Roles and functions of Atp6ap2 in the brain
2018-10-22AlexanderBrackeOlivervonBohlenundHalbach
Alexander Bracke, Oliver von Bohlen und Halbach
Institute of Anatomy and Cell Biology, Universitätsmedizin Greifswald, Greifswald, Germany
AbstractThe classical renin-angiotensin system (RAS) in the body has been studied intensively in the last decades,since it is known that this system is involved in the regulation of blood pressure. Since nearly all members of the classical RAS have also been identified within the brain in the last decades and due to the existence of the blood-brain barrier, a RAS within the brain (bRAS) that is largely independent from the peripheral RAS has been postulated. All members of the angiotensin family as e.g., angiotensin II, angiotensin IV and angiotensin II (1–7) along with the respective receptors (e.g., angiotensin II receptor type 1 (AT1), angiotensin II receptor type 2 (AT2), angiotensin IV receptor (AT4), angiotensin II (1–7) receptor (Mas)) have been identified within the brain. Moreover, a receptor capable of binding renin and the renin precursor prorenin with high affinity has also been detected within the brain. This protein functions as a membrane receptor for (pro)renin and also represents a V-ATPase subunit and is therefore termed (P)RR or Atp6ap2,respectively. In this review we shed light on the (known as well as putative) roles and functions of Atp6ap2 in the brain under physiological and pathophysiological conditions.
Key Words: prorenin receptor; renin-angiotensin; X-linked intellectual disability; hippocampus; behaviour;adult neurogenesis; animal model; human
Introduction
The classical renin-angiotensin system (RAS) in the body has been studied intensively in the last decades, since it is known that this system is involved in the regulation of blood pressure. Since nearly all members of the classical RAS have also been identified within the brain and due to the existence of the blood-brain barrier, a RAS within the brain (bRAS),that is largely independent from the RAS in the periphery,has been postulated. Thus, members of the angiotensin family as e.g. angiotensin II, angiotensin IV and angiotensin II(1–7) along with the respective receptors (e.g., AT1, AT2,AT4, mas) have been identified within the brain (von Bohlen und Halbach, 2005). Moreover, a prorenin receptor was cloned by Nguyen et al. (2002). The prorenin receptor ((P)RR) binds renin as well as prorenin (the inactive proenzyme form of renin). Interestingly, ((P)RP) binds renin with a lower affinity than prorenin (Phillips and de Oliveira, 2008).The binding of prorenin has been discovered to trigger the activation of the mitogen-activated protein kinase p42/p44. This activation is than followed by an up-regulation of profibrotic gene expression. In addition, the prorenin that has bind to the (P)RR undergoes a specific conformational change and, thereby, becomes catalytically active (Nguyen,2010) and, in addition, enhances the enzymatic activity to generate angiotensin I, which, thereafter, is converted to active angiotensin peptides, such as angotensin II and others.In the circulation, angiotensin II is a strong vasopressor and regulates water as well as electrolyte balance. In the brain, angiotensin peptides may exert various functions such as blood pressure regulation, pituitary gland hormone regulation and even sexual behavior (Wright and Harding,1995). Moreover it has been shown that different forms of angiotensins in the brain, especially angiotensin II (Denny et al., 1991; von Bohlen und Halbach and Albrecht, 1998),angiotensin (1–7) (Hellner et al., 2005) and angiotensin IV (Wayner et al., 2001) are also involved in mechanisms related to learning and memory (von Bohlen und Halbach,2005).
In general, binding of (pro)renin to the (P)RR activates angiotensin-independently intracellular pathways, such as pathways involving extracellular regulated protein kinase(ERK) or promyelocytic zincfinger (PLZF) (Nguyen et al.,2002; Schefe et al., 2006). Moreover, a fragment of the (P)RR has been identified to be identical with the M8-9 protein.This protein is known to associate with vacuolar H+-ATPases (V-ATPases) (Ludwig et al., 1998). Furthermore, it has also been demonstrated that the (P)RR is capable of binding V-ATPase subunits and is required to mediate Wnt signaling of Xenopus early central nervous system (CNS) development independent of (pro)renin (Cruciat et al., 2010).Therefore, the (pro)renin receptor is also termed Atp6ap2.
Since V-ATPases, in general, have been found to contribute to receptor-mediated endocytosis, as well as protein degradation via to acidification of organelles (Forgac, 2007), Atp6ap2 might have a role in sensing the levels of acidification of intracellular compartments and thereby can be involved in the regulation of the V-ATPase activity (Ichihara, 2012).(P)RR/Atp6ap2 functionality seems to incorporate renin-angiotensin-depended as well as independent aspects, providing a possible link between both (Sihn et al., 2010). Until yet,data concerning the subcellular expression of Atp6ap2, the downstream pathways and the intracellular cascades that are induced by the activation of Atp6ap2 in neuronal as well as non-neuronal cells with the brain are still missing. In other organs, like heart and kidney, the intracellular pathways are much better investigated. For getting a better insight in this topic, refer to the excellent reviews by Shin and collegues or Ishihara (Sihn et al., 2010; Ichihara, 2012).
Atp6ap2 mRNA has been detected in various organs, including heart, kidney, liver, pancreas, muscles, ovary, placen-ta and the adrenal gland, but it also has been reported that within the brain Atp6ap2 mRNA is expressed (Nguyen et al.,2002). Lack of Atp6ap2 leads to developmental alterations in vertebrates, such as abnormal body and eye pigmentation and necrosis in the CNS, and early embryonic lethality (Sihn et al., 2010). This may suggest, at least during development, an important role of Atp6ap2 within the nervous system. However, Atp6ap2 may also play a role in the adult brain.
Atp6ap2 Is Expressed in the Brain
A fact supporting the assumption that Atp6ap2 has specific functions in the brain that are not related to the known functions of Atp6ap2 in the periphery is that it has been demonstrated that Atp6ap2 mRNA is widely expressed in the adult mouse brain, including regions that are not involved in fluid and/or cardiovascular homeostasis. For example, strong Atp6ap2 mRNA signals were detected in the thalamus, the hippocampus and the cortex (Contrepas et al.,2009), but Atp6ap2 is also found, e.g., in the cerebellum, the medulla and the olfactory bulb (Dubos et al., 2015). In addition, Atp6ap2 was detected in neurons derived from the rat brainstem and hypothalamus (Shan et al., 2008).
This hints for a role of Atp6ap2 in the brain in functions that are not closely related to cardiovascular control andfluid homeostasis. This is comparable to the other roles of the bRAS, including functions that may be related to learning and memory or other specific brain functions (Wright and Harding, 1995, 2013; von Bohlen und Halbach and Albrecht,2006). This would argue for a expression of Atp6ap2 on neuronal cells in the brain. Indeed, Atp6ap2 protein expression was mapped to neuronal cells (Schäfer et al., 2013), but not to astrocytes (Dubos et al., 2015). However, within the substantia nigra a population of Atp6ap2 expressing astrocytes has been detected (Garrido-Gil et al., 2013, 2017). Nevertheless,Atp6ap2 protein has also been detected in microglia cells(Valenzuela et al., 2010) and seems, together with the angiotensin II receptor AT1, promote inflammatory responses and is thought to contribute in the induction of the pro-inflammatory M1-phenotype (Labandeira-Garcia et al., 2017).
Concerning the expression of Atp6ap2 in oligodendrocytes, only in a recent publication atp6ap2 expression was seen in cells with an oligodendritic phenotype, that were derived from human adipose tissue and inducted to proliferate and differentiate into a mixed population of brain cells(Makdissy et al., 2018). However, an expression of Atp6ap2 in oligodendrocytes in mature brain tissue has not been shown yet.
Alterations in Atp6ap2 Severely Affects the Human Brain
Some studies have linked altered splicing of the ATP6AP2 gene to pathophysiological and pathopsychological conditions. In 2002, Nguyen and coworkers have cloned the (pro)renin receptor (Nguyen et al., 2002). Only 3 years later a unique exonic splice enhancer mutation in a family with X-linked mental retardation (XLMR) and epilepsy was reported (Ramser et al., 2005). This mutation was found in the gene coding for the (pro)renin receptor. A subsequent functional analysis revealed that the mutated receptor still was able of binding renin and increasing catalytic activity of renin(similar to the non-mutated receptor), however, this was accompanied by an impairment of ERK1/2 activation (Ramser et al., 2005). In one generation of the family carrying this mutation, eight males were affected. All of the affected members had seizures. Moreover, the phenotype was associated with variable combinations of hyperactivity, impulsive and aggressive behaviour as well as ataxia and speech delay (Ramser et al., 2005). Interestingly, in that context, mutations in one of the two receptors capable of binding specifically angiotensin II, namely the angiotensin II receptor type 2 (short: AGTR2 or AT2), were also described to be associated with XLMR(Vervoort et al., 2002). Moreover, mice that were deficient for the angiotensin II type 2 receptor (AT2) are known to display impaired spatial memory and altered dendritic spine morphology in the hippocampus (Maul et al., 2008). This indicates a specific role of the bRAS in learning and memory and “higher” brain functions. However, whether there is a link between Atp6ap2 and AT2 is still enigmatic.
In 2013 it e.g. was described that altered splicing of Atp6ap2 causes X-linked parkinsonism with spasticity (XPDS),a slowly progressive disease with considerable phenotypic variability (Korvatska et al., 2013). The initial symptom in the affected individuals was spasticity. These individuals later developed a parkinsonian resting tremor, masked facies and bradykinesia (Korvatska et al., 2013). In this case the altered splicing leads to a reduction in the level of Atp6ap2 in the brain and it is was speculated by the authors of that study that this reduction may compromise the function of the V-ATPase (Korvatska et al., 2013). And, furthermore, in a paper from 2015, a splice site mutation in ATP6AP2 was reported that causes X-linked intellectual disability, epilepsy,and parkinsonism (Gupta et al., 2015). The MRI of the brain of one patient showed mild cerebellar atrophy and a reduction in the thickness of the cerebellar folia and the MRI of the brain of the other patient brain showed, in addition to the cerebellar atrophy and a reduction in the thickness of the corpus callosum, a global atrophy of the cerebral hemispheres (Gupta et al., 2015). Whether the Atp6ap2 variant described by Gupta and colleagues leads to a loss or gain of function or does affect the functionality of Atp6ap2 has not been analyzed yet. Together, these studies link disturbances in Atp6ap2 to pathophysiological as well as pathopsychological conditions and to alterations in the brain architecture.Moreover, a meta-analysis of genome-wide association studies and gene expression data revealed significantly reduced expression of the Atp6ap2 gene in case of Alzheimer’s disease (Goldstein et al., 2016). Thus, at least for the brain, lack of functional Atp6ap2 seems to be harmful.
Analyzing Atp6ap2 Functions in Neuronal Cell Cultures and in Animal Models
For a deeper understanding of the role and function of Atp6ap2 in the brain, suitable models are required. These may include the use of cell culture systems for analyzing the effects of a down-regulation or over-expression of Atp6ap2(Schäfer et al., 2015; Bracke et al., 2018).
Since it was thought that Atp6ap2 not only play roles in the CNS there was a broad interest in analyzing the physiology, morphology and behaviour of Atp6ap2 knockout mice. However, these efforts were hampered by the fact that an embryonic ablation of Atp6ap2 is lethal (Wendling et al.,2017) and that the tissue specific Atp6ap2 knockout mice have a life expectation that is too short for comprehensive studies (Krop et al., 2013). However, since the deficiency of Atp6ap2 in adults severely affects various organ systems, it demonstrates that Atp6ap2 is an essential gene implicated in basic cellular mechanisms and necessary for multiple organ function (Wendling et al., 2017).
Since a neuronal expression of Atp6ap2 has been demonstrated (Schäfer et al., 2013), the neuronal subtype that expresses Atp6ap2 has been analyzed in more detail and neuronal Atp6ap2 expression was not only seen in glutamatergic neurons but also in neurons that use the transmitter gamma-aminobutyric acid (GABAergic neurons) as revealed by its colocalization with CamK2alpha and/or GAD67 (Dubos et al.,2015). Based on this, conditional knockout mice (under the control of the CamK2alpha promotor), in which the knockout of the Atp6ap2 gene was restricted to glutamatergic neurons in the postnatal forebrain were created (Dubos et al., 2015).
Another strategy to investigate the role of Atp6ap2 in the brain is the use of transgenic mouse model in which Atp6ap2 is over-expressed. These mice are a viable and despite a dramatic increase in Atp6ap2 expression (nearly 100-fold in the renal system and even more in the cardiac system), no differences in albuminuria or in the systolic blood pressure between wild-type and Atp6ap2 overexpressing littermates were found (Rosendahl et al., 2014). Moreover, on the histological level, no signs of a cardiac or renalfibrosis in the Atp6ap2 transgenic mice were seen at an age of 12 months postnatally (Rosendahl et al., 2014).
Atp6ap2 and Animal Behavior
Both mouse models, (i) the mice, in which Atp6ap2 was conditionally deleted in the majority of postnatal glutamateric neurons in the forebrain and (ii) the transgenic mice that over-express Atp6ap2 survive into adulthood and can be analyzed on the behavioral level.
In the open-field, male conditional Atp6ap2 knockout mice showed, on one hand, a strong and significant increase in locomotor activity and, on the other hand, the time spent in the centre of the arena was comparable between the genotypes, suggesting normal anxiety-related behavior (Dubos et al., 2015).
In the Morris water maze (MWM), a test for hippocampus-dependant learning and memory, the conditional Atp6ap2 knockout mice, as compared to their controls, were able to learn the task, but the Atp6ap2 mutant mice showed reduced speed and higher latencies to reach the platform position (Dubos et al., 2015). In the probe trail of the MWM(platform was removed from the pool), the conditional ATP6ap2 knockout mice showed nearly the same number of crossings and a comparable preference for the target quadrant, indicating that the conditional Atp6ap2 knockout mice have normal spatial learning abilities (Dubos et al., 2015).
The heterozygous Atp6ap2 transgenic mice (that over-express Atp6ap2) have also been tested in the open field. In contrast to the Atp6ap2 knockout mice, the transgenic Atp6ap2 mice did not show, as compared to their controls, altered motor behavior. Thus, the two groups of mice travelled nearly the same distances within a specific time period in the openfield. Concerning their zone preference, the transgenic mice also did not differ from their controls, also suggesting normal anxiety-related behavior (Bracke et al., 2018).
In the MWM, the transgenic mice were also able to learn tofind the platform. There was no major difference in this learning behavior as compared to the respective control mice. Furthermore, no differences were found in the socalled “probe trail” of the MWM. Thus, in summary, the heterozygous Atp6ap2 transgenic mice did not show any altered performance in the MWM test (Bracke et al., 2018),indicating that in the probe trail heterozygous Atp6ap2 transgenic mice (over-expressing Atp6ap2) seemed not to differ from the conditional ATP6ap2 knockout mice.
Effects of Atp6ap2 on Adult Neurogenesis
Within the hippocampus, Atp6ap2 expressing neurons were not only found in the pyramidal layers of the CA regions(Schäfer et al., 2013; Dubos et al., 2015), but also within the dentate gyrus (DG). Most cells within the DG were positive for the marker of mature neurons NeuN, however, several cells were negative for that marker (Schäfer et al., 2013).These cells were located not only in the layer that is mainly composed of the granule cells of the DG, but also in the subgranular zone (SGZ) of the DG (Schäfer et al., 2013).
This SGZ is somewhat unique in the brain, since it represents a place where a ongoing generation of new neurons has been observed, even postnatally. This adult neurogenesis taking place in the hippocampus includes several steps from a presumably stem cell with radial glia-like characteristics to transiently amplifying progenitor cells that are lineage-determined. These cells divide and can differentiate in early postmitotic neuronal cells. These postmitotic cells get mature by integrating into the pre-existing network (Eriksson et al., 1998; Bruel-Jungerman et al., 2005; Klempin and Kempermann, 2007; Kempermann, 2008). It is thought that adult hippocampal neurogenesis can e.g. be influenced not only directly by behaviour but also by environmental factors(Kempermann et al., 1997; Eriksson et al., 1998; van Praag et al., 1999) and is related to neuronal plasticity and hippocampus-dependant learning and memory (Gould et al., 1999;van Praag et al., 1999; Goncalves et al., 2016). Thus, adult hippocampal neurogenesis is thought to consist of several developmental stages, including proliferation, differentiation, migration, targeting and synaptic integration (Kempermann et al., 2004; Ming and Song, 2011) that ends with the formation of functionally integrated postmitotic neurons in the hippocampal circuitry (see review: Ming and Song,2005). During the process of maturation, the cells express different markers that correlate with the differentiation steps from the early progenitor cell up to the postmitotic neuron(von Bohlen und Halbach, 2007, 2011).
By using specific markers of adult neurogenesis the timecourse and fate of newly born cells within the DG can be followed very precisely. For example, Ki-67, as well as phospho-histone H3 (PH3) represent established markers for proliferative events (regardless of the cell type). Other markers(e.g., nestin, GFAP, Sox2) are specific for early phases of adult hippocampal neurogenesis. In addition, markers are available that allow to distinguish cells in a later stage of neurogenesis(e.g., NeuroD or doublecortin (DCX)) by using immunohistochemistry (von Bohlen und Halbach, 2007, 2011).
Based on the presence of Atp6ap2 on NeuN-negative cells in the DG, and the assumption that Atp6ap2 may function as a putative regulator of developmental processes(Buechling et al., 2010; Cruciat et al., 2010; Hermle et al.,2010; Kanda et al., 2013), it can be assumed that Atp6ap2 is involved in processes related to adult hippocampal neurogenesis. Since different markers for adult neurogenesis are available, co-labeling experiments have been performed that demonstrate that early mitotic cells that are positive for Sox2 are negative for Atp6ap2, whereas young newly formed neurons in the DG that express the marker DCX also express Atp6ap2 (Schäfer et al., 2013), hinting for a possible specific role of Atp6ap2 in adult hippocampal neurogenesis.
Down-regulation of Atp6ap2 in the DG of young adult mice by stereotactic injections of sh-atp6ap2-RNA-expressing retroviruses, resulted in a marked reduction of DCX-labeled newborn neurons in comparison to animals that were injected with non-targeting shRNA-expressing retroviruses that served as control (Schäfer et al., 2015). Moreover, the DCX-positive neurons in the Atp6ap2 knockdown mice display altered morphology, including a reduced dendritic arborization and an altered position within the granule layer of the DG (Schäfer et al., 2015). In this context, it would be interesting to see whether a conditional knockdown of Atp6ap2 also affects adult neurogenesis in a similar fashion.So far, only the effects on adult neurogenesis induced by Atp6ap2 over-expression have been reported (Bracke et al.,2018). In contrast to what might be speculated, Atp6ap2 over-expression neither has a significant effect on the proliferative events taking place during adult hippocampal neurogenesis nor on events in relation to neuronal differentiation.Moreover, mice over-expressing Atp6ap2 did not show any obvious alteration in the morphology or positioning of the newly generated neurons in the DG (Bracke et al., 2018).
Aside from the hippocampus, there is a further region capable of adult neurogenesis, the subventricular zone. The cells that were generated in the SVZ migrate through the rostral migratory stream (RMS) to the olfactory bulb (OB),where they differentiate into interneurons (von Bohlen und Halbach, 2007). Currently, Atp6ap2 expressing cells belonging to this further system that is capable of adult neurogenesis have not been identified. However, it might be possible that the atp6ap2 expressing cells near the ventricular wall(see: allen brainmap: http://mouse.brain-map.org/gene/show/46336) belong to the RMS. However, this has not been investigated yet in detail.
Effects of Atp6ap2 on Cell Signaling and on Neuronal Morphology
In non-neuronal tissue, Atp6ap2 has e.g. been described to interact with the transcription factor PLZF protein and thereby promotes the translocation of PLZF to the nucleus. This in turn activates the transcription of the p85alphasubunit of the phosphatidylinositol-3 kinase (Schefe et al.,2006). Concerning cultured neurons it has been described that activation of Atp6ap2 leads to the phosphorylation of ERK1/2 (Contrepas et al., 2009). Moreover, the Wnt signaling pathway is modulated by Atp6ap2 with a profound effect on adult hippocampal neurogenesis (Schäfer et al., 2015).Interestingly in that context, down-regulation of Atp6ap2,but not over-expression of Atp6ap2, has an effect upon adult hippocampal neurogenesis.
Aside from adult neurogenesis, morphological changes in the shape of mature hippocampal neurons can contribute to altered neuronal signaling, neuronal plasticity and altered hippocampal functions. Dendrites of most principal neurons in the forebrain are covered by dendritic spines. Dendritic spines are known to represent the main sites of excitatory synaptic input for neurons, especially in the hippocampus(Megias et al., 2001). The receptors, especially those for the neurotransmitter glutamate, are enriched at the surface of spines and are concentrated nearby the presynaptic element.In close apposition to the presynaptic active zone, this postsynaptic zone is indicated by the postsynaptic density (PSD),an electron dense, membrane-associated structure (Nimchinsky et al., 2002). Dendritic spines are morphological structure that play important roles in neuronal plasticity, and they contribute to processes related to learning and memory (von Bohlen und Halbach, 2009). Abnormalities in the morphology of individual dendritic spines as well as changes in the number (or density) of dendritic spines are often associated with mental retardation (von Bohlen und Halbach, 2010).Since in some cases X-linked mental retardation can be due to mutations in the Atp6ap2 gene, it was speculated that Atp6ap2 might be involved in the maintenance or shape of dendritic spines or even affects neuronal morphology; however,over-expression of Atp6ap2 neither affected the number of dendritic spine on dendrites of mature pyramidal cells in the hippocampalfield CA1 nor the numbers of dendritic spines on dendrites of mature granule cells located in the (upper leaf of the) DG (Bracke et al., 2018).
Conclusion and Outlook
Atp6ap2 has been discovered in 2002. Since that time Atp6ap2 has been found to be expressed in several tissues, including neuronal tissues. Atp6ap2 can act as a receptor for renin and prorenin, with a higher affinity for prorenin (Nabi et al., 2006), allowing prorenin to cleave angiotensinogen to angiotensin I (Nguyen et al., 2002). Moreover, Atp6ap2 seems to interact with a variety of different other partners, includ-ing frizzled (FZ), partitioning defective 3 homolog (PAR3),phosphatase of regenerating liver-1 (PRL-1) and some others(for a more detailed review on the interaction partners of Atp6ap2, see Peters (2017)). The detailed signalling pathways are not fully understood and it may be possible that Atp6ap2 is tissue-specific involved in different pathways.
Within the postnatal brain, Atp6ap2 is expressed in a variety of specific brain areas ranging from the brain stem up to the cortex. The roles of Atp6ap2 in the brain are not well understood and much effort must be invested in the future to get more insight in Atp6ap2 signalling and its effects on neuronal development, maintenance of the cytoarchitecture of the brain and neuronal plasticity. Since disturbances in Atp6ap2 have been linked to intellectual disability in humans, investigations focusing on the role of Atp6ap2 could be important for the understanding of higher brain functions (Figure 1).
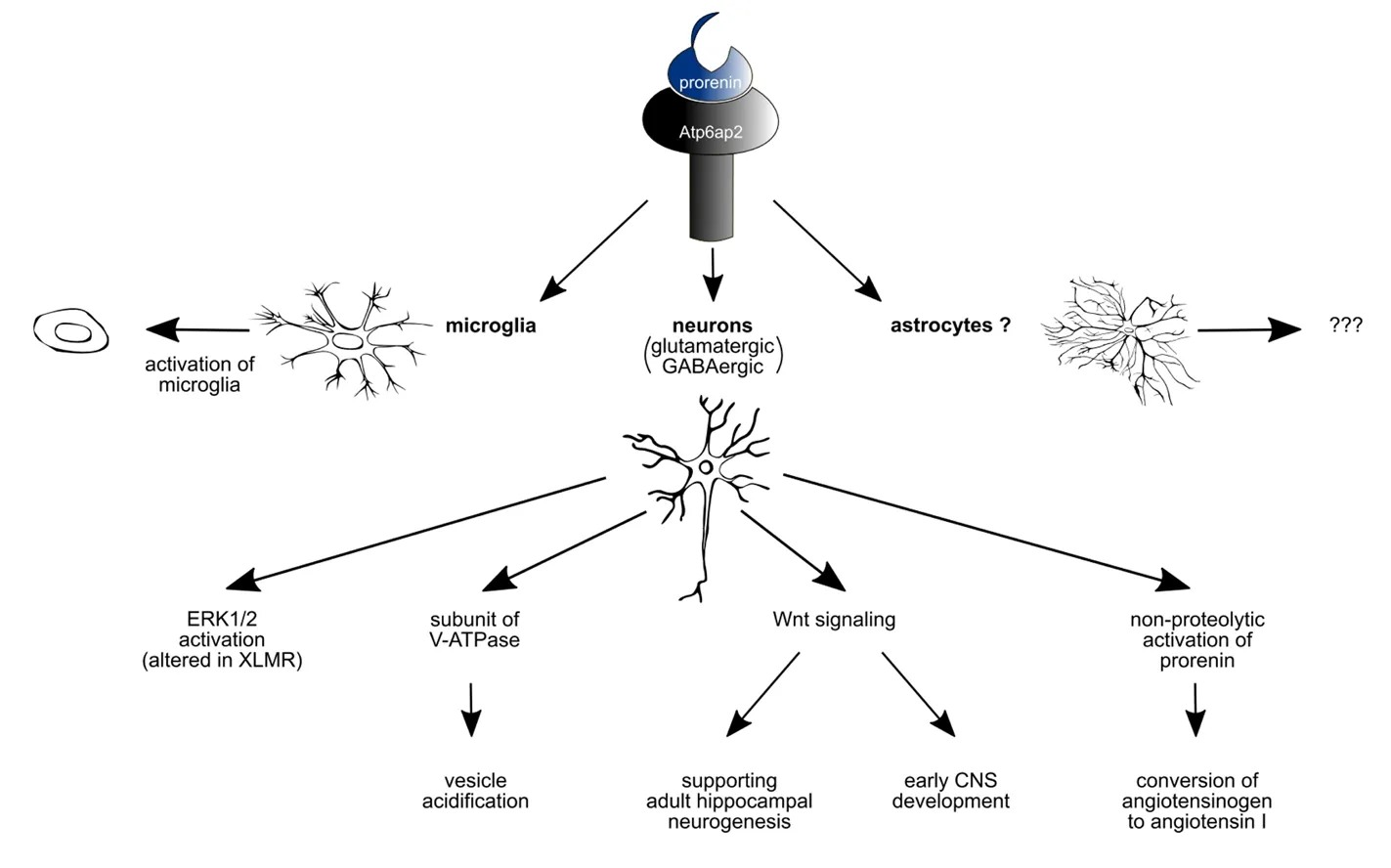
Figure 1 Prorenin mainly acts on microglia and neurons.
Acknowledgments:The authors wish to thank Katharina Bracke (Institute of Anatomy and Cell Biology, Universitätsmedizin Greifswald,Greifswald, Germany) for her help with the illustration.
Author contributions:Manuscript writing: AB and OBH.
Conflicts of interest:We declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Fu-Chou Cheng, Taichung Veterans General Hospital, Taiwan, China.
Additionalfile:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Huangqinflavonoid extraction for spinal cord injury in a rat model
- Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy
- Analysis of transcriptome sequencing of sciatic nerves in Sprague-Dawley rats of different ages
- Exogenous brain-derived neurotrophic factor attenuates cognitive impairment induced by okadaic acid in a rat model of Alzheimer’s disease
- Partial improvement in performance of patients with severe Alzheimer’s disease at an early stage of fornix deep brain stimulation
- Epigenetic marks are modulated by gender and time of the day in the hippocampi of adolescent rats:a preliminary study