Partial improvement in performance of patients with severe Alzheimer’s disease at an early stage of fornix deep brain stimulation
2018-10-22ZhiQiMaoXinWangXinXuZhiQiangCuiLongShengPanXiaoJingNingBaiXuanXuLinMaZhiPeiLingJianJunJiaXinGuangYu
Zhi-Qi Mao , Xin Wang , Xin Xu Zhi-Qiang Cui Long-Sheng Pan Xiao-Jing Ning, Bai-Xuan Xu, Lin Ma, Zhi-Pei Ling ,Jian-Jun Jia , Xin-Guang Yu
1 Department of Neurosurgery, Chinese PLA General Hospital, Beijing, China
2 School of Medicine, Nankai University, Tianjin, China
3 Department of Nuclear Medicine, Chinese PLA General Hospital, Beijing, China
4 Department of Radiology, Chinese PLA General Hospital, Beijing, China
5 Department of Neurology, Chinese PLA General Hospital, Beijing, China
Abstract
Abstract Deep brain stimulation is a therapy for Alzheimer’s disease (AD) that has previously been used for mainly mild to moderate cases. This study provides thefirst evidence of early alterations in performance induced by stimulation targeted at the fornix in severe AD patients.The performance of thefive cases enrolled in this study was scored with specialized assessments including the Mini-Mental State Examination and Clinical Dementia Rating, both before and at an early stage after deep brain stimulation. The burden of caregivers was also evaluated using the Zarit Caregiver Burden Interview. As a whole, the cognitive performance of patients remained stable or improved to varying degrees, and caregiver burden was decreased. Individually, an improved mental state or social performance was observed in three patients, and one of these three patients showed remarkable improvement in long-term memory. The conditions of another patient deteriorated because of inappropriate antipsychotic medications that were administered by his caregivers. Taken together, deep brain stimulation was capable of improving some cognitive aspects in patients with severe AD, and of ameliorating their emotional and social performance,at least at an early stage. However, long-term effects induced by deep brain stimulation in patients with severe AD need to be further validated. More research should focus on clarifying the mechanism of deep brain stimulation. This study was registered with ClinicalTrials.gov(NCT03115814) on April 14, 2017.
Key Words: Alzheimer’s disease; deep brain stimulation; fornix; cognition, memory; mood, performance; early stage; functional neurosurgery;dementia
Introduction
Alzheimer’s disease (AD) is the leading cause of dementia and one of the most challenging issues in public health(Scheltens et al., 2016). More than 40 million people are diagnosed with dementia globally, most of whom are older than 60 years, and this number is anticipated to double every 20 years until at least 2050 (Prince et al., 2013). AD is characterized by cognitive disorder, memory deficits, and a gradual loss of daily living activities. This disease results in heavy social burdens, and has generated considerable interest in public health, medical, and basic life science research(Querfurth and LaFerla, 2010; Ballard et al., 2011; Scheef et al., 2012; Gaiteri et al., 2016; Winblad et al., 2016). There are four main drugs used to treat AD: the cholinesterase inhibitors donepezil, rivastigmine, and galantamine, and the glutamate antagonist memantine (Scheltens et al., 2016).However, the therapeutic effects of these drugs are limited in many cases, and deep brain stimulation (DBS) has therefore become a novel therapy for AD.
DBS is generally used to treat patients with movement disorders, such as Parkinson’s disease (PD) (Castrioto et al., 2014), Meige’s syndrome (Horisawa et al., 2018), dystonia (Kupsch et al., 2006), and spasmodic torticollis (Volkmann et al., 2014). However, previous studies have shown that DBS can modulate dysfunctional brain circuits (Lozano and Lipsman, 2013) and rescue memory in mice (Hao et al., 2015). Based on thesefindings, DBS was manipulated in the clinic for the treatment of dementia to rescue memory deficits and cognitive impairment. Thirty years after thefirst case report by Turnbull et al. (1985), phase II clinical trials (Lozano et al., 2016) have provided long-term follow-up evidence of DBS in AD patients. Sankar et al. (2015)presented evidence that DBS in the fornix in humans can influence brain structures and greatly slow hippocampal atrophy. Furthermore, a previous study reported that DBS therapy for mild AD patients is relatively inexpensive(Mirsaeedi-Farahani et al., 2015). Nevertheless, key issues remain. First, current research has mainly been focused on animal experiments, and there is still a relative lack of evidence from clinical trials. Second, current clinical studies in this area mainly focus on changes in assessment scores,brain structures, or metabolism. Furthermore, most current studies of DBS are performed in patients with mild to moderate AD. Finally, partial improvement in the performance of AD patients remains poorly understood. However, severe AD patients, with more seriously impacted qualities of life, have a much more urgent demand for effective therapies.
This prospective study presents what we believe to be thefirst evidence of the effects of DBS on severe AD patients, infive cases. Comparisons were made between evaluation results from before and at an early stage after DBS, both in the whole group and individually. More importantly, changes in various dimensions were respectively reported and their underlying mechanisms are discussed later in this paper.
Subjects and Methods
Subjects
This is a prospective case study. Seven AD patients underwent bilateral fornix DBS (DBS-f) in the Neurosurgery Department at the General Hospital of People’s Liberation Army from June 2017 to March 2018. Among these patients, five cases of severe AD were included in this study,and we excluded two cases of moderate AD. Before surgery, the performance of each patient was evaluated with the Mini-Mental State Examination (MMSE) (Folstein et al., 1975), Montreal Cognitive Assessment (MoCA; Beijing Version) (Nasreddine et al., 2005; Yu et al., 2012), Clinical Dementia Rating (CDR) (Hughes et al., 1982; Morris, 1993),Barthel Index (Mahoney and Barthel, 1965; Cid-Ruzafa and Damian-Moreno, 1997), Functional Activities Questionnaire (FAQ) (Pfeffer et al., 1982), and Functional Independence Measurements (FIM) (Granger, 1986; Hsueh et al.,2002). Caregiver burden was assessed by the Zarit Caregiver Burden Interview (ZBI) (Zarit, 1985). Allfive patients (two males and three females) were aged 50–70 years and satisfi ed the criteria for dementia in the ICD-10 (Organization,1992). Their CDR scores were all 3.0. and they were primary dementia cases with a disease course of less than 10 years and a life expectancy of more than 2 years. No patients had comorbidities such as multiple sclerosis or seizures, and all patients had been given unsatisfactory conservative medication treatments lasting longer than 6 months. Patients had no structural brain abnormalities (intracranial tumors, infarction, or hematomas) or severe cardiovascular or respiratory diseases. Their visual and hearing abilities were normal and no patients had a history of psychoactive medication therapy.
This study was approved by the local ethics committee of the Chinese People’s Liberation Army (PLA) General Hospital (approval number: 2015-013-02), and registered with ClinicalTrials.gov (NCT03115814) on April 14, 2017. In consideration of the serious cognitive impairment in patients with severe AD, written informed consents were obtained from each caregiver.
Therapeutics

Figure 1 Severe brain atrophy in the whole brain, pre-surgery.

Figure 2 Location of electrodes in the fornix.

Figure 3 Sites of electrode and leading wires.
Imaging examinations were performed with a 3.0T (GE750,GE Healthcare, Waukesha, WI, USA) or 1.5T magnetic resonance imaging (MRI) scanner (Siemens Espree, Siemens,Erlangen, Germany) (Figure 1A–C). After pre-surgical preparations, each patient was given DBS targeting the bilateral fornix. The original MRI Dicom data were transferred into the Surgiplan Workstation (ELEKTA, Stockholm, Sweden) to preliminarily determine the bilateral fornix location(Figure 1). After a local anesthesia induction with 20 g/L lidocaine by percutaneous injection, the Leksell-G stereotactic frame was mounted on each patient’s head, followed by a cranial CT scan. The acquired original CT Dicom data were merged into the ELEKTA System with MRI data to calculate the bilateral coordinates for targeting the fornix. Then the left and right targets’ coordinates were converted into frame coordinates. The mean left frame coordinate of thefive cases was (108/107.2/122.9) and the right was (94.4/107.3/122.6).In the operating room, each patient was given a general anesthesia in the supine position. Subsequently, a semi-circular incision was made 10 cm posterior to the superciliary arch,followed by bilateral bone burring, which was performed by a 14 nm drill. Lead-point microelectrodes were inserted into the bone burrs after positioning the insertion tube and detecting electrophysiological signals from the fornix. Next,quadripolar DBS electrodes (PINS L301, Beijing PINS Medical Co., Ltd., Beijing, China) were implanted into the bilateral fornix after recording the electrophysiological signals.Localfield potentials from the fornix could then be detected.Soon afterwards, a test of the electrodes targeting the bilateral fornix was performed, followed by removal of the insertion tube and implantation of the single-channel internal pulse generator (PINS G101A, Beijing PINS Medical Co.,Ltd.) into the bilateral infraclavicular area and connecters via lead extensions.
In general, the DBS electrodes were implanted parallel to the bilateral anterior ventral fornix, medial to the hypothalamus, with the largest ventral contact surface 2 mm above the optic tract surface, 5 mm from the midline (Laxton et al.,2010) (Figures 2 and 3).
Patients were discharged from hospital if there were no complications, and electrical stimulation was given within 2 weeks after surgery. The monopolar stimulation was given a ventral connection, with a frequency of 130 Hz, pulse width of 90 ms, and voltage gradually increasing from 1 V to 5 V.Medication after surgery was given to each patient at the same dose as before surgery.
Assessments and statistical analysis
In this study, a short-term (1.5–3 months) follow-up was given to each patient at an early stage after DBS surgery. The cognitive status of each patient and caregiver burden were evaluated using the same assessments as in the pre-operative testing (MMSE, MoCA-BJ, CDR, Barthel Index, FAQ, FIM,and ZBI; all items were evaluated by the same experienced neurologists (Jianjun Jia’s group) as in the pre-DBS assessments). The ZBI was performed by the same caregiver for each patient as in the pre-DBS assessments. In this study, the Wilcoxon signed-rank test for matched pairs was performed to compare the scores between before and recently after surgery. Statistical significance was defined as a probability value of P < 0.05. All mathematical operations were performed with SPSS 17.0 (SPSS, Chicago, IL, USA).
As mentioned earlier, changes in certain characteristics of each patient, such as long-term memory and mental states,could not be well reflected by a patient’s scores. In these cases, these characteristics were summed up through consultation with caregivers and through patient observation by two experienced practitioners.
Results
Patient characteristics
Among thefive patients (two males, three females), cases 1 to 4 were followed up at 3 months of DBS, while case 5 was followed up at 1.5 months of DBS. The mean age of patients was 59 years (range: 53–65 years). The mean course of the disease was 7.6 years (range: 5–10 years). Case 2 had a family history of dementia, while other patients had no relevant family histories. The functions that were initially impaired were mainly related to memory, orientation, and temperament. All patients had long-term history of conservative medication therapy: Cases 1, 2, 4, and 5 took oral drugs such as nimodipine, oxiracetam, memantine, and donepezil, while case 3 was given traditional Chinese medication.However, all conservative therapeutic trial medications were inefficient. Consequently, patients receiving conservative therapeutic trial medications developed severe AD (Table 1).
Whole performance of patients after DBS treatment
On the whole, there was varying improvement in all assessment scores except CDR after surgery, indicating that most cases gained an enhancement in some aspect of cognition.The largest change (25%) in scores occurred in the MMSE and the MCoA in the visual angle of the whole group, but there was no significant difference in these two scores (P >0.05). The probable reason was that the whole improvement was contributed by case 2. CDR scores remained at 3 in all cases, demonstrating that the grade and phase of the disease probably could not be reversed in the early stages of DBS.ZBI, which was defined as caregiver burden in the study,was significantly improved in the whole group (P < 0.05) as well as in each case, indicating that the psychological burden of caregivers was relieved to a certain degree.
Among individuals, the most considerable amelioration at an early stage of DBS was in case 2, where MMSE and MCoA scores increased from 3 to 6 and 2 to 3, respectively.In contrast, case 4 was the only patient whose condition deteriorated after surgery. In terms of his activity of daily living (ADL), case 4’s Barthel index fell from 55 to 45, which can be considered as a degeneration in basic activities of daily living (BADL). His FAQ score rose from 28 to 30, revealing a decrease in instrumental ADL, and the decrease from 61 to 56 in FIM was evidence of both BADL and cognitive impairment. The caregiver burden did not increase along with the degeneration of case 4; on the contrary, the ZBI score decreased slightly following DBS (from 75 to 72). Case 5, the most severe case in the whole group, demonstrated increases in some of her scores (the Barthel Index, FAQ, and FIM) to different degrees. There was also a reduction in the ZBI score from her caregiver (Table 2).
Partial cognitive improvement in each patient
Participants enrolled in the present study had already lost most of their ADL and were taking medications with helpfrom their caregivers. There was no significant improvement in the group at an early stage of DBS in ADL. Nevertheless,all cases had alterations in certain dimensions, according to both their caregivers’ and our own observations. The improved functions in this group were mainly in mental state,language, long-term memory, and calculations. Cases 1, 3,and 5 had an improved mental state after the surgery, and case 2 had an astonishing amelioration in long-term memory. The details of each case are summarized as follows:
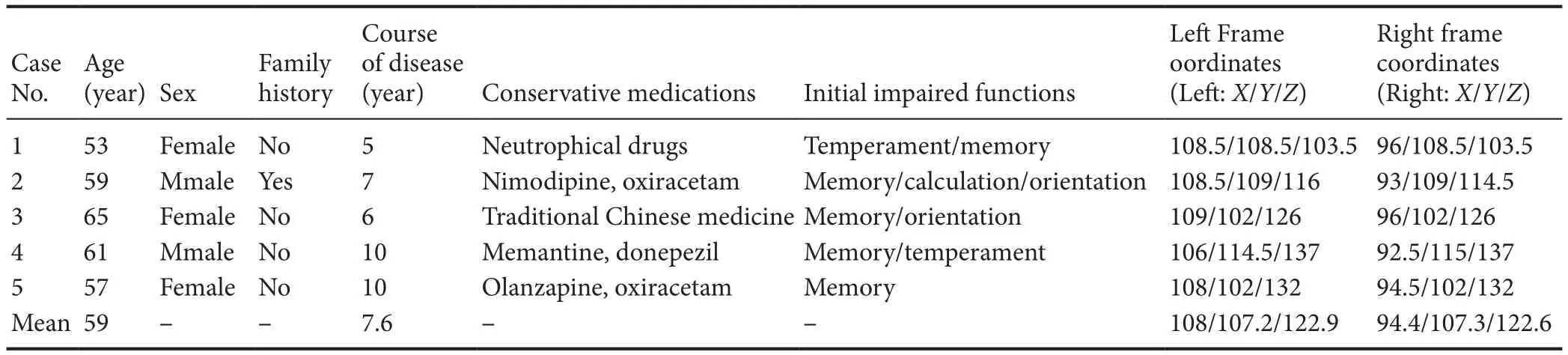
Table 1 Clinical characteristics offive included cases

Table 2 Pre-DBS and post-DBS performance evaluation results infive included cases
Case 1, a 53-year-old female, was a right-handed peasant.Her most obviously improved aspects of cognition at the early stage of DBS were in mood and verbal dexterity. She suffered from initial manifestations after witnessing the death of her pet dog, including an alteration in character and a decline in memory. She gradually lost interest in going outside and communicating with others. Neurotrophic medications were ineffective in her course of disease. Her symptoms had been exacerbated in the past 2 years: an aggravated loss of memory, language disorder, a degenerative coordination in daily activities, impaired eyesight, and an intention tremor, especially in agitation. Her medical examinations on admission were as follows: deterioration of calculations, impaired spatial and temporal orientation, severe loss of short- and long-term memories, and no pathological disorders. Her pre-DBS MRI indicates whole brain atrophy(Figure 1A–C). In hospital, she was given bilateral DBS and electrode implantation (PINS L301) (Figures 2 and 3).
Her mood obviously improved after DBS and she was eager for communication with others, which was in contrast with her introversion and depression before surgery. She could communicate on her own initiative when running into acquaintances, although she was not able to express herself clearly or form an integrated sentence. Verbal dexterity improved along with the change in mental status. The intention tremor of her hands was also alleviated, especially while eating. There was no significant difference in short- or long-term memory after surgery. She was able to eat independently, but relied on her caregivers for clothing, emiction, and excretion.
Case 2, a 59-year-old male, was a right-handed farmer.Long-term memory was the most substantial improvement in this patient. The patient was admitted to hospital because of a progressive decline in memory, calculation, and orientation over 7 years. During his disease course, he was given nimodipine and oxiracetam as conservative therapy. His father suffered from AD during his lifetime. Before DBS therapy, case 2’s short-term memory was seriously impaired:he usually forgot things only several minutes after they occurred. According to his caregivers’ observations, his shortterm memory showed negligible improvement following DBS. However, it was intriguing that his long-term memory improved markedly. For instance, he could bring many names he had previously forgotten to mind, including his relatives and friends and the children in his hometown. Another exceptional change was in his self-care ability. For example, he needed help to put on clothes before surgery, but he could manage it independently in the early stage of DBS.Additionally, his calculating ability was slightly better and he could solve the simplest mathematical problems. Furthermore, sleep quality of case 2 improved noticeably following surgery, which contributed to his physical and mental condition, and vice versa.
By contrast, he acquired a destructive power which had rarely manifested before: he destroyed a lot of furniture,such as a television. He tended to mistake young foreigners as his own descendants, especially in tense situations. Apart from these negative impacts, he sometimes attempted to prove himself by helping with cleaning, which was not very successful. In regard to his temperament, he remained introverted and lonely, similar to before. Before surgery, he did not experience thirst, hunger, or sleeplessness and did not consider others’ feelings, and these aspects remained the same in the post-DBS period.
Case 3, a 65-year-old female, was a right-handed farmer.She had been suffering from a decline in memory and orientation for 6 years. Deterioration appeared 2 years ago in spite of the administration of traditional Chinese medication. She was recognized as having “a total loss of self-care ability, loss of calculation, and worsening of previous symptoms” in admission. In addition, she was easy to rage and tended to curse others in ADL.
She showed an improvement in mood after surgery, and tended to be friendly and more likely to smile. Although she was still not able to express herself effectively, she looked forward to interacting with her relatives more. She sometimes talked to herself, which was incomprehensible to her caregivers, and she slept better than before, which gave her caregivers more time to rest. However, she was still unable to do almost all daily activities, such as eating and dressing herself, independently. Incontinence was another problem that bothered her caregivers. There was little change in her memory after surgery.
Case 4, a 61-year-old male, was a right-handed farmer. This was the only patient who performed worse after the surgery.This patient suffered from progressive memory loss and became bad-tempered gradually over 2 years at the beginning of his AD. After an inefficacious conservative therapy of oral medication, he developed symptoms of intermittent mania.He was easily excited and had a sense of euphoria shortly after the DBS therapy, so he was given quetiapine, an atypical antipsychotic medication, to control these symptoms. Shortly after DBS, he was reluctant to eat by himself and needed caregivers to feed him. Moreover, he did not show any interest in the greetings of others. Unfortunately, he had a violent tendency and was easy to be raged. He needed full-time attendance in his normal daily activities.
Case 5, a 57-year-old female, was a retired chemistry teacher. She progressively developed severe AD with total memory impairment, from the initial stage involving a slight loss of memory and character changes. She was considered to have lost nearly all the orientations of character, time, and space on admission. After DBS, she had a much better mood than before. She was also easily excited, similar to case 4.
Less satisfactory was that she ate more meals every day and slept less than before. Furthermore, she was afraid of going upstairs and downstairs on her own, probably because of impaired visuospatial ability. According to the narration from her caregiver, she was unable to carry out almost all daily activities, and still suffered from incontinence.
Discussion
Prior clinical trials have documented the feasibility of DBS for AD, including its safety and its effects on cognition and brain glucose metabolism, as summarized in Table 3. For example, Laxton et al. (2010) provided evidence in a phase I trial of DBS in the fornix/hypothalamus in AD that a continuous stimulation could induce an early reversal of impaired glucose metabolism in the temporal and parietal lobes,which lasted 12 months after the surgery. In addition, evaluations of some patients suggested possible improvements and a slowing of cognitive decline at 6 and 12 months. In a phase II trial, Lozano et al. (2016) reported that DBS was associated with elevated cerebral glucose utilization at 6 months, and that no differences existed in cognition after surgery as a whole. In addition, no serious complications were reported in the two above-mentioned studies. The safety of DBS in the bilateral fornix for AD treatment was further validated in a study by Ponce et al (2016) , which demonstrated that DBS was tolerated by mild, probable AD cases in a double-blind, randomized, multi-center study.However, the limited number of available studies have mainly investigated DBS in mild to moderate AD cases. To our knowledge, ours is thefirst clinical trial of DBS therapy in severe AD patients. A partial improvement in certain aspects of cognition and a reversal of memory deficits were achieved in some of the cases enrolled in the trial, and no severe neurological adverse events occurred in the early stages of DBS. Our paper gives a preliminary validation of the feasibility of DBS in severe AD cases, in terms of safety and effects, and enriches the current knowledge of DBS in different grades of AD.
There are various potential DBS targets for dementia,such as the fornix, hypothalamus, NBM, anterior nucleus of the thalamus, and hippocampus (Laxton and Lozano,2013); however, the fornix is recognized as the leading target of DBS for cognitive and memory deficits (Sankar et al., 2014). The fornix and associated structures, along with hippocampal commissures, mammilo-thalamic tracts, thalamo-cingulate connections, posterior cingulate cortex (PCC),and retrosplenial cortex (RSC) constitute the Papez Circuit,according to a diffusion spectrum imaging (DSI) in vivo visualization by Wei et al. (2017). Moreover, cholinergic axons from the septal area to the hippocampus are conveyed by the fornix (Sankar et al., 2014), and lesions in the fornix are reported to produce memory impairments (Thomas et al., 2011). Based on the afore mentioned reasons, this study recognized the fornix as the target of DBS and anticipated a partial cognitive improvement in patients.
In the present study, a considerable improvement in longterm memory was observed in case 2, who became capable of recalling long-forgotten acquaintances. This result verified the hypothesis that DBS can rescue memory deficits,which had been described in various previous studies in both individuals and in animal models (Hamani et al., 2008;Hao et al., 2015). One possible factor that contributed to the cognitive improvement of case 2 may have been the activation of certain areas and brain networks concerning memory. Hamani et al. (2008) reported memory enhancement induced by DBS in a patient receiving hypothalamic stimulations for his morbid obesity; in this study, activation of the hippocampal formation and the para-hippocampal gyrus region was mapped by standardized low-resolution electromagnetic tomography (sLORETA). Apart from the regulation of hippocampal activity, another two possible factors involved in memory improvement may be an alteration in synaptic plasticity and an increase in neurogenesis induced by DBS. It is generally considered that memory traces are encoded and stored mainly based on the changes in the strength of synaptic connections between neurons, and synaptic plasticity disruption occurs in many neurodegenerative diseases (Cobb, 2015). A study by Hao et al. (2015) based on mouse models of Rett syndrome found that DBS rescued hippocampal memories including contextual fear memory and spatial learning and memory, which indicates that DBS might mitigate cognitive dysfunction in this type of intellectual disability disorder. In addition to the promising result,this study also observed long-term potentiation, a type of synaptic plasticity associated with particular types of learning, which was impaired in the Rett model but enhanced by DBS treatment (Hao et al., 2015). Moreover, neurogenesis was detected in hippocampal areas after DBS targeting of the fornix (Hao et al., 2015), which indicated that cognitive and memory decline may be rescued, at least in part, by the regeneration of neurons induced by DBS.
Another highlight from our study is that three of ourfive cases had improved mood and social performance after DBS. These patients had gradually developed an eccentric,unsociable, or irritable character, but they became more friendly, sociable, and more likely to smile with the persistent and chronic stimulation. This result is thought to be somewhat similar to results from the treatment of depression with DBS. In a study by Bergfeld et al. (2016), 25 cases with treatment-resistant depression were given DBS targeted at the ventral anterior limb of the internal capsule, and a significant reduction in depression occurred in 10 cases.Another target for DBS in depression includes the STN and subcallosal cingulate (Dandekar et al., 2018). However, few studies of depression have chosen the fornix as a stimulating target. It was therefore an interesting phenomenon that better mood could also be brought about by stimulation targeting the fornix, a structure initially recognized as associated with cognition. DBS modulates brain activity directly in limited brain structures, but also adjusts interconnected brain network connectivity (Dandekar et al., 2018). In this study, the improvement in mood was probably due to modulation in the functional connectivity brain networks in our AD cases. Among the brain networks, the default mode network (DMN), which involves a collection of brain
regions, is unique for its exhibition of greater activity during resting states than during task performance (Zhang and Raichle, 2010). Moreover, AD-related connectivity alterations have been found in DMN in a previous study (Zhang and Raichle, 2010), and a study by Ho et al. (2015) reported that there was an association between adolescent depression and inflexibly elevated DMN connections. An appropriate DBS target has been hypothesized to change this particular network concerning mental states and social behavior. Nevertheless, the exact mechanism seems elusive and there has not been a consensus about this explanation regarding mental and behavioral improvements.

Table 3 Human studies of DBS for dementia
In the present study, the deterioration of case 4 was thought to be the result of his improper use of quetiapine.This atypical antipsychotic drug was misused by his son, an emergency doctor, to treat the initial dysphoria and euphoria after DBS. In general, this mental state was detected in many cases shortly after DBS and returned to normal after a period without any intervention. In regards to the safety of DBS, no severe adverse events were observed in this study, which is in agreement with the clinical trials by Ponce et al. (2016).
There are several limitations of the current study that should be acknowledged. First, the number of cases in our study was relatively small, and a larger sample size is needed in further investigations. Second, although at an early stage an improvement was gained by most cases to differing extents in cognition, memory, mental states, and social behavior, long-term outcomes for severe AD patients are still unknown and there is an urgent need for long-term follow-up evidence. Furthermore, the mechanisms of changes in various aspects such as cognition and memory are still unknown, and future work focused on these mechanisms is required. Finally, a large improvement in performance was achieved by a particular patient, which highlights the need for future research that investigates which individual patient factors can be used to predict improvements in cognition and memory.
Author contributions:Study conception: ZQM and XGY; study implement: ZQM, XX, ZQC, LSP, XJN, BXX, LM, ZPL; data analysis: JJJ,ZQM and XW; paper writing: ZQM, WX, and XGY. All authors approved thefinal manuscript.
Conflicts of interest:None declared.
Financial support:This study was supported by a grant from the National Natural Science Foundation of China (No. 8187052509 ) and a grant from the National Key Research and Development Plan of China(No. 2017YFC0114005). Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper
Institutional review board statement:This study was approved by the Local Ethics Committee of the Chinese People’s Liberation Army (PLA)General Hospital. ZQM and XW contributed equally to this work. This study was registered with ClinicalTrials.gov (NCT03115814) on May 6, 2015.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms from their caregivers. In the form the caregivers have given their consent for the patients’ images and other clinical information to be reported in the journal. The caregivers understand that the patients’ names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement:This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were re-viewed by the biostatistician in the PLA General Hospital, China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:For data sharing, individual participant data will be available after deidentification. The study protocol was available as publication (Mao et al., 2017). In order to gain access, data requestors will need to sign a data access agreement, and proposals should be directed to markamoqi@126.com.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy
- Analysis of transcriptome sequencing of sciatic nerves in Sprague-Dawley rats of different ages
- Exogenous brain-derived neurotrophic factor attenuates cognitive impairment induced by okadaic acid in a rat model of Alzheimer’s disease
- Epigenetic marks are modulated by gender and time of the day in the hippocampi of adolescent rats:a preliminary study
- Dysphagia in patients with isolated pontine infarction