Epigenetic marks are modulated by gender and time of the day in the hippocampi of adolescent rats:a preliminary study
2018-10-22VivianeRostirolaElsnerLauraReckCechinelLouisianaCarolinaFerreiradeMeirelesKarineBertoldiIonaraRodriguesSiqueira
Viviane Rostirola Elsner , Laura Reck Cechinel Louisiana Carolina Ferreira de Meireles Karine Bertoldi Ionara Rodrigues Siqueira
1 Programa de Pós-Graduação em Ciências Biológicas: Fisiologia, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brasil
2 Programa de Pós-Graduação em Biociências e Reabilitação, Centro Universitário Metodista-IPA, Porto Alegre, Rio Grande do Sul, Brasil
3 Curso de Fisioterapia, Centro Universitário Metodista-IPA, Porto Alegre, Rio Grande do Sul, Brasil
Abstract Although the involvement of gender in epigenetic machinery in peripheral tissues during the neonatal period has been suggested, the gender-related epigenetic profile of brain areas during the adolescent period is rarely exploited. Furthermore, the influence of time of day on hippocampal acetylation marks has been demonstrated in young adult and aged rats; however, there are no studies reporting epigenetic changes in the adolescent period. Therefore, this study aimed to investigate the effects of gender on hippocampal DNA methyltransferase 1 content and histone deacetylase (HDAC) activity of adolescent rats at different time points, specifically early morning and afternoon. Both epigenetic markers increased significantly in the hippocampi of female rats compared to the male group, an indicator of reduced transcriptional activity. In addition, HDAC activity during the early morning was higher compared to afternoon groups in both male and female rats, while DNA methyltransferase 1 content was not altered by the time of day. Ourfindings demonstrate that hippocampal DNA methylation and histone acetylation status can be influenced by gender during the adolescent period, while the time of the day impacts HDAC activity.
Key Words: adolescent rats; DNA methyltransferase 1; histone deacetylase; hippocampus; time of the day;gender; epigenetic marks
Introduction
Exposure to early-life adversity, such as chronic mild stress and social isolation, is a major risk factor for psychiatric disorders during adulthood in a gender-dependent manner (Fone and Porkess, 2008; Toth et al., 2008). Although animal models and clinical evidence have shown stressors can affect adolescent females and males differently, females have been widely neglected in experimental studies (McCormick et al., 2010).
Furthermore, epigenetic changes have been associated with long-term programming and sex-specific effects of early exposure (Luoni et al., 2016). It is possible that the gender-dependent epigenetic profile can contribute to behavioral and molecular outcomes in early experiences. Indeed, sex differences in profiles of DNA methyltransferases (DNMT)expression and DNA methylation status have been reported,especially in liver and reproductive tissues; however, few studies have investigated brain areas (Xu et al., 2014). DNA methylation that is linked with gene transcription repression is catalyzed by DNMTs. The amygdalae of female newborns at postnatal day 1 (PND1) have been shown to have increased levels of DNA methyltransferase 3a (DNMT3a),without any gender differences at PND10; and the authors have also shown that sexual steroid hormone treatment decreases the DNMT3a expression without any effect on DNMT1 in the amygdala of neonatal rodents (Kolodkin and Auger, 2011), suggesting that sexual hormones were unable to directly impact DNMT1 content. Despite thesefindings,little is known regarding the fundamental gender differences in hippocampal DNA methylation machineries during adolescence. It is noteworthy that DNMT1 is the most active,with a preference for hemimethylated sites as well as de novo methylation (Reik et al., 1999).
Interestingly, we have previously demonstrated that time of day modulates the epigenetic machinery. Specifically,higher levels of HDAC activity were observed in the early morning when compared to the afternoon period in the hippocampi of both young adult and aged Wistar rats (Elsner et al., 2011; Sant’Anna et al., 2013); however, to our knowledge,the early stages of development have not been investigated.Therefore, this study aimed to analyze the effects of gender and time of day on DNMT1 content and HDAC activity in the hippocampi of adolescent rats.
Materials and Methods
Ethics statement
The Local Animal Ethics Committee (CEUA/UFRGS) approved all handling and experimental conditions (nr.21449),and the study was conducted in accordance with Arouca Brazilian law (11794/2008) as well as the NIH “Guide for the Care and Use of Laboratory Animals” (NIH publication No.80–23, revised 1996).
Animals
Male and fem ale adolescent Wistar rats (PND39, mean body weight 180 g, n = 26), provided by and housed at the Centro de Reprodução e Experimentação de Animais de Laboratório(CREAL) at the Universidade Federal do Rio Grande do Sul(UFRGS), were used in the study. The animals were housed five per cage (Plexiglass cages, dimensions: 40 × 33.3 × 17 cm3), maintained under standard conditions (12:12 hours light:dark cycle, 22 ± 2°C), and provided with water and food ad libitum.
The rats were randomized to the male early morning group, male afternoon group, female early morning group,or female afternoon group (n = 6–7 per group).
Brain tissue extraction
To investigate the effect of time of the day on epigenetic modulation, rats were sacrificed by decapitation at different time points in the day: early morning (approximately 8–9 a.m.) and afternoon (approximately 1–2 p.m.). The hippocampi were carefully and quickly dissected on ice, immediately snap-frozen in liquid nitrogen, andfinally stored at-80°C. For the epigenetic assays, the samples were prepared as previously described by Elsner and colleagues (2011).
Epigenetic measurements
The global HDAC activity and DNMT1 content were measured using specific ELISA Assay Kits (Fluorometric Detection catalog # 17-372, Upstate Biotechnology, Temecula, CA, USA; Colorimetric Detection, catalog # P-3011,EpiQuik®, Farmingdale, NY, USA, respectively). All procedures were conducted according to the manufacturer’s instructions. The data are expressed as pmoles of HDAC and DNMT1 amount (ng/mg protein). The protein content of the homogenates was quantified using the Coomassie brilliant blue colorimetric method, with bovine serum albumin as the standard (Bradford, 1976).
Then the prince inquired why she was wandering about all by herself, and she told him that since her mother s death she was so sad that whilst her father was away she preferred being alone
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software(SPSS, Chicago, IL, USA). The data are expressed as mean± standard deviation. The results were analyzed by twoway analysis of variance (ANOVA) with gender and time of day as independent variables, followed by post hoc Duncan multiple range tests, when appropriate. In all tests, P < 0.05 indicated statistical significance.
Results
The effects of gender and time of day on hippocampal HDAC global activity
Two-way ANOVA showed a significant effect of gender since female adolescent Wistar rats had higher global HDAC activity than males as highlighted in Figure 1 (F(1,19)=35.144, P < 0.001). Moreover, the influence of time of day in HDAC activity was observed; this parameter was enhanced in the early morning compared to the afternoon period (F(1,19)= 6.377, P = 0.022). In addition, there was an interaction between gender and time of day (F(1,19)=9.623, P = 0.007).
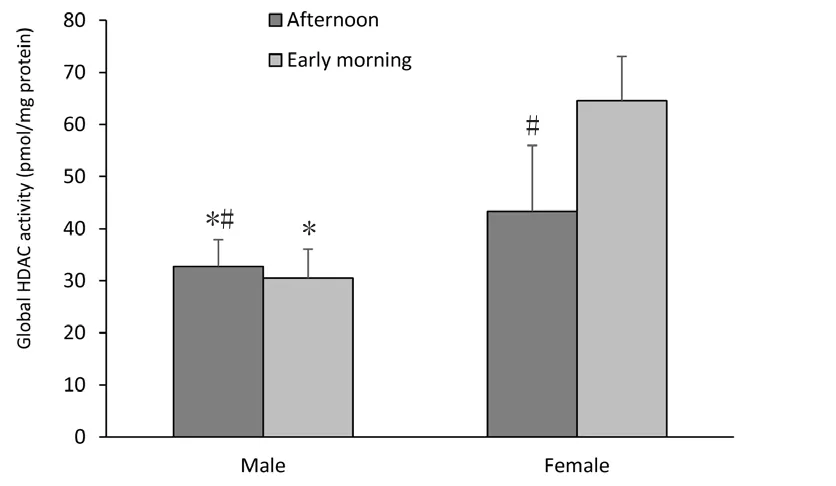
Figure 1 Histone deacetylase (HDAC) global activity in hippocampi of female and male Wistar rats.
The effects of gender and time of day on hippocampal DNMT1 activity
A two-way ANOVA also showed that the hippocampi of adolescent female Wistar rats exhibited higher levels of DNMT1 than those of males (F(1,21)=9.789, P = 0.006; Figure 2). However, this epigenetic marker was not influenced by time of day (P > 0.05).
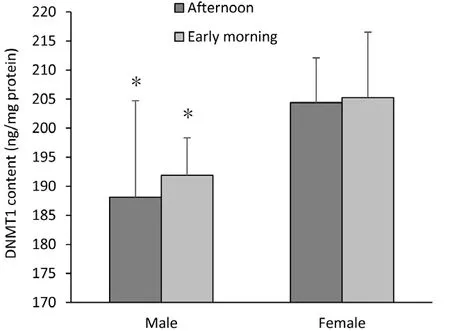
Figure 2 DNA methyltransferase 1 (DNMT1) content in hippocampi of female and male Wistar rats.
Discussion
In the current study, we provide novel insights into the basis for gender differences in the brain, demonstrating changes in epigenetic modulation, measured by both histone acetylation and DNA methylation markers, in the hippocampi of male and female adolescent rats. Distinct from previous studies that focused on pharmacological treatment with hormones in newborn rodents, our study explored the effect of gender on histone acetylation and DNA methylation machineries.
Hippocampal DNMT1 content was higher in the female group than in the male group, an indicator of reduced transcriptional activity. Kosten and colleagues (2014) also found DNA hypermethylation of specific genes in the hippocampi of female adolescents, which may have relevance for mental health and behavior. Furthermore, a recent study investigated DNA methylation marks in adolescent rats that were submitted to repeated exposure to an adverse caregiving environment during infancy. Interestingly, they showed that maltreated females had greater DNA methylation of brain-derived neurotrophic factor (BDNF) exon IV DNA in the hippocampus compared to the males (Doherty et al.,2016). Moreover, Li and colleagues (2016) demonstrated that male rats have better spatial learning performance compared to females; neonatal treatment with DNMT inhibitor,5-Aza, reversed sexual differences in the water maze test. A pronounced effect in females of neonatal 5-Aza treatment on forced swimming and openfield tests was also reported(Li et al., 2016). The higher DNMT1 levels observed in females are related to their susceptibility to DNMT inhibitor treatment. Further, our findings with brain areas partially agree with those obtained from liver, as levels of DNMT1 and DNMT3b expression were lower in adult male mice than females (Li et al., 2016). Taken together, ourfindings add evidence that the epigenetic profile may contribute to certain sexually dimorphic characteristics.
It is possible that DNMT content can contribute to the sex-dependent DNA methylation profiles. Although the involvement of sexual steroid hormones on DNA methylation markers remains to be explored, it is possible to exclude their role in DNMT1 content, since treatments with estradiol and dihydrotestosterone decreased DNMT3a expression in the amygdala of females without effecting DNMT1 expression (Kolodkin and Auger, 2011). It may be inferred that higher basal DNMT1 can contribute to this gender-dependent protective mechanism, maintaining genomic stability after insults. Koturbash and colleagues (2011) suggested that gender-specific epigenetic changes may be correlated to the prevalence of radiation-induced cancers in males. It has been reported that high-grade gliomas following radiotherapy are more prevalent in males than in females (Carret et al., 2006). In addition, ionizing radiation is able to increase DNMT3a levels in the female frontal cortex, and concomitantly higher basal DNMT1 was observed, which prevented radiation-induced DNA methylation loss, while no changes were observed in males (Koturbash et al., 2011).
It is known that epigenetic mechanisms are not isolated events but rather interact and influence each other (Gupta et al., 2010). Accordingly, our results indicated an important link between histone acetylation and DNA methylation parameters in mediating brain sexual differentiation, since increased HDAC activity, an indicator of hypoacetylation status, was also observed in the female group.
This finding can be related to those obtained by Tsai and colleagues (2009) showing that during neonatal brain development female mice have lower histone H3 acetylation levels in the hippocampus and cortices compared to males. In agreement, we also found lower levels of histone H4 acetylation at the estrogen receptor alpha (ERα) in the bed nucleus of the stria terminalis from female rats at postnatal 21 compared to males (Matsuda et al., 2011). We might suggest that female-related hypoacetylation status may be associated with the higher HDAC activity here described. Moreover, higher levels of DNMT1 and HDAC in the hippocampi of females compared to the male group could be linked to X-inactivation,since one of two X chromosomes is epigenetically inactivated in each female cell. Both DNMT1 and HDAC can be mechanisms for maintaining X silencing (Morey et al., 2010).
Taken together, it is reasonable to suppose that the adolescent female brain possesses higher HDAC activity and/or increased DNMT content, indicating transcriptional activity repression. Although the relevance of our data is currently unknown, we propose that the epigenome, via modulation of histone acetylation and DNA methylation signals, may contribute to the expression of specific genes appropriate for the feminization or masculinization of brain phenotypes and sex-specific behaviors.
It was reported that the infusion of a HDAC inhibitor into the cerebral ventricles of newborn males impaired sexual behavior in adulthood (Matsuda et al., 2011). Indeed, the involvement of HDAC in female characteristics was evaluated in androgenized females, since the exposure of newborn females to testosterone increased acetylation levels of histone 3 on lysine 9 and 14 in the hippocampus; this profile was considered as a masculinized acetylation profile (Tsai et al., 2009). It is remarkable that treatment with a HDAC inhibitor, valproic acid,was able to disrupt their testosterone-induced masculinization profile in some brain areas (Murray et al., 2009; Auger et al.,2011). However, sexually dimorphic microRNAs (miRNAs),another epigenetic marker, was found in the hippocampus(Koturbash et al., 2011). Conversely, Matsuda and colleagues(2011) demonstrated that the binding of HDAC2 and HDAC4 to gene promoters was higher in males than in females. It is possible that lower levels of HDAC can compensate for its higher affinity, which is observed in male samples.
Another remarkable point to discuss is the effect of time of day on the epigenetic parameters evaluated. We showed that HDAC activity was higher in the hippocampi of male and female adolescent rats in the early morning compared to the afternoon, corroborating thefindings observed in both young adult and aged rats (Elsner et al., 2011; Sant´Anna et al., 2013). Additionally, DNMT1 content was unaltered by time of day, in accordance with our data obtained from the hippocampi of young adult rats (Elsner et al., 2017). To date,this is thefirst evidence demonstrating the impact of time of day on epigenetic markers in the adolescent rat brain.
Altogether, these data led us to hypothesize that histone acetylation status can be influenced by time of day in all stages of development, while DNMT1 seems to be insensitive to circadian rhythm. It is widely known that the potency and toxicity of drugs are also influenced by the circadian rhythm (Debon et al., 2004). Therefore, our findings re-garding HDAC activity support the idea that the effects of HDAC inhibitor administration may depend on the time/times of day of administration; and the most appropriate time of day for the administration of these drugs in rodent models seems to be early morning (Elsner et al., 2011;Sant´Anna et al., 2013).
Conclusion and limitations
In summary, our results demonstrated gender differences in hippocampal DNA methylation and histone acetylation machineries, specifically the HDAC and DNMT1 content, which are epigenetic marks related to transcriptional activity repression. In addition, HDAC activity is influenced by time of day.Notably, the limitations of the present study should be considered. First, we evaluated only one time point during postnatal development. Future research should be done considering other stages of development as well, including histomorphological analysis in order to investigate the gender differences reflected by adolescent brain function and the epigenome. Furthermore, we only measured two epigenetic signals, suggesting that other studies might consider parameters such as histone H3 and H4 acetylation levels,DNA methylation levels, modifications in histone methylation status, RNA editing, genomic imprinting, miRNA regulation, and the expression of specific genes. In addition,the measurements were done in the whole hippocampus,suggesting that other studies may observe these changes in specific regions, i.e., CA1, CA2 and CA3. We believe that our preliminary findings may guide future studies in this field with perspectives on target interventions that could epigenetically respond to gender differences.
Author contributions:Research project conception, execution, data analysis and manuscript writing: VRE; research project execution, data analysis and manuscript review: KB, LRC and de Menezes LCF; project conception,data analysis and manuscript review and critique: IRS. All authors approved thefinal version of the paper.
Conflicts of interest:None declared.
Financial support:This work was partially supported by grant 476634/2013-0 from Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq /Brazil. CNPq fellowships (to IRS, VRE, and KB); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES fellowships (to LCM); Programa de Bolsas de Iniciação Científica – UFRGS (to LRC). None of the funding bodies played any role in the study other than to provide funding.
Institutional review board statement:This study was approved by the Local Animal Ethics Committee (CEUA/UFRGS) (nr.21449), and was conducted in accordance with Arouca Brazilian law (11794/2008) as well as the NIH “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 80-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Jigar Pravinchandra Modi, Florida Atlantic University, USA.
Additionalfile:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy
- Analysis of transcriptome sequencing of sciatic nerves in Sprague-Dawley rats of different ages
- Exogenous brain-derived neurotrophic factor attenuates cognitive impairment induced by okadaic acid in a rat model of Alzheimer’s disease
- Partial improvement in performance of patients with severe Alzheimer’s disease at an early stage of fornix deep brain stimulation
- Dysphagia in patients with isolated pontine infarction