New models to study vulvodynia:hyperinnervation and nociceptor sensitization in the female genital tract
2018-10-22ChristineM.Barry,KalyaniK.Huilgol,RainerV.Haberberger
Vulvodynia is a prevalent form of chronic pain, most commonly affecting the vaginal vestibule (vestibulodynia) (Pukall et al., 2016). Women with vulvodynia describe intense pain in response to light touch of the affected region, such that sexual function and other activities can be severely limited. Medical costs associated with vulvodynia are high, exceeding $21 billion annually in the United States (Xie et al., 2012). The high level of direct medical costs has been linked to high treatment failure rates. Many women with the disorder consult multiple practitioners and undergo multiple courses of treatment with limited benefit.
First-line treatments for management of vulvodynia, according to an expert committee of the Fourth International Consultation on Sexual Medicine, are psychological interventions,pelvic floor physiotherapy and surgical removal of painful tissue, with progression from less invasive to more invasive treatments if initial treatments fail. Further interventions including capsaicin, botulinum toxin and interferon are also recommended (Goldstein et al., 2016). Clinical evidence does not support the use of lidocaine, topical corticosteroids and antidepressant medications in management of vulvodynia, and further evidence is required before anti-inflammatory medications, hormonal treatments or anticonvulsant medications can be recommended (Goldstein et al., 2016). The need for further clinical trials of established treatments is well recognised, and more fundamentally, studies to improve understanding of vulvodynia pathophysiology, so that treatments can be appropriately targeted.
Vulvodynia is usually idiopathic but certain pathologicalfindings are well described. Symptomatic vaginal tissue contains increased numbers of nervefibres (hyper-innervation), including peptidergic and non-peptidergic axons, though noradrenergic sympatheticfibres do not appear to contribute to hyper-innervation in women with vulvodynia (Bohm-Starke et al., 2001;Tommola et al., 2016; Liao et al., 2017). In addition to hyper-innervation, nociceptor sensitisation is also evident, and patients have allodynia in response to mechanical and thermal stimuli of symptomatic regions, with reduced pain thresholds to both heat and cold (Bohm-Starke et al., 2001). Symptomatic tissue from patients also contains increased numbers of macrophages,T cells and B cells (Tommola et al., 2016; Liao et al., 2017). Some women with vulvodynia also demonstrate hypertonicity of pelvicfloor muscles, which is considered to be secondary to increased pain sensitivity (Goldstein et al., 2016; Pukall et al., 2016). While the aetiology and pathophysiology of vulvodynia are unclear, a widely held view is that infection or trauma triggers sprouting and sensitisation of nervefibres with persistence of innervation changes once inflammation has resolved.
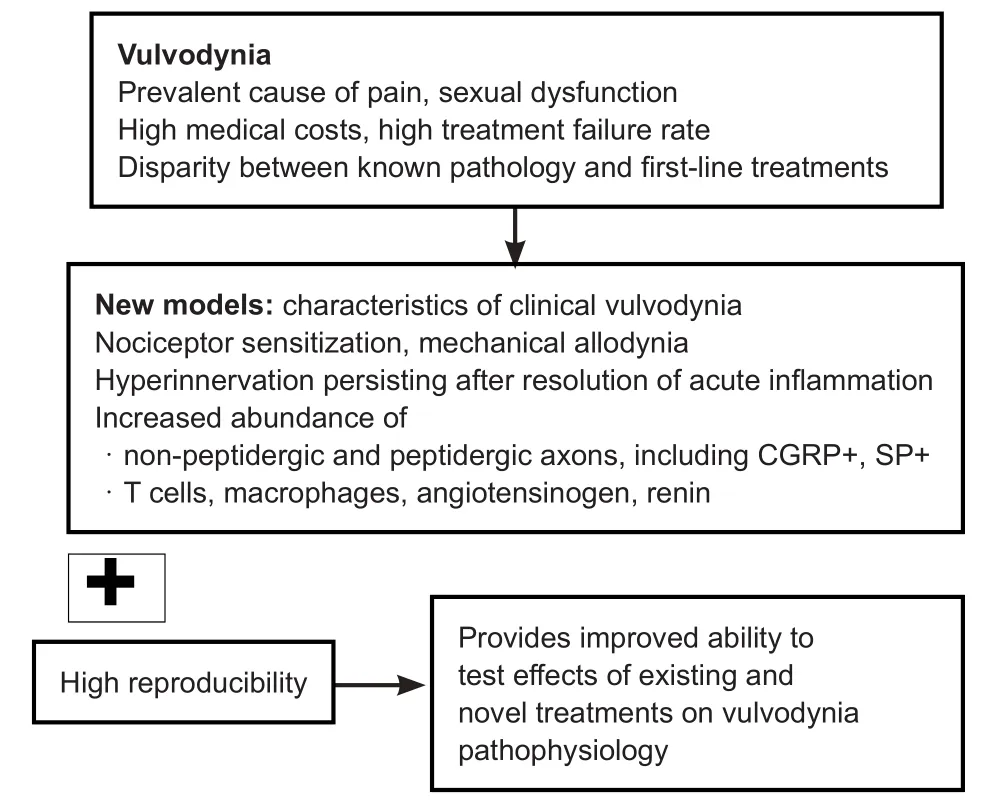
Figure 1 Key pathological and pathophysiological features of vulvodynia also evident in recently developed models.
Recently developed models are aiding our understanding of mechanisms that contribute to proliferation and sensitisation of sensoryfibres in vulvodynia (Figure 1). We have shown that hyper-innervation in the mouse vagina is induced by microinjection of the pro-inflammatory agent complete Freund’s adjuvant (CFA) (Sharma et al., 2018). Hyper-innervation was prominent at 7 days and continued to be present at 28 days following a single administration of CFA. Oedema was evident at 7 days and resolved at 28 days. Hyper-innervation involved multiple types of nervefibres, including nervefibres that were immunoreactive for calcitonin gene related peptide (CGRP+fibres), substance P (SP) and vasoactive intestinal peptide, in addition to nervefibres that were identified by the pan-neuronal marker protein gene product 9.5 (PGP9.5) and not immunoreactive for CGRP (PGP9.5+CGRP-fibres). Putative sympatheticfibres were identified in the mouse vagina by immunoreactivity for tyrosine hydroxylase (TH) and TH+fibres did not contribute to hyper-innervation in this model. These findings regarding proliferation of CGRP+, SP+and PGP9.5+but not TH+fibres are consistent with the reported innervation changes in women with vulvodynia (Bohm-Starke et al., 2001; Tommola et al.,2016; Liao et al., 2017). We also identified substantial infiltration of CD68 positive, putative macrophages and proliferation of vaginal blood vessels in this mouse model (Sharma et al., 2018).Mast cells were identified in the vagina but were relatively few,and their presence was not increased following administration of CFA and development of hyper-innervation. A similar model in rats found CFA injected into the posterior vestibule produced hyper-innervation involving peptidergic and non-peptidergic neurons, hypersensitivity, and increased presence of macrophages and T cells (Chakrabarty et al., 2018). An important feature of these new models of vaginal hyper-innervation and nociceptor sensitisation is that inter-individual differences within each treatment group are small, consistent with these models’utility for investigating the impact of interventions.
Studies using the CFA model of vulvodynia in rats have shown interactions between macrophages and sensory fibres contributing to the pathophysiology, and an important mechanism involves local production of angiotensin II (Liao et al., 2017; Chakrabarty et al., 2018). Activation of angiotensin II type-2 receptors (AT2R) appears to contribute to both hyper-innervation and nociceptor sensitisation. AT2Rs are expressed by a substantial proportion of small and medium sized sensory neurons (Anand et al., 2013). Treatment of cultured dorsal root ganglion (DRG) neurons with angiotensin II enhances neurite outgrowth and enhances their response to capsaicin, the transient receptor potential V1 (TRPV1) cation channel agonist, whereas treatment with an AT2R antagonist reduces neurite outgrowth and inhibits the response to capsaicin (Anand et al., 2013). The precursors of angiotensin II,renin and angiotensinogen are found in increased abundance in symptomatic tissue from patients and in the CFA model of vestibulodynia, in association with the increased vestibular macrophages and T cells (Liao et al., 2017; Chakrabarty et al.,2018). Media conditioned by pain-associated vestibular tissue produced enhanced axonal sprouting of cultured dorsal root ganglion neurons, and this response was prevented by blockade of the actions of angiotensin II by an AT2R antagonist or by an angiotensin II antibody (Liao et al., 2017). Consistent with this, inhibition of peptidases that convert angiotensin I to angiotensin II,i.e., angiotensin converting enzyme (ACE)or mast cell chymase, reduced neurite sprouting of cultured DRGs incubated in conditioned media. There are additional mechanisms through which a local renin-angiotensin system could impact on nociceptive signaling. Angiotensin II inhibits release of acetylcholine which has many actions in the peripheral and central nervous system to inhibit nociceptive signaling.Reduced availability of ACE could delay degradation of SP and enhance local neurogenic inflammation. It is not clear if these mechanisms play a role in vulvodynia. Regardless, in the CFA model of vestibulodynia, rats treated with an AT2R antagonist had fewer immune cells expressing renin or angiotensinogen,and had reduced hyper-innervation (Chakrabarty et al., 2018).
In addition to helping identify novel interventions for vulvodynia, these models may be useful to understand the impact of existing treatments and improve their application. For example, the models could be applied in studies to maximise analgesic effects of botulinum toxin for treatment of vulvodynia, in addition to reducing superimposed pelvicfloor muscle spasm. Existing evidence indicates several mechanisms may be involved. Studies of the rat temporalis muscle show botulinum toxin reduced sensitivity to mechanical stimulation and reduced neurogenic inflammation by inhibiting release of glutamate and CGRP (Gazerani et al., 2010).In vitrostudies show botulinum toxin inhibits angiogenesis and inhibits proliferation and migration of keratinocytes (Gugerell et al.,2016). Inhibition of angiogenesis is likely to inhibit development of accompanying perivascular nociceptive nerve fibres,contributing to hyperinnervation. In the CFA model of vaginal hyperinnervation, vascular proliferation was accompanied by proliferation of perivascular nerve fibres, many of which showed neurochemical characteristics of nociceptors (Sharma et al., 2018). Keratinocytes are known to participate in crosstalk involving nociceptors and immune cells but research regarding keratinocyte-nociceptor interactions in vulvodynia is lacking. However, macrophage-nociceptor interactions are implicated in vulvodynia (Liao et al., 2017; Chakrabarty et al.,2018), and macrophage-nociceptor communication mediated by exosomes is interrupted by botulinum toxin (Simeoli et al.,2017). It was recently shown that macrophages contribute to nociceptor sensitisation after phagocytosis of exosomes released by DRG neurons in response to activation of TRPV1, which is expressed by a high proportion of nociceptive neurons. Specific microRNAs were identified in these exosomes and they caused altered mRNA and gene expression in macrophages, resulting in altered macrophage functional state towards the pro-inflammatory, M1 phenotype (Simeoli et al., 2017). Further studies are required to clarify if any of these mechanisms contribute to beneficial effects of treatment with botulinum toxin reported by some patients with vulvodynia, and such studies are critical to improve targeting of existing treatments and establishment of appropriate clinical trials.
Two decades since hyper-innervation and nociceptor sensitisation were described as key features of vulvodynia, there remains nofirst-line recommended treatment that specifically addresses this pathophysiology. Recently developed, highly reproducible rodent models of vulvodynia are important advancements,and already show promise towards improved understanding of mechanisms that contribute to the development and persistence of this disorder, and towards identifying potential targets for intervention (Figure 1).
A project described in this work regarding innervation changes in the murine vagina in response to inflammation was supported by a grant from the Centre for Neuroscience, Flinders University,Australia.
Christine M. Barry*, Kalyani K. Huilgol, Rainer V. Haberberger
Anatomy and Histology, College of Medicine and Public Health,Flinders University, Adelaide, Australia (Barry CM, Huilgol KK,Haberberger RV)
Centre for Neuroscience, Flinders University, Adelaide, Australia(Barry CM, Haberberger RV)
*Correspondence to:Christine M. Barry, PhD,christine.barry@flinders.edu.au.
orcid:0000-0002-5227-3524 (Christine M. Barry)
Received:2018-04-23
Accepted:2018-06-26
doi:10.4103/1673-5374.241455
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Fabricio Ferreira de Oliveira, Universidade Federal de Sao Paulo, Brazil.
Additionalfile:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy
- Analysis of transcriptome sequencing of sciatic nerves in Sprague-Dawley rats of different ages
- Exogenous brain-derived neurotrophic factor attenuates cognitive impairment induced by okadaic acid in a rat model of Alzheimer’s disease
- Partial improvement in performance of patients with severe Alzheimer’s disease at an early stage of fornix deep brain stimulation
- Epigenetic marks are modulated by gender and time of the day in the hippocampi of adolescent rats:a preliminary study