was the effect of low-δ7Li melt in filtration.On the other hand,Li concentration of olivine should be higher than the normal mantle because of external Li addition.These features are inconsistent with olivine in the Shangzhi peridotites which show generally normal mantlelike δ7Li and Li concentrations(Fig.5d).Thus,this possibility is short of strong evidence although it cannot be totally ruled out.
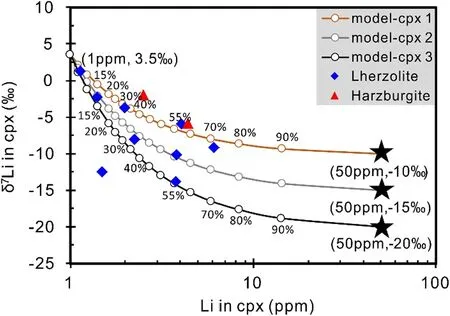
Fig.8 Binary mixing of Cpx of variable degrees by assumed subduction-related melts with low δ7Li values(-10‰ to-20‰).See text for more detailed explanation
We consider that the low δ7Li and high Li contents for Cpx and some bulk peridotites from Shangzhi were generated by kinetic isotope fractionation due to Li diffusion from host magma/percolating melt/ fluid shortly before or coincident with their entrainment into the host magmas(Jeffcoate et al.2007;Rudnick and Ionov 2007;Aulbach et al.2008;Magna et al.2008;Su et al.2014b).This percolating melt/ fluid would generate a chemical potential gradient to drive diffusion of Li from melt into peridotite.6Li would preferentially diffuse out of melt conduits into wall-rock peridotites during this process(Lundstrom et al.2005)and result in the decreasing of δ7Li for Cpx and bulk peridotite from Shangzhi.At the same time,low Li concentrations and normal mantle-like isotopic compositions of olivine in the Shangzhi samples suggest olivine was little affected by external Li addition.This may relate to much lower diffusivity of Li in olivine and larger grain sizes of olivine relative to Cpx(Richter et al.2003,2014).
In order to quantify how Li isotope ratios and concentrations change due to chemical diffusion from the percolating melt/ fluid,we conduct a modeling calculation applying the equation from Lai et al.(2015):
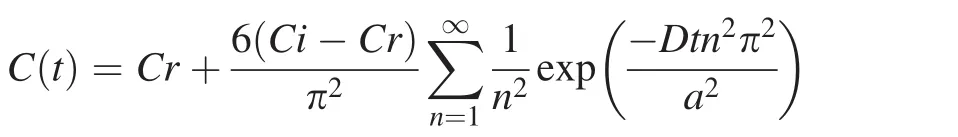
In this equation,‘‘C(t)’’represents the Li concentration in a bulk mineral at a given time; ‘‘Ci’’represents the original concentration in the mineral core; ‘‘Cr’’represents the Li concentration at the mineral surface,which is in equilibrium with the percolating melt; ‘‘D’’represents the diffusion coefficient of Li in a mineral;‘‘t’’represents time and‘‘a’’represents the radius of a mineral.We assume that the crystal is a sphere and the grain radius is ‘‘r’’.We also assume the melt-rock interaction occurred at 1200°C and remained constant over the relatively short period of melt percolation.Li diffusivities in Cpx and olivine at 1200°C are 2.1×10-11and 2.29×10-15m2/s,respectively(Coogan et al.2005;Dohmen et al.2010).The partition coefficient of Li in Cpx and olivine are 0.27 and 0.35,respectively(Brenan et al.1998).The diffusion coefficients of both6Li and7Li are related by the expression D7/D6=(m6/m7)β,where m6and m7are atomic masses(i.e.,6 and 7)(Richter et al.2003).A value for β of 0.27 is used in this model(Richter et al.2014).The grain radiuses are 0.5 mm for Cpx and 1.5 mm for olivine,respectively.
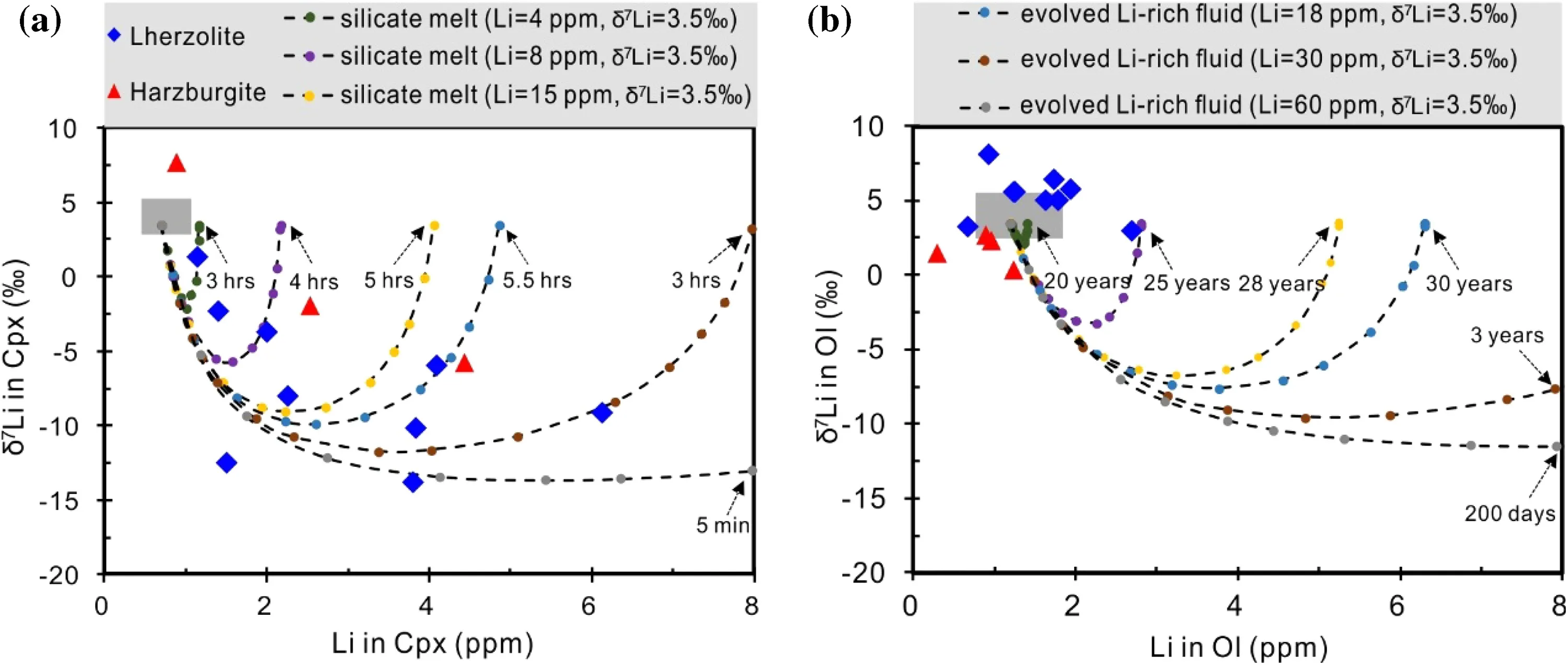
Fig.9 Modeled trajectories of Li concentrations against δ7Li for Cpx a and olivine b experiencing Li diffusion from silicate melts or Li-rich fluids with normal mantle-like δ7Li and variable Li concentrations.Each curve represents the pathway of a mineral with elapsed time.Grey fields show range of ‘‘normal mantle’’concentrations and δ7Li for Cpx and olivine.See text for more detailed explanation
The initial Li concentration and δ7Li for Cpx are assumed to be 0.7 ppm and 3.5‰,respectively,which are within the range of normal mantle(Seitz and Woodland 2000;Magna et al.2008;Pogge von Strandmann et al.2011;Lai et al.2015).The initial Li concentration and δ7Li for olivine are assumed to be 1.2 ppm and 3.5‰,respectively,which are within the range of pristine upper mantle values(Seitz and Woodland 2000;Magna et al.2008;Pogge von Strandmann et al.2011;Lai et al.2015).Silicate melt and fluid are used in our calculation.As discussed earlier,incompatible trace element enrichment in Cpx is possibly related to silicate melt metasomatism.It is reasonable to speculate that a possible relationship exists between Li isotope fractionation and silicate melt in filtration.The Li isotope composition of these percolating melts is speculated to be the same as that of common mantle melts(3.5‰).Li concentrations of silicate melts are assumed to be ranging from 4 to 15 ppm,which are similar to those of typical MORB and OIB(Chan and Frey 2003;Elliott et al.2006;Tomascak et al.2008;Magna et al.2008;Marschall et al.2017).Li concentrations of fluids(18–60 ppm)are speculated to be much higher than typical silicate melts(Wunder et al.2006).These fluids may come from degassing and the fractional crystallization of the magma due to the high solubility of Li in fluids(Rudnick and Ionov 2007)or from exsolution of Li-rich fluids from the magma due to a high partition coefficient between fluid and melt(DLifluid/meltup to 13)(Webster et al.1989)during exsolution of a chlorite-bearing fluid(Teng et al.2006;Aulbach and Rudnick 2009;Schuessler et al.2009).
The calculated results show that Li diffusion from silicate melts or Li-rich fluids leads to a strong enrichment of Li and a sharp decrease of δ7Li for Cpx in a very short time(several minutes to~5 h,Fig.9a).In contrast,it would take much more time to lower δ7Li for olivine(several years,Fig.9b).The low δ7Li and high Li contents of Cpx from the Shangzhi peridotites can be readily explained by Li diffusion from silicate melts or Li-rich fluids in a very recent time(Fig.9a).The Cpx with light δ7Li values and Li enrichment is also observed in a suite of spinel peridotites from Hannuoba,Fanshi,Hebi areas in the North China Craton(NCC),which was explained to be a consequence of diffusive fractionation caused by recent Li ingress from in filtrating melts/ fluids (Tang et al.2007b,2011).Compared to the published Li isotopic data of bulk minerals from NCC,Cpx in the Shangzhi peridotites shows similar variations in Li abundances and δ7Li(Fig.5c,d).Furthermore,in situ isotopic profiles across minerals from Hannuoba,Beiyan,Kuandian xenoliths from NCC,and Dayishan xenoliths from NE China revealed clear Li isotopic zoning in Cpx,displaying increasing Li concentration and decreasing δ7Li from core to rim,which can be attributed to Li diffusive ingress into Cpx from metasomatic melts/ fluids(Tang et al.2007b;Xu et al.2013a,b;Xiao et al.2015;Su et al.2017b).Thus,these observations provide further evidence that diffusive fractionation of Li isotopes during peridotite-melt/ fluid interaction plays an important role in producing Li enrichment and light δ7Li of Cpx in the Shangzhi peridotites.On the other hand,normal mantle-like isotopic compositions and low Li concentrations of olivine from the Shangzhi peridotites imply that olivine was little affected by such shorttime melt/ fluid in filtration because of much lower diffusivity of Li in olivine(Richter et al.2003,2014).Lastly,the light Li isotopic compositions preserved in the bulk samples also indicate that the percolated melts/ fluids have not had enough time to isotopically equilibrate with the bulk peridotite.
6 Conclusions
Li isotopic fractionation in the Shangzhi mantle xenoliths is mainly related to Li diffusion from silicate melts or Lirich fluids shortly before or coincident with their entrainment into the host magmas.Low δ7Li and high Li contents of Cpx from the Shangzhi peridotites can be explained by Li diffusion from such melts/ fluids in a very short time(several minutes to several hours).In contrast,normal mantle-like isotopic compositions and low Li concentrations of olivine from the Shangzhi peridotites indicate that olivine was little affected by such short-time melts/ fluids in filtration.The negative correlation between δ7Li and Fo for olivine is consistent with variable degrees of partial melting rather than melt in filtration.
AcknowledgementsWe thank Prof.Yi-Lin Xiao and Hai-Yang Liu from the University of Science and Technology of China and Ting Zhou from the Institute of Geochemistry,Chinese Academy of Sciences for their assistance in analysis of Li isotopes.This study was funded by the strategic priority research program(B)of the Chinese Academy of Sciences(XDB18000000),NSFC(41573009;41373042,41203031).Open research fund of the State Key Laboratory of Ore Deposit Geochemistry of China(SKLODG Grant#201204).We thank three anonymous reviewers for their constructive reviews which greatly improved the quality of this manuscript.
杂志排行
Acta Geochimica的其它文章
- Trace element composition of magnetite from the Xinqiao Fe-S(-Cu-Au)deposit,Tongling,Eastern China:constraints on fluid evolution and ore genesis
- Theoretical calculation of equilibrium Mg isotope fractionation between silicate melt and its vapor
- Zinc isotope fractionation under vaporization processes and in aqueous solutions
- The partitioning patterns of nutrients between pods and seeds of Zanthoxylum fruits impacted by environmental factors
- Geological significance of nickeliferous minerals in the Fule Pb-Zn deposit,Yunnan Province,China
- Iron isotopic analyses of geological reference materials on MCICP-MS with instrumental mass bias corrected by three independent methods
