Prevalence of white matter hyperintensities increases with age
2018-10-22FengJuanZhuangYanChenWenBoHeZhiYouCai
Feng-Juan Zhuang , Yan Chen , Wen-Bo He Zhi-You Cai
1 Department of Neurology, Renmin Hospital, Hubei University of Medicine, Shiyan, Hubei Province, China
2 Department of Neurology, Chongqing General Hospital, University of Chinese Academy of Sciences, Chongqing, China
Abstract
Abstract White matter hyperintensities (WMHs) that arise with age and/or atherosclerosis constitute a heterogeneous disorder in the white matter of the brain. However, the relationship between age-related risk factors and the prevalence of WMHs is still obscure. More clinical data is needed to confirm the relationship between age and the prevalence of WMHs. We collected 836 patients, who were treated in the Renmin Hospital, Hubei University of Medicine, China from January 2015 to February 2016, for a case-controlled retrospective analysis. According to T2-weighted magnetic resonance imaging results, all patients were divided into a WMHs group (n = 333) and a non-WMHs group(n = 503). The WMHs group contained 159 males and 174 females. The prevalence of WMHs increased with age and was associated with age-related risk factors, such as cardiovascular diseases, smoking, drinking, diabetes, hypertension and history of cerebral infarction. There was no significant difference in sex, education level, hyperlipidemia and hyperhomocysteinemia among the different age ranges. Thesefindings confirm that age is an independent risk factor for the prevalence and severity of WMHs. The age-related risk factors enhance the occurrence of WMHs.
Key Words: nerve regeneration; white matter; prevalence; severity; age; risk factor; retrospective analysis; cerebrovascular; neural regeneration
Introduction
White matter hyperintensities (WMHs) of presumed vascular origin are commonly referred to as white matter changes or WMHs on T2-weighted magnetic resonance imaging(T2-MRI) and hypointensities on computed tomography(CT) in cerebral white matter areas (Williams et al., 2010;Seneviratne et al., 2013; Aribisala et al., 2014; Shim et al.,2015). The clinical manifestations of WMHs that arise with age and/or atherosclerosis often present with subtle functional impairment, cognitive impairment, dementia and with a high prevalence of neuropsychiatric disorders, such as major depressive disorder, bipolar disorder, schizophrenia, multiple sclerosis and normal pressure hydrocephalus(Grangeon et al., 2010; Yoon et al., 2014; Benedictus et al.,2015; Chang et al., 2015; Smith et al., 2016). A large volume of WMHs is a risk factor for cerebrovascular events and vascular cognitive impairment (Akisaki et al., 2006; Smith et al.,2008; Debette and Markus, 2010).
Cerebrovascular risk factors such as age, hypertension, diabetes mellitus, hyperhomocysteinemia, hyperlipidemia, and hypersensitive C-reactive protein are known risk factors for WMHs (Avci et al., 2015). WMHs are frequent findings on T2-MRI in the brains of elderly individuals but are also seen in middle-aged individuals (Williams et al., 2010). The relationship between the prevalence of WMHs and age remains obscure; therefore, it is essential to clarify this relationship with clinical data. This retrospective analysis investigated clinical data of WMHs patients attending our hospital in Central China from January 2015 to February 2016 to clarify the relationship between the prevalence of WMHs and age. This retrospective analysis also analyzed the relationship between the prevalence of WMHs and risk factors on the basis of age.
Subjects and Methods
Study design
This retrospective cohort analysis was conducted at the Renmin Hospital, Hubei University of Medicine, China. WMHs patients were diagnosed by MRI, and WMH was confirmed in T2-MRI. Control subjects did not present WMH in T2-MRI. The trial was approved by the Institutional Medical Ethics Committee of the Renmin Hospital, Hubei University of Medicine.
Subjects
Theflow chart of this study is shown in Figure 1. We selected 836 Han Chinese subjects, including WMHs patients (n= 333) and non-WMHs subjects (n = 503). The MRI data of patients (≥ 16 years old) were obtained from the MRI Examination Center and the Department of Neurology.All collected cases were examined with conventional MRI sequences (including GRE sequence T1 and T2, T2 FLAIR,or diffusion weighted imaging). WMHs were diagnosed as areas of bright, high signal intensities noted on MRI T2-weighted and proton density sequences. All collected WMHs were a consequence of age and/or arteriosclerosis.Cases were excluded based on the following criteria: an ethnic origin other than Han, WMHs resulting from brain injury, infectious brain diseases, immunological brain diseases,congenital brain diseases or other brain disorders.
Data collection
The clinical and demographic characteristics of all patients were obtained from the MRI Examination Center and Hospital Medical Records Information Management System of Renmin Hospital, Hubei University of Medicine, including age, gender, risk factors, initial vital symptoms, laboratory parameters, and clinical outcomes.
The volume burden of WMHs was investigated with the Fazekas Scale (Fazekas et al., 1987). The Fazekas Scale is a simple visual rating scale used to rate the degree of WMHs,which contain periventricular hyperintensities (PVH) and deep white-matter hyperintensities (DWMH). PVH and DWMH are considered separately and rated from 0–3 on a four-point scale. Each case in this study has a score of at least 2 for either PVH or DWMH [PVH: 0 = absent, 1 = caps or pencil-thin lining around ventricles, 2 = smooth halo around ventricles (6–10 mm regular margins), 3 = irregular halo > 10 mm; DWMH: 0 = absent, 1 = punctate foci, 2 =beginning confluence, 3 = large confluent areas].
Statistical analysis
Statistical analysis was performed using SPSS for Windows Version 18.0 (SPSS, Chicago, IL, USA) and Microsoft Excel 2010 (Microsoft, Seattle, WA, USA). Significance of differences between the mean values in the two groups was verifi ed using Student’s t-test. A chi-square test was introduced to analyze the associations between two different variables.A P-value of less than 0.05 was considered statistically significant.
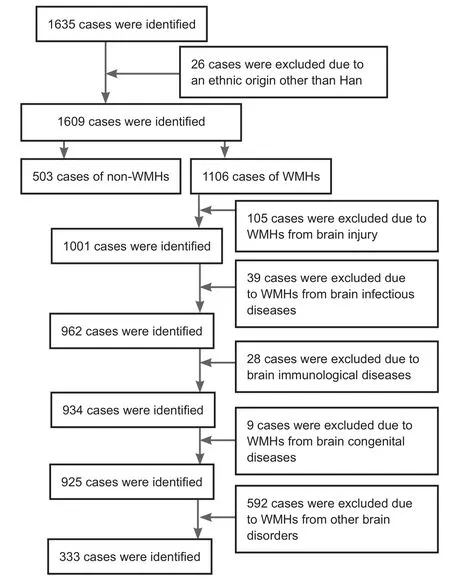
Figure 1 Studyflow chart.
Results
Clinical characteristics of WMHs patients
The clinical characteristics of WMHs patients are listed in Table 1. These mainly included dizziness, headache, limb weakness, limb numbness, unstable walking, speech disorder, blurred vision, and disorders of consciousness. The most common clinical manifestation was dizziness.
Spatial distribution of WMH features
The distribution of WMH features according to MRI examination is listed in Table 2. The most common location of WMHs was the frontal lobe. Thereafter, the order for the most common location of WMHs is: basal ganglia, parietal lobe, semiovale center, pons, temporal lobe, thalamus, occipital lobe, corona radiate, brainstem, opisthencephalon and insular lobe.
Age distribution for WMHs patients
We collected 836 cases, including 333 WMHs patients and 503 non-WMHs subjects. There were six age-range groups(less than 40, 41–50, 51–60, 61–70, 71–80 and more than 80). The 333 WMHs patients contained 159 males and 174 females.
Table 3 shows that the prevalence of WMHs increases with age. The same results were found in male and female subjects (Table 3). The chi-square test showed that the prev-alence of WMHs increased with age (P < 0.0001). Fisher’s exact test showed that the prevalence of WMHs was higher in the 41 to 50 age-range group than in the less-than-40 group (P < 0.0001), and higher in the 51 to 60 age-range group than in the 41 to 50 age-range group (P < 0.0001),and higher in the 61 to 70 age-range group than in the 51 to 60 age-range group (P = 0.0018), and higher in the 71 to 80 age-range group than in the 61 to 70 age-range group (P =0.0010). These results indicate that the prevalence of WMHs increases with age.
Association between risk factors and the prevalence of WMHs
Table 4 shows that the prevalence of WMHs is associated with age-related risk factors: age (P < 0.0001), cardiovascular diseases (P < 0.0001), smoking (P = 0.0098), drinking (P= 0.0151), diabetes (P = 0.0038), hypertension (P = 0.0326),and history of cerebral infarction (P < 0.0001). There was no significant difference in sex (P = 0.8043), education level (P= 0.3042), hyperlipidemia (P = 0.605) and hyperhomocysteinemia (P = 0.3321) among different age ranges.
The volume burden of WMHs assessed by the Fazekas scale is shown in Table 5. Chi-square analysis indicated that there was a significant difference between the 61 to 70 agerange group and the 71 to 80 age-range group (P = 0.0105).The Fazekas scale scores were associated with increasing age(Table 5) (P < 0.0001). The trend of the increasing WMHs volume burden (Fazekas scale scores) with advancing age is shown in Table 5.
Discussion
WMHs are often referred to as being areas in cerebral white matter that appear hyperintense on T2-MRI and hypointense on CT. WMHs are a common pathophysiological feature that influence cognitive, mental and physical function in the adult and elderly population. WMHs are associated with various geriatric disorders, including cerebrovascular diseases (Poggesi et al., 2008; Pu et al., 2009),cardiovascular diseases (Te et al., 2015), hypertension (Henry Feugeas et al., 2005), diabetes (Poggesi et al., 2008), obesity,cognitive impairment and dementia (Mok et al., 2011; Raji et al., 2012; Doi et al., 2015; Cooke et al., 2016), and psychiatric disorders (Thomas et al., 2004; Meurs et al., 2016;Murray et al., 2016). This one-hospital, clinical, retrospective analysis demonstrated that the prevalence of WMHs increases with age. This clinical investigation of Han Chinese individuals identified a relationship between the prevalence of WMHs and age-related risk factors (age, cardiovascular diseases, smoking, drinking, diabetes mellitus, hypertension and history of cerebral infarction) on the basis of different age groups. Moreover, the severity of WMHs (the WMHs volume burden measured with Fazekas scale scores) also increased with age. Thus, this analysis indicates that aging is an independent contributor to the prevalence and severity of WMHs. The age-related risk factors enhance the occurrence of WMHs.
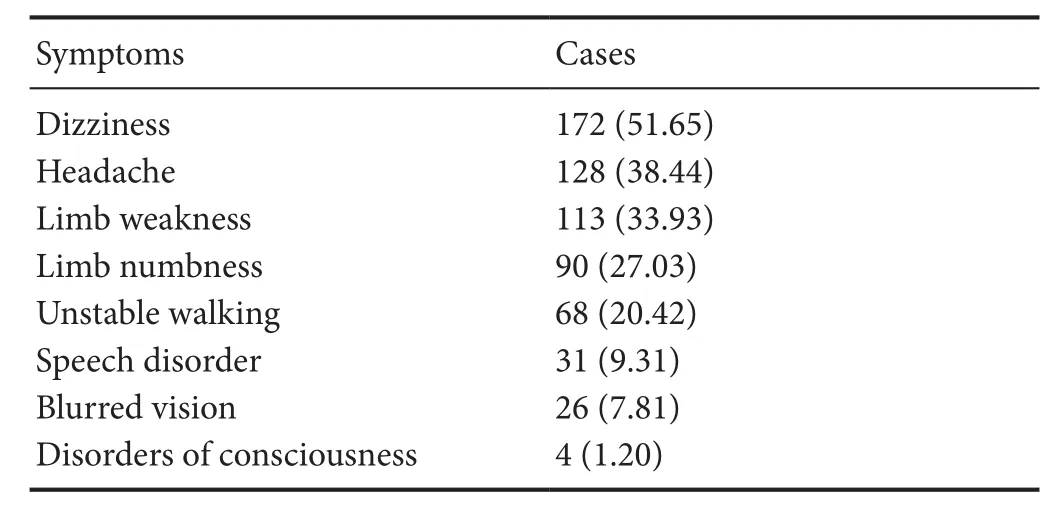
Table 1 Clinical characteristics of 333 patients with white matter hyperintensities
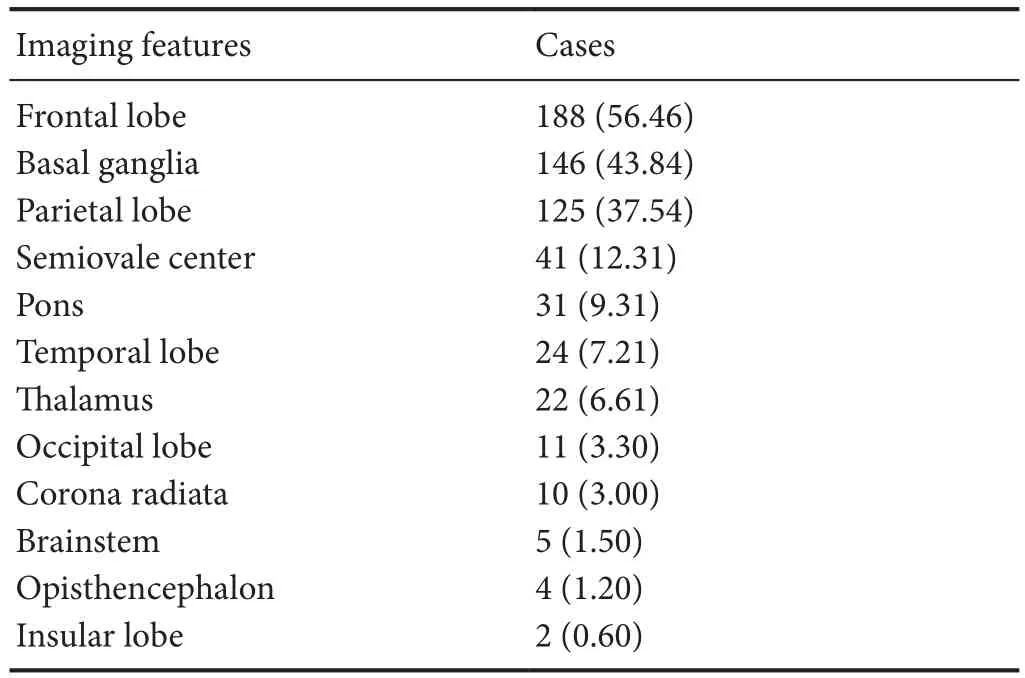
Table 2 Distribution of white matter hyperintensity features according to magnetic resonance imaging in 333 patients with white matter hyperintensities
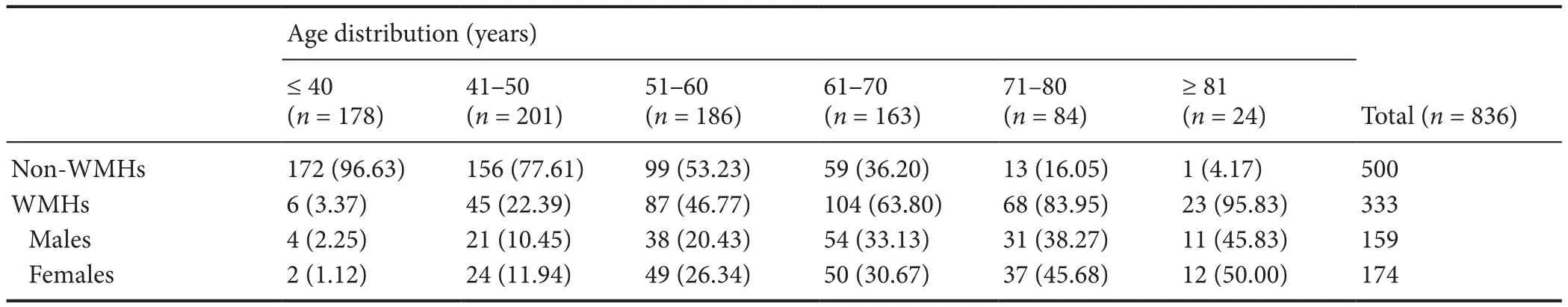
Table 3 Distribution of white matter hyperintensities (WMHs) by age range and sex

Table 4 Association between risk factors and the prevalence of white matter hyperintensities (WMHs) on the basis of age
Compelling imaging results have revealed that WMHs is an age-related chronic ischemia process that is a common occurrence in the elderly. Major risk factors for WMHs are age and hypertension (Miki and Sakamoto, 2013).The pathological changes of WMHs are characterized by demyelination, loss of glial cells, spongy appearance, and age-related vascular pathology (occlusion of small vessels by venous collagenosis and arteriolar tortuosity) (Brown et al., 2002a, b), which are all involved in the process of aging.WMHs are common in the world’s population, such as in Japanese (Honda et al., 2015; Yamawaki et al., 2015), Caucasian (Corbin et al., 2004), African-American (Nyquist et al.,2014), Caribbean black (Corbin et al., 2004), and Chinese populations (Tsai et al., 2013, 2015). Numerous clinical studies have shown that the prevalence of WMHs increases with age (Pantoni et al., 2005; van Straaten et al., 2006).Our investigation further confirmed that the prevalence and progression of WMHs is an independent contributor to disability in the elderly and increases with age, indicating that age is an independent contributor to the prevalence and progression of WMHs. The implications of these findings indicate that WMHs are a biomarker of aging that may help to develop preventive strategies to decrease the burden of disability from WMHs in later life.
A number of studies have noted that the etiology of WMHs is heterogeneous, but is most likely ischemic and neurodegenerative in nature, resulting from cortico-subcortical disconnections and loss of brain cells. All these vascular risk factors will promote previous pathogenesis, and are important in the occurrence of WMHs. These clinical results revealed that age-related risk factors (such as age, cardiovascular diseases, smoking, drinking, diabetes, hypertension and history of cerebral infarction) increase the prevalence of WMHs. However, the prevalence of WMHs is unrelatedto gender, education, hyperlipidemia or hyperhomocysteinemia, although several studies support the notion that hyperlipidemia and hyperhomocysteinemia are risk factors for WMHs (Uehara et al., 1999; Wong et al., 2006; Kuo et al.,2010; Marseglia et al., 2015; Shang et al., 2015). These studies reported that patients with a history of hyperlipidemia had less severe WMHs at the time of stroke and that hyperlipidemia may play a relatively protective role in cerebral small-vessel diseases (Jimenez-Conde et al., 2010). Therefore, the role of hyperhomocysteinemia and hyperlipidemia in the progress of WMHs remains unclear.
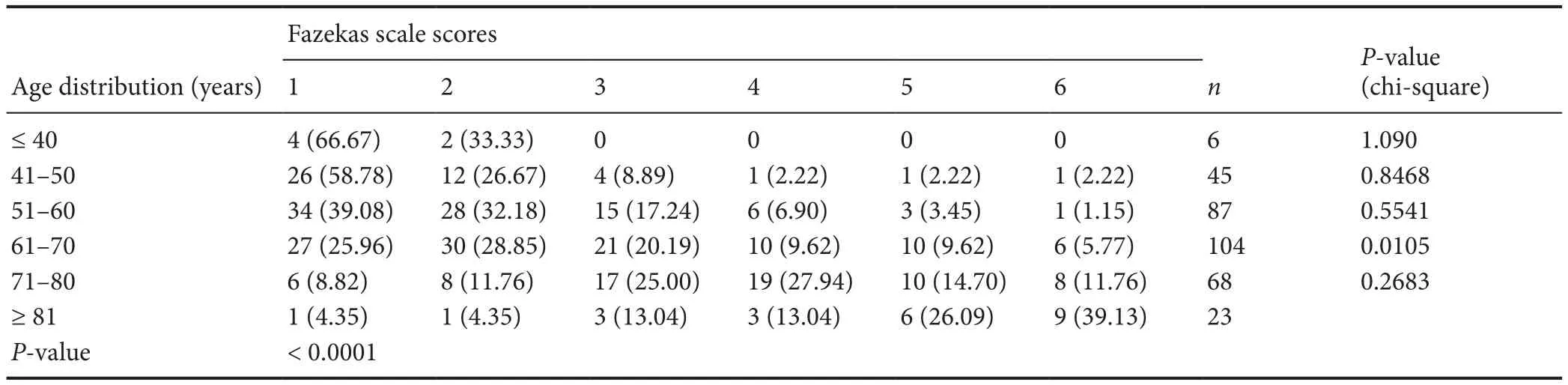
Table 5 Association between age distribution and Fazekas scale scores (WMHs volume burden)
Overall, age is an independent contributor to the prevalence and severity of WMHs. The age-related risk factors increase the occurrence of WMHs. However, this study is not a randomized controlled trial, and further studies and longitudinal data are needed to confirm the temporal sequence of events and these conclusions, and to discover the exact role of age-related risk factors in the occurrence of WMHs.However, as this is a retrospective analysis, there may be bias in subject selection and stratification.
Author contributions:Study design and paper writing: ZYC; study implementation, data collection and analysis: FJZ and YC; data collection and analysis, and discussion of result interpretation: WBH. All authors approved thefinal version of the paper.
Conflicts of interest:None declared.
Financial support:This study was supported by the Natural Science Foundation of Hubei Province of China, No. 2015CFB260; the Health and Family Planning Scientific Research Project of Hubei Province of China, No. WJ2015MB219 (to WBH and ZYC). The funding bodies played no role in the study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement:The experiments were approved by the Institutional Medical Ethics Committee of Renmin Hospital, Hubei University of Medicine, China.
Reporting statement:The study complied with the STrengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting guideline.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Renmin Hospital, Hubei University of Medicine, China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:The requirement for informed consent was waived due to the retrospective nature of this study.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Daoli Zhu, Nantong University, China; Haiqiang Qin, Capital Medical University, China.
Additionalfile:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy
- Analysis of transcriptome sequencing of sciatic nerves in Sprague-Dawley rats of different ages
- Exogenous brain-derived neurotrophic factor attenuates cognitive impairment induced by okadaic acid in a rat model of Alzheimer’s disease
- Partial improvement in performance of patients with severe Alzheimer’s disease at an early stage of fornix deep brain stimulation
- Epigenetic marks are modulated by gender and time of the day in the hippocampi of adolescent rats:a preliminary study