The performance of the Noblesse multi-collector noble gas mass spectrometer for40Ar/39Ar geochronology
2018-10-26·,2···
·,2···
Abstract Noblesse multi-collector noble gas mass spectrometer is specially designed for multi-collection of Ar isotopes with different beam sizes,especially for small ion beams,precisely,and hence is perfectly suitable for 40Ar/39Ar geochronology.We have analyzed widely used sanidine,muscovite,and biotite standards with recommended ages of~ 1.2–133 Ma,with the aim to assess the reliability of Noblesse for40Ar/39Ar dating.An ESI MIR10 30W CO2laser was used for total fusion or incremental heating samples.Extracted gases were routinely purified by four SAES NP10 getters(one at~ 400°C and others at room temperature).A GP50 getter and a metal cold finger cooled by liquid N(-196°C)were also attached for additional purification if necessary.The Ar isotopes were then measured by Noblesse using Faraday or multiplier according to the signal intensities.Over a period of 1.5 months 337 air calibrations produced a weighted mean 40Ar/36Ar of 296.50± 0.08(2σ,MSWD=4.77).Fish Canyon sanidine is used to calculate J-values,which show good linear relationship with position in irradiation.The age of four mineral standards(Alder Creek sanidine,Brione muscovite,Yabachi sanidine,and Fangshan biotite)are within error of the accepted ages.Five Alder Creek sanidine aliquots yielded an age range of 1.174–1.181 ± 0.013 Ma(2σ)which broadly overlaps the established age of the standard and the uncertainty approaches those of the foremost Ar/Ar laboratories in the world.The weighted mean ages of four Brione muscovite aliquots(18.75± 0.16 Ma,2σ), five Yabachi sanidine aliquots(29.50± 0.19 Ma,2σ),and three Fangshan biotite aliquots(133.0± 0.76 Ma,2σ)are consistent with the recommended values of these standards,and the uncertainties are typical of modern Ar/Ar laboratories worldwide.
Keywords Ar/Ar geochronology⋅Multi-collector⋅High precision⋅Noblesse⋅Age standard
1 Introduction
The40Ar/39Ar dating method is arguably the most widely applied and precise methods of geochronology.It is predicated on the radioactive decay of40K to40Ar and has largely superseded the K–Ar technique(Merrihue and Turner 1966;McDougall and Harrison 1999).The K–Ar method,as originally formulated,relies on direct measurement of K and Ar abundances in two separate splits of a dateable material.This approach is limited by a number of potential factors including sample inhomogeneity,initial excess argon,and Ar-loss and-gain after cooling below the Ar closure temperature.The40Ar/39Ar dating technique is based on the conversion of39K to39Ar in samples by neutron activation(n,p reaction)in a nuclear reactor.The39Ar abundance serves as a proxy for40K (requires assumption about the40K/39K ratio)and permits the simultaneous measurement of both the parent and daughter nuclide in a single argon isotope measurement.A standard mineral(monitor)with an independently determined age is used to precisely calculate the amount of39K that has been converted to39Ar.The monitors are evenly distributed amongst the irradiated sample so that the neutron fluence(39K production rate,J)can be calculated at a given sample position.Subsequent40Ar/39Ar measurements for an unknown sample can then be used to calculate age.
Ar isotope determinations for40Ar/39Ar geochronology are typically determined using single-collector magnetic sector and mass spectrometers.Data collection is performed using a ‘‘peak-jumping’’procedure where the magnetic field strength or the ion acceleration potential is changed to focus the individual Ar isotopes onto a single collector.A new generation of multiple-collector magnetic sector mass spectrometers have been developed for40Ar/39Ar dating.One design uses five Faraday detectors equipped with 1011and 1012Ω resistors(GV Instruments ARGUS V;Mark et al.2009).A variant of this instrument includes a low mass electron multiplier(Thermo Scientific ARGUS VI;Kim and Jeon 2015;Phillips et al.2017;Bai et al.2018).The Nu-Instruments Noblesse mass spectrometer has an array of ion-counting electron multipliers for Ar isotope abundance determinations(Cosca 2007;Coble et al.2011;Jicha et al.2010,2016).The third mass spectrometer has an array of five movable detectors,which can each be switched between an ion-counting electron multiplier and a Faraday cup(GV Instruments HELIX-MC and Thermo Scientific HELIX-MC plus,Marrocchi et al.2009;Zhang et al.2016).Isotopic ratio measurement using multi-collector mass spectrometers have better precision than single collector instruments,and they dramatically reduce the size of samples that are necessary for40Ar/39Ar analysis.They also increase sample throughput and permit more robust analytical strategies,such as better background and discrimination control,replicate analyses,etc.In this contribution we describe the Nu instruments Noblesse mass spectrometer that is located in the State Key Laboratory of Ore Deposit Geochemistry,Institute of Geochemistry Chinese Academy of Sciences(IGCAS),and we review its performance.
2 Mass spectrometer description
The Nu instruments Noblesse mass spectrometer is a single focusing magnetic sector instrument with a 75°angle flight tube,two ion-optic lens arrays and a Nier-type ion source.The focusing of the ion beam and collector-slit spacing produce broad, flat-topped peaks with steep sides that afford improved resolution compared to the previous generation of single-collector instruments.The Nu Noblesse at IGCAS has a sensitivity of 14.0 A/mol Ar in static mode using the Faraday detector.The analytical volume of 2229±22 mL includes SAES NP10 getters mounted on source and detector blocks.A Faraday cup(FAC)with 1011Ω resistor occupies the high mass position,while three secondary electron multipliers(ETP)operating in ioncounting(IC)mode are fixed in an array designated IC0,IC1,and IC2(from high to low mass).Electrostatic filters are located at the entrance of the IC1 and IC2 detectors in order to suppress scattered ions.Background levels of Ar in the extraction-purification system(isolated for 5 min)are typically 5.5×10-16mol40Ar,9.8×10-19mol39Ar,1.1×10-18mol38Ar, 1.7×10-17mol37Ar,4.4×10-18mol36Ar.Blanks that completely simulate the analysis procedure are measurably higher:7.5×10-16,5.0×10-17, 1.7×10-18, 1.9×10-17, and 7.6×10-18mol for40Ar through36Ar.
The mass resolving power(MRP),or the ability of the instrument to resolve isobaric interferences,is defined as m/Δm,where m is the peak mass and Δm is the mass difference between 5%and 95%peak height as determined by the side of the peak(Ireland 2013).The IGCAS Noblesse has MRP that is typically 2200–2800.This does not allow for the resolution of the interferences produced by HCl+and hydrocarbon species that are present at the high mass side of the Ar peaks.This is especially important for12C3+and H35Cl+at mass 36.Peak heights are measured on the least affected low-mass peak sides,and these are accounted for by equivalent measurements in the blank.
An ultra-high vacuum gas purification line is directly attached to the mass spectrometer.Calibration gases are contained in two gas reservoirs that include pipettes for producing reproducible gas amounts.Gas is extracted either using an ESI MIR10 30W CO2laser or a doublewalled vacuum furnace.The gas released from samples is purified by exposure to two SAES NP10 getters.A metal cold finger cooled by liquid N(-196°C)and a GP50 getter are used for ‘‘dirty’’samples,such as whole rock basalts.Single crystals(e.g.sanidine,mica)can be fused with the laser,and~ 1–5 mg of K-bearing minerals can be heated incrementally.Samples are melted in a single step using laser power of 5–6 W for 30 s.For incremental heating extraction,the sample is irradiated by the laser beam in approximately ten steps ranging from 0.6 to 6 W for 90 s in a raster scan mode.The active gases are consumed by the NP10 getters for 5 min.One getter is held at~ 400 °C(0.65 A),and the other kept at room temperature.For ‘‘dirty’’samples,the gas is exposed to the cold finger and the GP50 getter for an additional 5 min.Blanks are measured for every three analyses of samples.Purification procedure and timings are identical to those used for sample analysis.Air standards are measured after every ten analyses in order to determine mass discrimination.The raw data of blank,air,standard,and sample are input into ArArCalc software(Koppers 2002),and regressed to inlet time.The J-value and sample age are determined from the measured Ar isotope composition after the corrections are made for interfering nuclear reactions,mass discrimination,procedural blanks,and concentration of atmospheric Ar.Each age includes the error assigned to the J parameter,in this case 0.5%.
3 Performance
Typical multi-collection analysis methods for the IGCAS Noblesse are summarized in Table 1.Each method requires unique detector and source settings in order to optimize peak shape and maximize beam intensity(Fig.1).For analysis of atmospheric Ar,the40Ar,38Ar and36Ar beam intensities are measured simultaneously on the Faraday cup,IC1 and IC2,respectively.For typical40Ar/39Ar measurement of samples,40Ar and39Ar are usually analyzed on the same detector in peak-jumping mode(for example method 2 and method 4;Table 1)to minimize the influence of IC gain fluctuation during analysis.In order to generate similar beam intensities of40Ar and39Ar,irradiation time and sample amounts are carefully calculated according to their potassium contents and estimated ages.Samples with significantly different40Ar and39Ar beam intensities require use of the Faraday and IC1,respectively(e.g.,method 3;Table 1).The beam intensity ratio intercalibration(BI-IC)method(Turrin and Swisher III 2010),which measures the appropriate40Ar beam size(3–15 mV)among Faraday and electron multipliers,was applied to determine the IC gains.They were measured before experiments each day and typically reported averages of 0.993±0.002 (IC0), 0.934±0.002 (IC1) and 0.993± 0.002(IC2)for 10 days(1σ,10 runs from June 8th to 18th,2017)and showed no correlation with beam size(Fig.2).
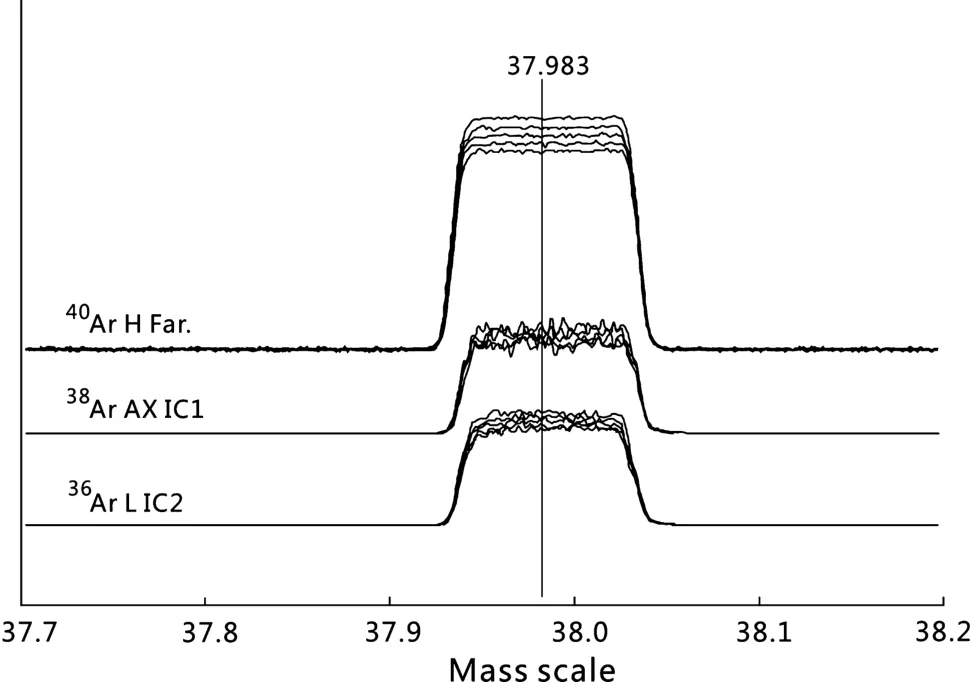
Fig.1 Peak scans showing peak top and peak side stability during continuous scanning of an air calibration shot(initially~100 mV for40Ar,25 s per scan and~135 s interval between 2 scans).Gas consumption within the mass spectrometer accounts for the decreasing beam intensities
4 Air standards
The Ar isotope analysis of a typical air standard is shown in Fig.3.All beam intensities decreased with time.They showed slight concave upward forms that reflect the consumption of gas by ionization and detection.Exponential lines were fitted,but the Ar isotope ratios showed no obvious change with time.Figure 4a,b show40Ar(Faraday)and36Ar(electron multiplier)beam intensities plotted against the precision of measurements.Faraday beam intensities derived from 1.1×10-11mol Ar have a measurement precision of 0.002%. For analysis of 2.1×10-14mol the measurement precision was 0.46%.For the electron multiplier,3.7×10-14mol Ar was measured with a precision of 0.03% whereas 7.4×10-17mol Ar was measured with a precision of 0.58%.Pipettes of air yielding 7.9×10-13mol40Ar produced a beam intensity of~110 mV on the Faraday cup that had a precision of 0.02%,while 2.7×10-15mol36Ar produced~2.3×104counts per second(cps)on theelectron multiplier(equivalent to 0.37 mV on the Faraday)and had a precision of 0.1%.
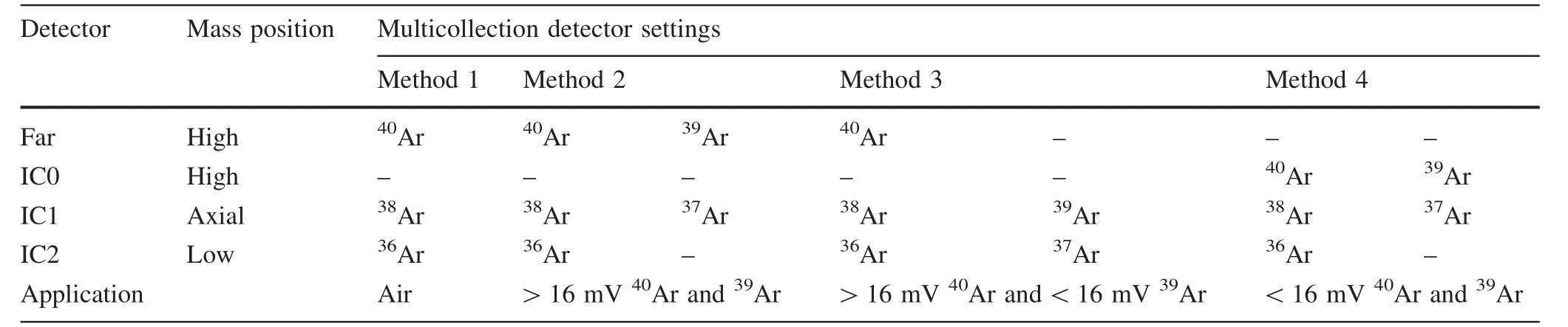
Table 1 Typical multi-collection analysis methods for a Noblesse con figured with one Faraday(FAC)and three ion-counting electron multipliers(IC)
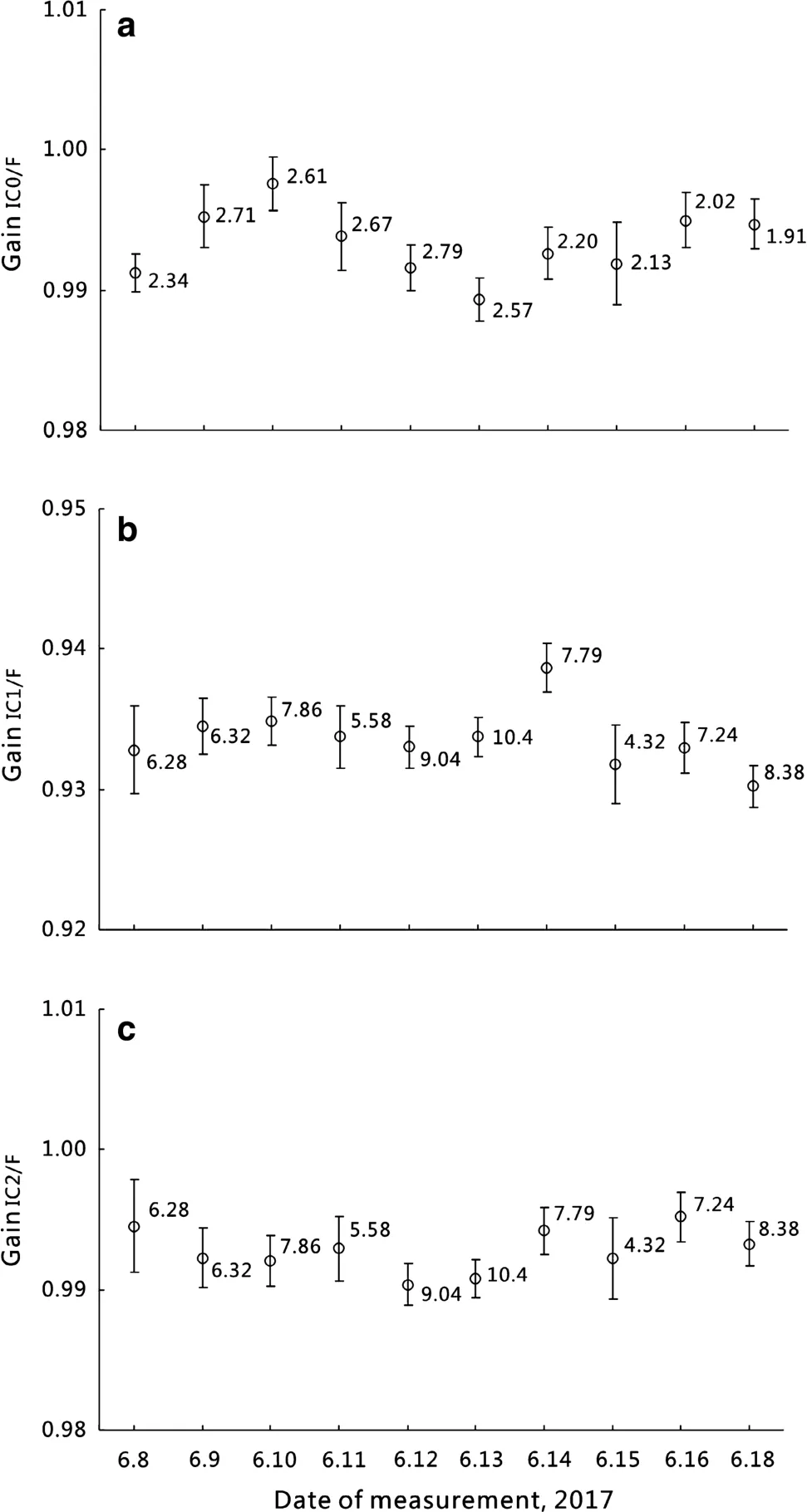
Fig.2 a–c IC gains with 1 sigma uncertainties measured using(BIIC)method(Turrin and Swisher III 2010)remain almost constant within 10 days of sample analysis and exhibit no correlation with beam size.The intensity of40Ar on Faraday(mV)of each analysis is adjacent to each data point
The40Ar/36Ar of 337 air calibrations measured between October 18th and November 30th 2016 have a normal distribution and yield a weighted mean of 296.50±0.08(Fig.5;2σ,MSWD=4.77).This corresponds to a mass discrimination value of 1.001738±0.000543,according to the atmospheric argon40Ar/36Ar(298.56±0.31;Lee et al.2006).
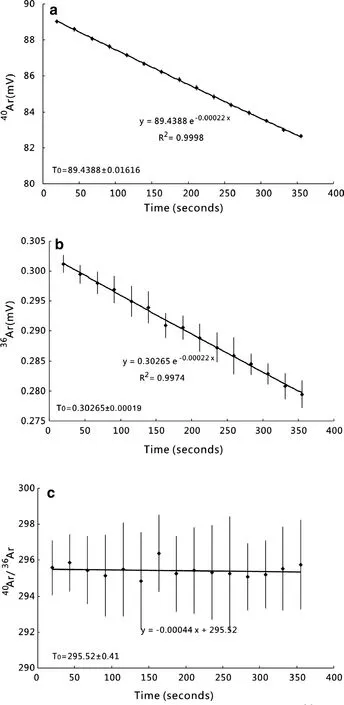
Fig.3 a Raw40Ar peak evolution.Data conform to an exponential fit.b Raw36Ar peak evolution.Data conform to an exponential fit.c Multi-collector40Ar/36Ar collected online
The effect of Ar partial pressure on source sensitivity and mass fractionation was assessed by the analysis of varying amounts of atmospheric Ar.There was no resolvable difference in the measured40Ar/36Ar ratio by varying the40Ar signal size from 1538 mV(1.1×10-11mol)to 2.94 mV(2.1×10-14mol;n=72)(Fig.4c).During analysis of40Ar/39Ar mineral age standards,there was no significant control on the40Ar/39Ar by signal size.Uncertainty in the determination of40Ar/36Ar was governed by the determination of the small ion beam intensities,i.e.,36Ar.For instance,for36Ar+beam intensities,uncertainty increased quickly with decreasing beam size below 10-15mol(Fig.4b).
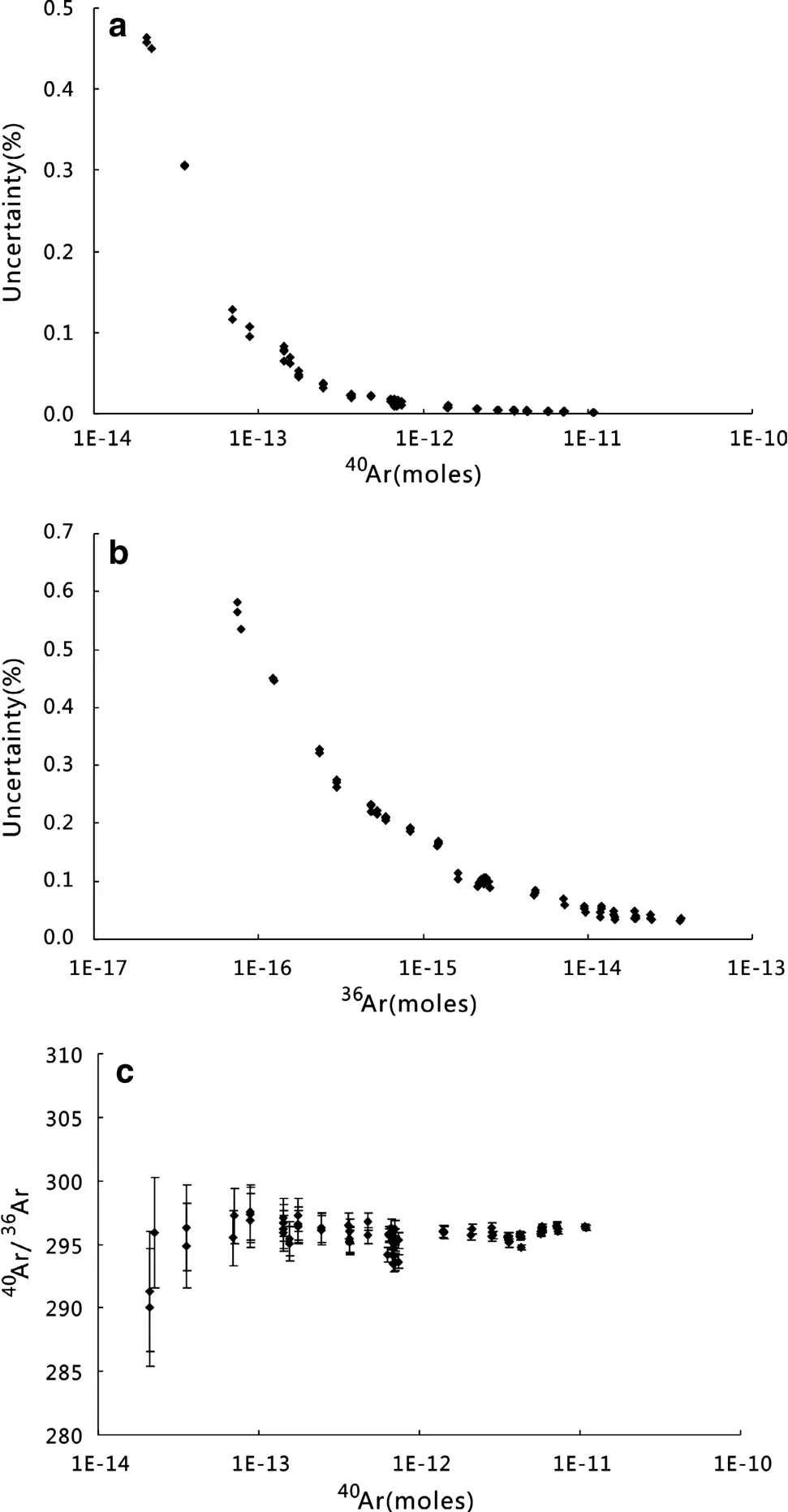
Fig.4 Plots showing the uncertainty for measurements of varying ion beam size for a Faraday,b multiplier,and c40Ar/36Ar linearity with changes in40Ar signal size.The range in ion beam intensities covers the complete size range for sample40Ar analyzed at IGCAS
5 Age standards
Here we report data from five well-characterized40Ar/39Ar age standards in order to assess the performance of Noblesse.We have analyzed Alder Creek sanidine(ACs;e.g.,1.181±0.001 Ma,Phillips et al.2017;1.185±0.001 Ma,Niespolo et al.2017;1.185±0.004 Ma,Kim and Jeon 2015;1.178±0.002 Ma,Phillips and Matchan 2013;1.185±0.002 Ma,Rivera et al.2013),Brione muscovite(Bern4M;18.74±0.20 Ma,Hall et al.1984),Fish Canyon tuff sanidine(FCs;e.g.,28.126±0.019 Ma,Phillips et al.2017;28.01±0.04 Ma,Phillips and Matchan 2013;28.172±0.028 Ma,Rivera et al.2011;28.294±0.036 Ma,Renne et al.2011;28.201±0.046 Ma,Kuiper et al.2008),Yabachi sanidine(YBCs;RYBCs FCs=1.044296±0.003968,Wang et al.2014),Fangshan biotite(ZBH25;133.0±0.3 Ma,Sang et al.2006).
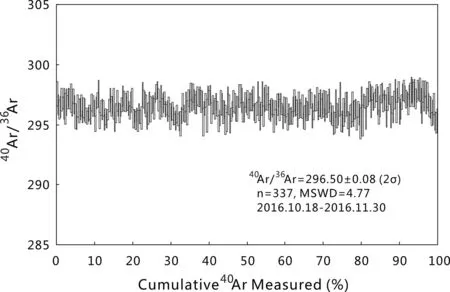
Fig.5 Measurement of40Ar/36Ar ratios of modern atmospheric argon in the laboratory over 1.5 months
All samples were wrapped in Al foil to form~4 mm diameter×1 mm thick wafers.The wafers were stacked in quartz vials(Fig.6)with the Fish Canyon sanidine standard used as the neutron flux monitor.The vial was 25 mm long and had an inner diameter of 5.0 mm.The vials were vacuum-sealed and put inside a quartz canister.The canister was wrapped in 0.5 mm thick Cd foil in order to shield from slow neutrons thus minimize undesirable interference reactions generated during irradiation.The canister was irradiated in position E4 of the 49-2 Nuclear Reactor(49-2 NR),Beijing for 24 h,with a neutron flux of 2.65×1013n(cm2s-1).The correction factors for interfering isotopes obtained from the irradiation of pure CaF2and potassium salt K2SO4were(39Ar/37Ar)Ca=1.02×10-3,(36Ar/37Ar)Ca=2.69×10-4,and(40Ar/39Ar)K=6.64×10-3,respectively.
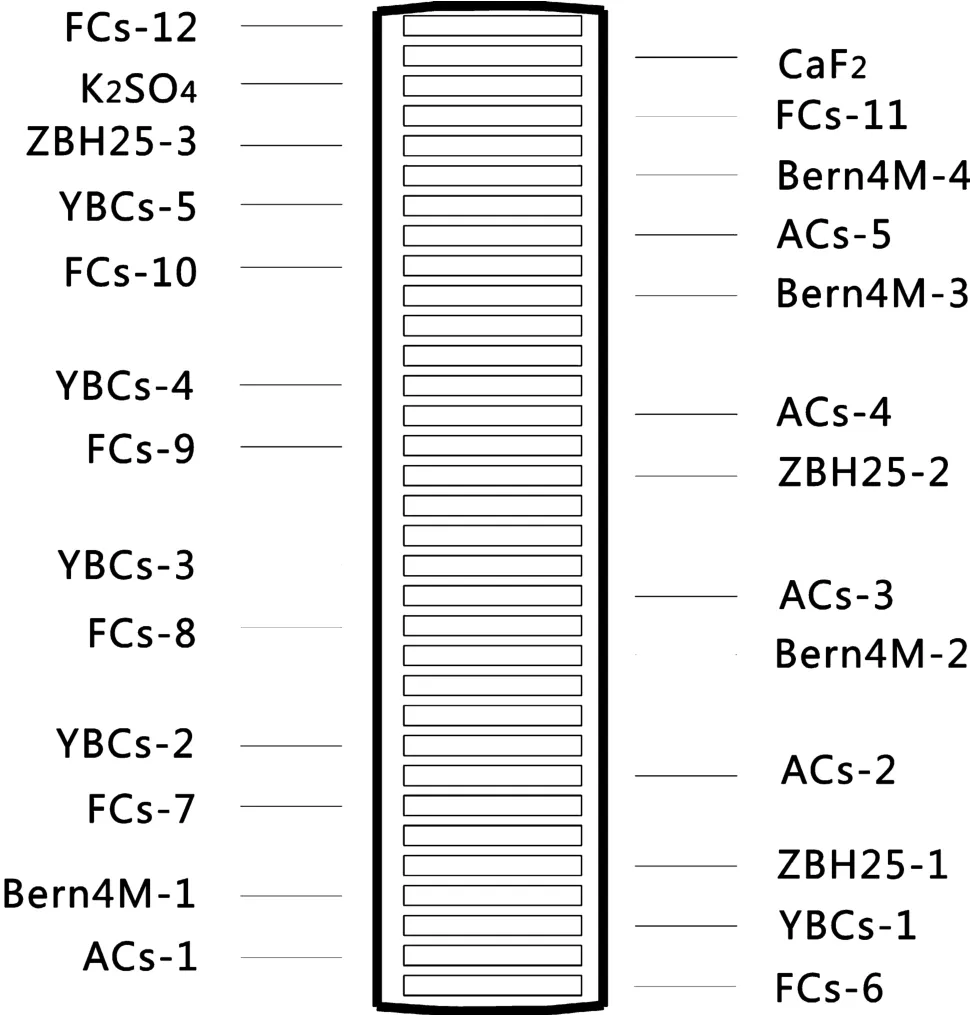
Fig.6 Packing list of vial II:ACs,FCs,YBCs,Bern4M,ZBH25,K2SO4and CaF2in a sealed quartz vial
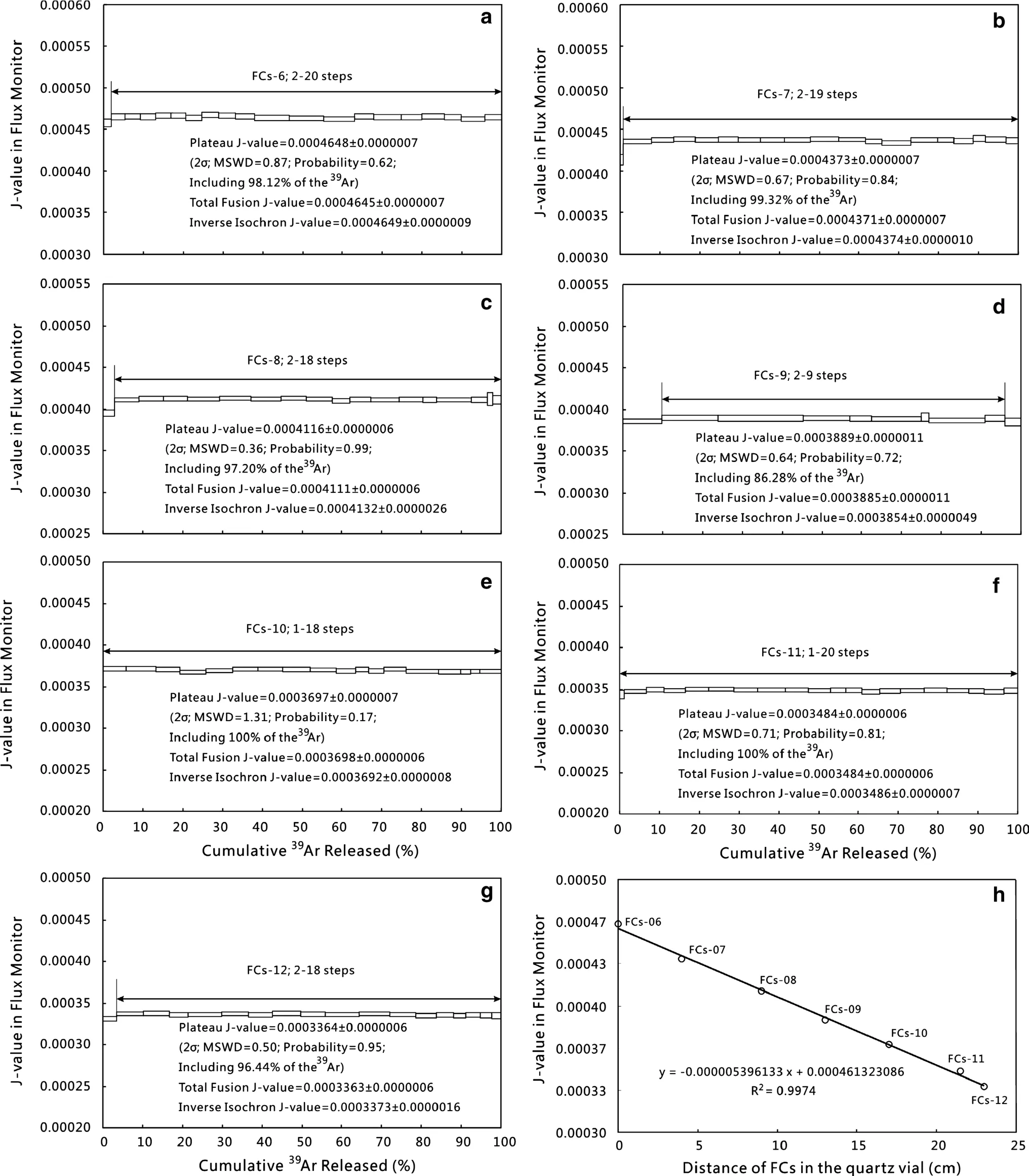
Fig.7 a–g The J-value step-heating spectra for FCs and the box heights on the plateau diagram reflect the 2σ uncertainties for each step;h vertical flux gradient of standard monitor FCs of vial II
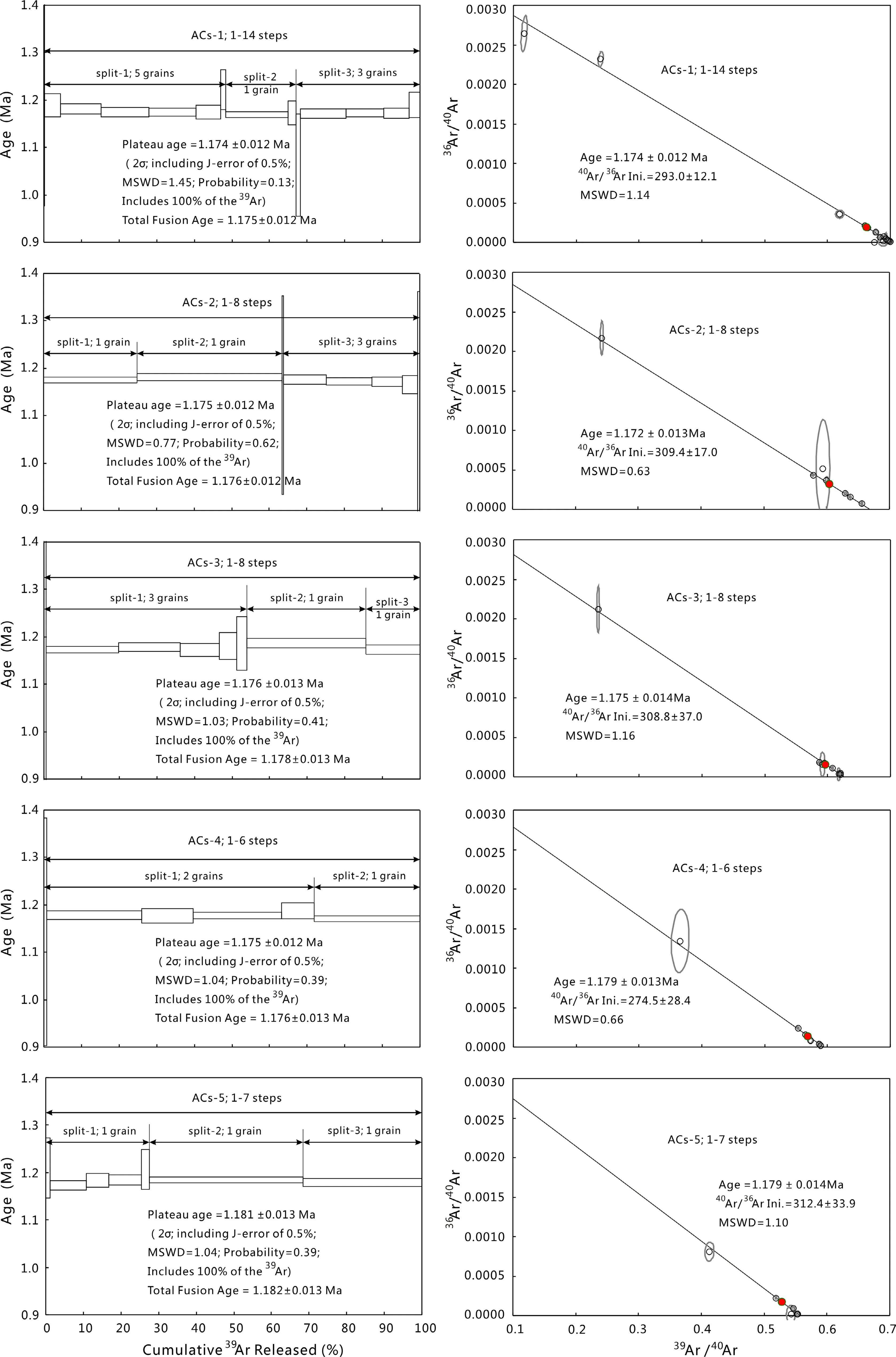
Fig.8 The40Ar/39Ar step-heating spectra and inverse isochrons for ACs.The box heights on the plateau diagram reflect the 2σ uncertainties for each step
After irradiation,seven FCs aliquots(Fig.6,denoted FCs-6 to FCs-12)were step-heated to obtain the J-values using the age of 28.294±0.036 Ma(Renne et al.2011).J-values(Fig.7a–g)display a nearly linear relationship with position[y=(-0.000005396133)×(+0.00046132 3086),R2=0.9974;Fig.7h].The J-values of the samples were calculated according to their positions.ACs,Bern4M,YBCs,and ZBH25 were analyzed as the same way as FCs.40Ar/39Ar age spectra,normal isochrons,inverse isochrons,and total fusion age(TFA)are all within error of each other for ACs,Bern4M,YBCs,and ZBH25(Figs.8,9,10,11).Ages and uncertainties were derived from the40Ar/39Ar age spectra of each standard and total fusion analysis in order to directly compare with K–Ar dating of standards.
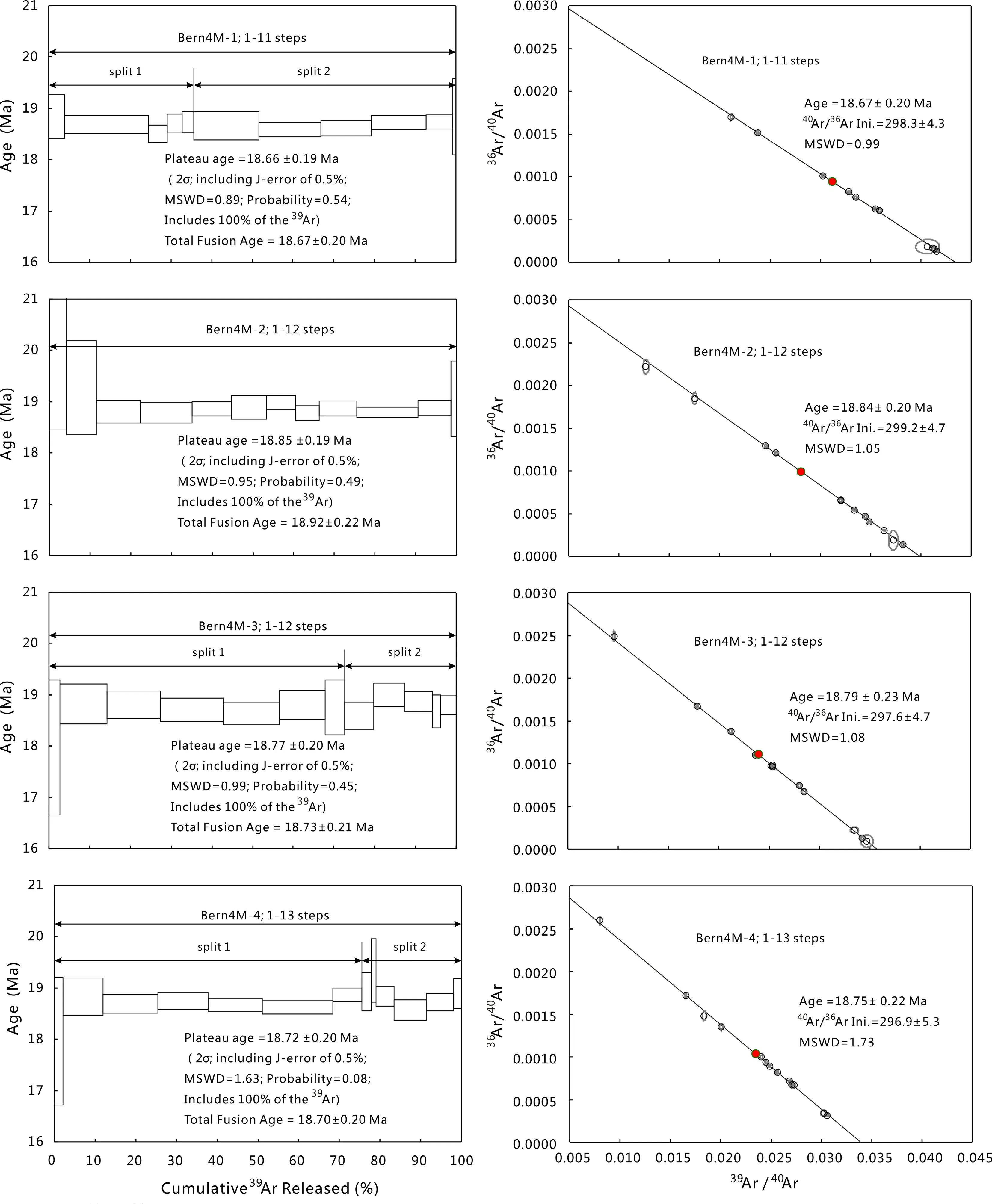
Fig.9 The40Ar/39Ar step-heating spectra and inverse isochrons for Bern4M.The box heights on the plateau diagram represent the 2σ uncertainties for each step
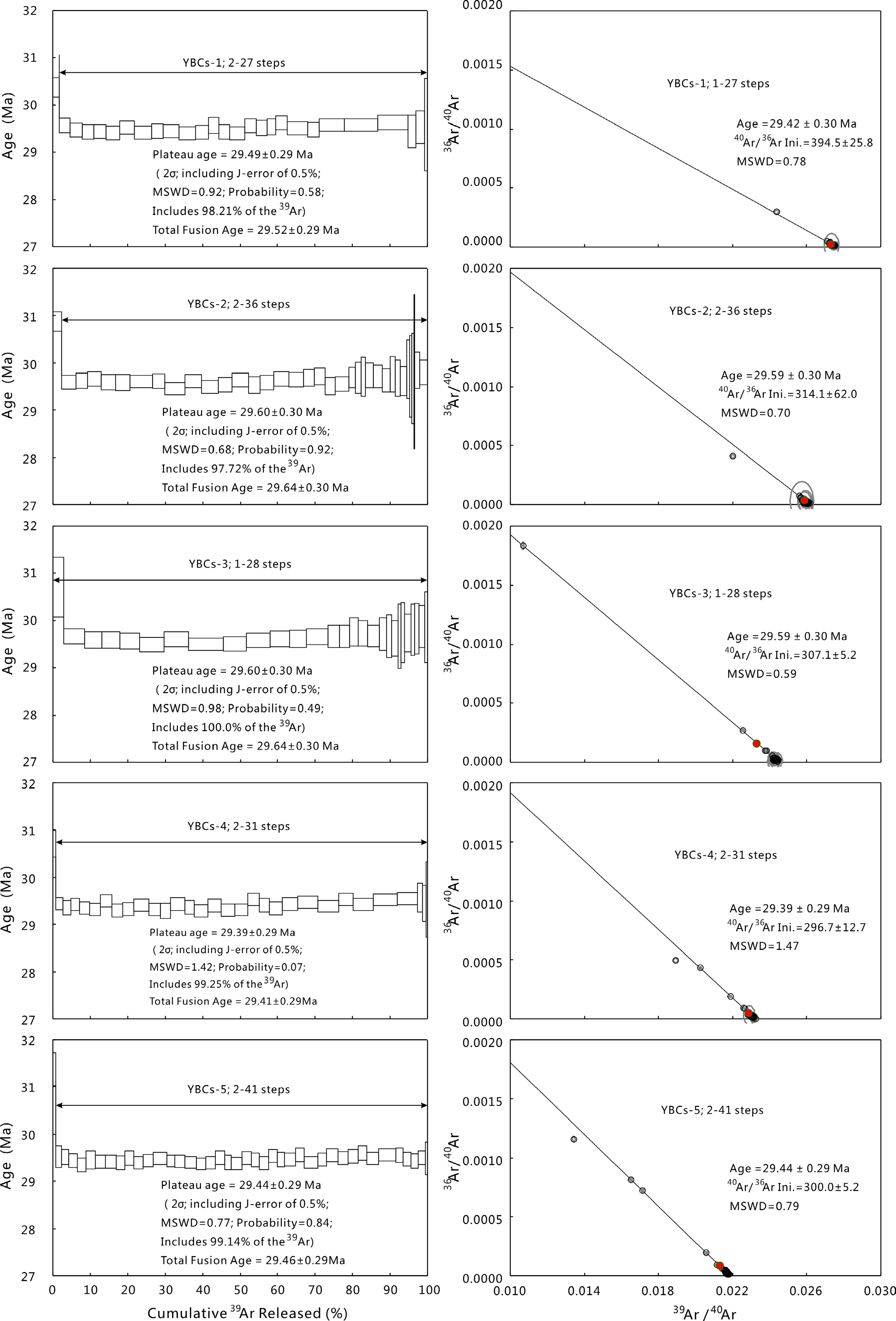
Fig.10 The40Ar/39Ar step-heating spectra and inverse isochrons for YBCs.The box heights on the plateau diagram represent 2σ uncertainties for each step
Step-heating of multi-grain samples(2–5 grains)and total fusion of single crystals were conducted on splits offive aliquots of ACs(Fig.8,denoted ACs-1 to ACs-5).One split with single grain of aliquot ACs-5 was also analyzed by laser step-heating.The results of single-grain measurements are consistent with those of multi-grain.All analyses yielded an age range of 1.174–1.181 ± 0.013 Ma(2σ, probability=0.13–0.62, MSWD=0.77–1.45,TFA=1.175–1.182 ± 0.013 Ma).This overlapped the established age of the standard(e.g.,1.181±0.001 Ma,Phillips et al.2017;1.185±0.001 Ma,Niespolo et al.2017;1.185±0.004 Ma,Kim and Jeon 2015;1.178±0.002 Ma,Phillips and Matchan 2013;1.185±0.002 Ma,Rivera et al.2013)and the uncertainty approaches those of the foremost Ar/Ar laboratories in the world.
Four aliquots of Bern4M(Fig.9,denoted Bern4M-1 to Bern4 M-4)were subjected to laser step-heating analysis.These analyses produced ages of 18.66–18.85 ± 0.20 Ma(2σ, probability=0.08–0.54, MSWD=0.89–1.63,TFA=18.67–18.92 ± 0.22 Ma)and a weighted mean age of 18.75± 0.16 Ma(2σ).The ages(inverse isochron,plateau and total fusion age)are within the uncertainty of the accepted age and the error is slight improvement on previously published data(18.74±0.20 Ma,Hall et al.1984).
YBC sanidine is from a phonolite of the Yabachi volcanic field in central Tibet,China.It was jointly developed by four laboratories in Australasia and Eurasia for a new standard mineral for40Ar/39Ar dating.Based on FCs age of 28.02±0.16 Ma(Renne et al.1998),the recommended age of YBC sanidine is 29.286±0.206 Ma,or neglecting the decay constant error,29.286±0.045 Ma(Wang et al.2014).Detailed laser step-heating experiments of 27-41 steps were carried out on five aliquots of YBC sanidine(Fig.10,denoted YBCs-1 to YBCs-5).These analyses resulted in ages of 29.39–29.60 ± 0.30 Ma(2σ,probability=0.07–0.92,MSWD=0.68–1.42,TFA=29.41–29.64±0.30 Ma)and a weighted average age of 29.50±0.19 Ma(2σ)relative to a FCs age of 28.294± 0.036 Ma(Renne et al.2011).According to the inter-calibration factors between YBCs and FCs(RYBCsFCs=1.044296±0.003968,Wang et al.2014),the recommended age of YBCs should be 29.547±0.206 Ma based on FCs age of Renne et al.(2011).Our analytical result is consistent with the established age and its uncertainty is slight improvement on previously published data.
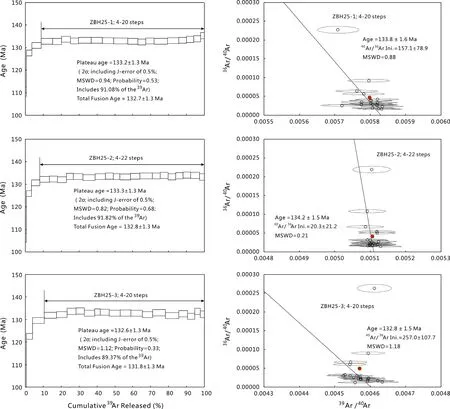
Fig.11 The40Ar/39Ar step-heating spectra and inverse isochrons for ZBH25.The box heights on the plateau diagram represent the 2σ uncertainties for each step
ZBH25 biotite was separated from Fangshan granodiorite complex in the southwest of Beijing,China.It was developed for K–Ar and40Ar/39Ar age determination,and it is used to calculate J-values during irradiation in some laboratories in China.The K–Ar age is 132.9± 1.3 Ma and the calibrated40Ar/39Ar age of ZBH25 is 133.0±0.3 Ma relative to a Ga1550 age of 98.8 Ma(Sang et al.2006).Detailed laser step-heating experiments of 20-22 steps were conducted on three aliquots of ZBH25 biotite(Fig.11,denoted ZBH25-1 to ZBH25-3).These analyses showed age spectrums with increasing ages in the three lowest temperature steps followed by 17–19 stable age steps and yielded integrated ages of 132.6–133.3± 1.3 Ma for 89.4%–91.1%of39Ar released(2σ, probability=0.33–0.68, MSWD=0.82–1.12,TFA=131.8–132.8 ± 1.3 Ma)and a weighted mean age of 133.0± 0.76 Ma(2σ).The age coincides with the published value,and its uncertainty is at the same level.
6 Summary
We have established a40Ar/39Ar dating system based on a Nu instruments Noblesse mass spectrometer and a CO2laser heating apparatus at IGCAS.The system was configured to accurately and precisely date small masses of minerals and volcanic rocks.Five aliquots of Alder Creek sanidine produced ages ranging from 1.174±0.012 to 1.181±0.013 Ma and a weighted mean age of 1.176± 0.006 Ma(2σ).Four Bern4M aliquots produced ages of 18.66± 0.19–18.85± 0.19 Ma and a weighted mean age of 18.75± 0.16 Ma(2σ).Five YBCs aliquots yielded ages ranging from 29.39±0.29 to 29.60±0.30 Ma and a weighted mean age of 29.50± 0.19 Ma(2σ).Three aliquots ZBH25 gave ages of 132.6± 1.3–133.3± 1.3 Ma and a weighted mean age of 133.0± 0.76 Ma(2σ).The results overlap the established age of these mineral standards and the analytical uncertainties are at the same level with those of the foremost Ar/Ar laboratories world-wide.
AcknowledgementsWe are very grateful to Huaiyu He,Wenbei Shi,Liekun Yang(Institute of Geology and Geophysics,CAS)and Xiujuan Bai(China University of Geosciences,Wuhan)for helpful advice and John Saxton,Bo Gao(Nu instrument)for technical support.We thank Fei Wang and Huaning Qiu(reviewers)for their constructive comments.This study was jointly funded by The National Key R&D Program of China(2016YFC0600405)and The National Natural Science Foundation of China(Grant No.40903022).
杂志排行
Acta Geochimica的其它文章
- Trace element composition of magnetite from the Xinqiao Fe-S(-Cu-Au)deposit,Tongling,Eastern China:constraints on fluid evolution and ore genesis
- Theoretical calculation of equilibrium Mg isotope fractionation between silicate melt and its vapor
- Zinc isotope fractionation under vaporization processes and in aqueous solutions
- The partitioning patterns of nutrients between pods and seeds of Zanthoxylum fruits impacted by environmental factors
- Geological significance of nickeliferous minerals in the Fule Pb-Zn deposit,Yunnan Province,China
- Iron isotopic analyses of geological reference materials on MCICP-MS with instrumental mass bias corrected by three independent methods
