Investigation on the Thermal Stability of Deep Eutectic Solvents
2018-09-18CHENWenjunXUEZhiminWANGJinfangJIANGJingyunZHAOXinhuiMUTiancheng
CHEN Wenjun , XUE Zhimin , WANG Jinfang , JIANG Jingyun , ZHAO Xinhui , MU Tiancheng ,*
epartment of Chemistry, Renmin University of China, Beijing 100872, P. R. China.
ollege of Materials Science and Technology, Beijing Forestry University, Beijing 100083, P. R. China.
Abstract: In recent years, deep eutectic solvents (DESs) have attracted considerable attention. They have been applied in many fields such as dissolution and separation, electrochemistry, materials preparation, reaction,and catalysis. The DESs are generally formed by the hydrogen bonding interactions between hydrogen-bond donors (HBDs) and acceptors (HBAs).Knowledge of the thermal stability of DESs is very important for their application at high temperatures. However, there have been relatively few studies on the thermal stability of DESs. Herein, a systematic investigation on the thermal stability of 40 DESs was carried out using thermal gravimetric analysis (TGA),and the onset decomposition temperatures (Tonset) of these solvents were obtained. The most important conclusion drawn from this work is that the thermal behavior of DESs is quite different from that of ionic liquids. The anions or cations of ionic liquids decompose first, followed by the decomposition of the opposite ion at elevated temperatures. On the other hand, the DESs generally first decompose to HBDs and HBAs at high temperatures through the weakening of the hydrogen bond interactions. Subsequently, the HBDs with relatively low boiling points or poor stabilities undergo volatilization or decomposition; the HBAs also undergo volatilization or decomposition but at a higher temperature. For example, the most commonly used HBA choline chloride (ChCl) begins to decompose at around 250 °C. The hydrogen bond plays an important role in the thermal stability of DESs. It hinders the“escape” of molecules and requires greater energy to break than pure HBAs and HBDs, which causes the Tonset of DESs to shift to higher temperatures. Note that the thermal stability of HBDs has a crucial effect on the Tonset of DESs. The HBDs would decompose or volatilize first during TGA because of their relatively poor thermal stability or lower boiling points. The more stable the HBDs are, the greater would be the Tonset values of the corresponding DESs. Further, the effects of anions on HBAs, molar ratio of HBAs to HBDs, and heating rate in fast scan TGA have been discussed. As the heating rate increased, the TGA curves of DESs shifted to higher temperatures gradually, and the temperature hysteretic effect became prominent when the rate reached 10 °C·min−1. From an industrial application point of view, there is an overestimation of the onset decomposition temperatures of DESs by Tonset, so the long-term stability of DESs was investigated at the end of the study. This study could help understand the thermal behavior of DESs (progressive decomposition) and provide guidance for designing DESs with appropriate thermal stability for practical applications.
Key Words: Deep eutectic solvents; Choline chloride; TGA; Thermal stability; Hydrogen bond
1 Introduction
Ionic liquids have drawn much attention in the last few decades1–3. However, ionic liquids have some drawbacks,which hinder their industrial applications. For example, the synthesis of ionic liquids is generally not so easy and not so benign because a lot of organic solvents are required. Besides that, it is difficult to purify the ionic liquids, and the residue ions (such as halogen ions) and water are not easy to be removed4,5. Deep eutectic solvents (DESs), also known as ionic liquid analogues, are a type of solvents composed of a mixture that form a eutectic with a much lower melting point than either of the individual components6. They are usually obtained by the complexion of a quaternary ammonium salt with a metal salt or hydrogen bond donor (HBD)7,8. Many DESs are environment benign, designable, non-volatile, high thermal stable and could be easily synthesized by simple operation conditions9. They could greatly avoid the drawbacks of ionic liquids. Therefore, they have been successfully applied in many fields10–15, such as dissolution and separation,electrochemistry (metal electrodeposition and electropolishing),materials preparation, reaction and catalysis. For industrial applications, DESs must endure certain high temperature for a period of time. Therefore, it is a prerequisite to know the thermal stability of DESs. The stability of ionic liquids has been thoroughly investigated before. For example, the quantitative investigation on the vaporization and decomposition of ionic liquid [EMIM][Tf2N] by thermogravimetric analysis-mass spectrometry (TG-MS) was carried out in our group16. Then the thermal stability and decomposition mechanism of ionic liquids were investigated by thermal gravimetric analysis (TGA) and TG-MS, and kinetic parameters of k, Eaand T0.01/10hthat refers to the temperature at which 1% mass loss occurs in 10 h, were obtained17–19. Also,ionicity of protic ionic liquids and electrochemical stability of ionic liquids and DESs were studied20,21. A comprehensive review on the chemical stability of ionic liquids have been published22. However, few works on the thermal stability of DESs have been published23. The works on the thermal stability of DESs are scare and are carried out at different conditions. Therefore, the Tonsetvalues obtained from different sources are incomparable, and the effects of molecular structure on the thermal stability of DESs are not clear. Thus, in this study, a systematic investigation on the thermal stability of DESs was carried out. The Tonsetand Tpeakvalues of DESs with different molecular structures were obtained by TGA. Thermal analysis shows that DESs have quite different thermal degradation behavior comparing to ionic liquids and they present gradual decomposition. In general, this investigation is useful for understanding the thermal behaviors of DESs, and it could provide guidance for high temperature applications of DESs.
2 Experimental section
2.1 Apparatus and chemicals
TGA and differential scanning calorimetry (DSC) analysis were carried out at thermogravimetric analyzer Q500 (TA Instrument company, USA), and differential scanning calorimeter DSC 8000 (PerkinElmer, USA), separately.
Choline chloride (98%) was purchased from J&K Scientific Ltd. Pentaerythritol (98%) was purchased from Shanghai Macklin Biochemical Co., Ltd. Choline bromide (98%),choline iodide (98%), N,N'-dimethyl thiourea (97%), and trimethyl thiourea (98%) were purchased from TCI (Shanghai)Development Co., Ltd. Urea (99%, Sinopharm, China),N-methylurea (97%), thiourea (99%), N-methyl thiourea (98%),acetamide (98.5%), N-methylacetamide (99.9%), succinimide(98.5%), ethylene glycol (98%, Sinopharm, China), glycerol(99%, Sinopharm, China), D(+)-glucose (98%), D-fructose(98%), xylitol (98%), 1,6-hexanediol (97%), oxalic acid (98%),malonic acid (98%), succinic acid (99.5%), glutaric acid (99%),adipic acid (99%), formic acid (98%), acetic acid (99.5%,Sinopharm, China), acrylic acid (98%), 2-furoic acid (98%),salicylic acid (99.5%), 2,2-bis(hydroxymethyl)propionic acid(98%), citric acid (99.5%, Sinopharm, China), and maleic acid(99%) were purchased from Sinopharm Chemical Reagent Co.,Ltd. All chemicals were used as received without further purification.
2.2 Experimental method
2.2.1 Preparation of DESs
All DESs were synthesized according to the literature24–30. The1H and13C NMR analysis (Bruker AM400 MHz spectrometer) indicated that no detectable impurities and degradants existed in the new synthesized DESs. All the DESs were used without further purification. The structure and name of the components of DESs are listed in Fig. 1.
2.2.2 Characterization of thermal stability of DESs
Thermogravimetric Analyzer Q500 (TA Instrument company, America) was applied to investigate the thermal stability of DESs in nitrogen atmosphere. The short-term stability of DESs (about 15–20 mg with the mass precision±0.1 μg) were measured at ramp mode (ramp 5 °C·min−1to 350 °C, N2flow 40 mL·min−1, platinum pan). The long-term stability were conducted at isothermal mode (heating 10 °C·min−1, from room temperature to specified isothermal temperature and then isothermal for 1 h, N2flow 40 mL·min−1, platinum pan) at different isothermal temperature range for different type of DESs. The decomposition of each DESs sample was performed for three times with the deviations of Tonsetbelow 1.5%.
3 Results and discussion
3.1 The thermal degradation of DESs
Unlike ionic liquids, DESs undergo progressive decomposition with the temperature increasing. As shown in Fig. 2, the short-term stability of choline chloride (ChCl)-urea was measured at ramp mode. The decomposition is divided into two steps. At around 150 °C, urea began to decompose while ChCl began to degrade at about 250 °C. As we all know, hydrogen bond is a type of weak intermolecular force, and it could be broken when temperature increases to some point. After that,HBDs with relatively poor thermal stability would decompose firstly at their characteristic decomposition temperature,followed by ChCl as the remaining component decompose at around 250 °C to 300 °C. We could not get the k and Eaof DESs through the isothermal method like the ionic liquids, so the Tonsetis used to evaluate the short-term stability of DESs.Tonsetand Tpeakcould be obtained from fast scan TGA. Tonsetis the intersection of the baseline weight and the tangent of the weight dependence on the temperature curves as max decomposition rate occurs17. Tpeakis the temperature at which the sample has max degradation rate, and it could be obtained from the peak in differential thermal gravimetric analysis(DTG) curves. The results are given in Table 1. It should be pointed out that if there are one more peaks in DTG curves, we only select the temperature corresponding to the first peak.

Fig. 1 Structure and name of HBDs and ChCl.
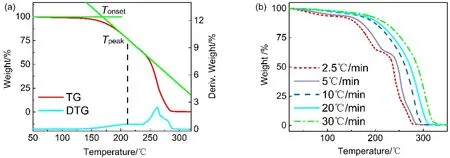
Fig. 2 Fast scan TGA curve for (a) ChCl-urea and (b) ChCl-urea under different heating rate.

Table 1 Decomposition temperature of DESs and HBDs derived from TGA.
As Table 1 shows, the formation of DESs would improve the thermal stability (vaporation or decompostion) of HBDs. The ΔTonsetvalues between most DESs and HBDs are positive,which means the enhanced thermo-stability of HBDs.However, there are some DESs with negative ΔTonsetvalues especially for malonic acid, which may relate to relatively poor ability of HBDs to form hydrogen bond with HBAs. The range of ΔTonsetvalues is from −18.2 °C to 129.3 °C. It is important to highlight that oxalic acid has great ability to form strong hydrogen bond network with HBAs, and the ΔTonsetvalues are 124.4, 127.0 and 129.3 °C for ChCl, choline bromide (ChBr)and choline iodide (ChI), separately.
3.2 The effect of heating rate on the Tonset
If the heating rate is too fast, the temperature hysteretic effect would be serious. As can be seen from Fig. 2, the effects of 2.5, 5, 10, 20 and 30 °C·min−1on the Tonsetof ChCl-urea were analyzed. As the temperature rise rate increasing, the TGA curve moved to high temperature, which means overestimating the thermal stability of ChCl-urea. What is surprising is that ChCl-urea underwent two-step decomposition at a low heating rate (2.5 °C·min−1and 5 °C·min−1). However, the two steps overlapped when the rate reached 10 °C·min−1, and this difference may arise from the temperature hysteretic effect. The heating rate is so high that the inner temperature of sample could not catch up with the temperature around furnace, which leads to the hysteretic effect. Such behavior could account for errors in the measurement of Tonsetand Tpeak. Finally, we choose 5 °C·min−1as the heating rate to investigate the thermal behaviors of DESs.
3.3 The effect of hydrogen bonds on the thermal stability of DESs
There are strong hydrogen-bond networks in DESs, which may affect the thermal decomposition behaviors of them31.During the experiment, the hydrogen bonds hinder the “escape”of molecules, which makes the decomposition more difficult.So, DESs need more energy to break the bonds and then the maximum degradation temperature shifts to higher direction.As we know, the melting points of DESs are significantly lower than each of components26. The stronger the hydrogen bonds are, the lower the melting point would be7. So the melting point differences (Δmp) between DESs and the corresponding HBDs are used to characterize the hydrogen-bond intensity,while the ΔTonsetvalues are used to characterize the thermal stability of DESs32–35. That is to say, there is correlation between the intermolecular force (hydrogen bonds) and the thermal stability of DESs. Through this method, we have successfully investigated the relationship between the thermal stability of DESs and the hydrogen bonds in system.
Fig. 3 shows that ΔTonsetand Δmp of the selected 20 DESs have an approximately linear relationship. It proves again that there is a positive correlation between the thermal stability and hydrogen bonds. From industrial application point of view, we could prepare DESs with great stability through selecting appropriate HBDs to form strong hydrogen-bond networks with suitable HBAs.
3.3.1 The effect of anions in hydrogen-bond acceptors(HBAs)
The anions of HBAs influence the thermal stability of DESs.The ChCl, ChBr and ChI were selected to analyze the effect of Cl−, Br−and I−ions on the thermal stability of DESs. Overall,the difference of ΔTonsetvalue for these anions is small (Fig. 3),and ChCl has a relatively great ability to form hydrogen-bond networks with different HBDs. The atomic radii of Cl−, Br−and I−increase with the increasing of atomic number, I−> Br−>Cl−. Meanwhile the electronegativity of Cl is greater than Br and I, which may affect the formation of hydrogen bonding.The Cl−ion tends to form relatively strong hydrogen bonds with HBDs and has advantage to form eutectic solvents comparing to Br−and I−. Since ChCl is nontoxic and biocompatible, it could be widely used for preparation of DESs.
3.3.2 The effect of HBDs
Obviously, HBDs play a vital role in the thermal stability of DESs, which mainly depends on the weak intermolecular interaction. The hydrogen-bond donor, forms a eutectic solvent with ChCl through hydrogen bonds. On the one hand, HBDs would decompose or volatilize firstly during the thermogravimetric analysis, because of their relatively poor thermal stability or lower boiling points. For example, for ChCl-glycerol, glycerol begins to decompose or evaporate at 175.5 °C, while the ChCl starts to degrade at about 250 °C.According to this, more stable DESs could be prepared bymeans of selecting more stable HBDs. For instance,ChCl-acetic acid volatilizes rapidly even at room temperature.Meanwhile, the Tonsetof ChCl-adipic acid is around 232.3 °C,which owes to the high heat endurance of adipic acid. On the other hand, the abilities of HBDs to form hydrogen bonds with HBAs are crucial for the Tonsetvalues of DESs. The stronger the hydrogen bonds are, the greater the ΔTonsetvalues are. It must also be mentioned that oxalic acid has a strong ability to form hydrogen bonds with ChCl. The ΔTonsetvalue of ChCl-oxalic acid is 124.4 °C, which is higher than other DESs with the HBAs of ChCl. Therefore, we could prepare DESs with appropriate thermal stability. It is important to highlight that the ΔTonsetvalues of three DESs with malonic acid as HBD are negative, which means that the formation of DESs reduces the Tonsetof malonic acid unexpectedly. Note that there are abundant intra-molecule hydrogen bonds in malonic acid (Fig.3) that need more energy to break and then improve the thermal stability. On the contrary, the addition of ChCl lowers the chance to form intra-molecule hydrogen bonds, which results the Tonsetvalue decrease.

continued Table 1
3.3.3 The effect of molar ratio
Besides HBAs and HBDs, the molar ratio of HBAs and HBDs also influence the thermal stability of DESs. Fig. 3 shows the TG curves for ChCl-urea with different molar ratio(2 : 1, 1 : 1, 1 : 2, 1 : 4 and 1 : 8). As we all know, ChCl and urea would form a eutectic solvent with the molar ratio of 1 : 2,and the thermal decomposition curve could divide into two steps. From the thermal stability point of view, ChCl is more heat stable than urea, so the Tonsetof ChCl-urea would decrease with the ratio of urea increasing. As for 2 : 1 and 1 : 1, there is a lack of urea, which results that the ChCl could not form enough and appropriate hydrogen bonds. So it seems that the system is a mixture of impurity (redundant ChCl) and “DES”, which means that it is not a strict DES system, although some hydrogen bonds are formed and the approximate eutectic mixture is prepared. As for 1 : 4 and 1 : 8, the urea is excess for the formation of DES with ChCl, and the system seems like a mixture of DES and urea. Urea with relatively poor thermal stability would influence the thermal behavior of system. Last but not least, fast scan TGA could apply a route to quickly analyze the component of DES sample.
3.4 The long-term thermal stability of DESs
The thermal stability of DESs is one of important parameter that limits the maximum operation temperature. The onset temperature is often used to define the thermal stability of ionic liquids36,37. However, it is well known that the onset temperature may lead to an overestimation of the upper operation limit38. The temperature of TG analysis increases rapidly at a fixed rate, which makes the actual critical temperature passed through rather quickly without a measurable mass loss. In a previous study, we had found that ionic liquids would degrade at temperature significantly lower than the onset temperature within a long period. Other reports also draw this conclusion39,40. Similarly, it is necessary to investigate the long-term thermal stability of DESs, especially for industrial applications, in which DESs must endure certain high temperature for a period of time. In this work, isothermal TG measurement at different temperature intervals were used to determine the long-term stability of DESs. The selection of temperature intervals of DESs is based on the Tonsetvalues. The Tonsetof ChCl-ethylene glycol is 90.3 °C, while it lost 15%weight after keeping it for 50 min at 70 °C, and the weight loss would be greater for a longer time. This further proves that there is an overestimation on the thermal stability by Tonset. Thisconclusion also could be drawn from the investigation of ChCl-urea and ChCl-glycerol. Apparently, it is necessary to investigate the long-term stability of DESs for their practical application.
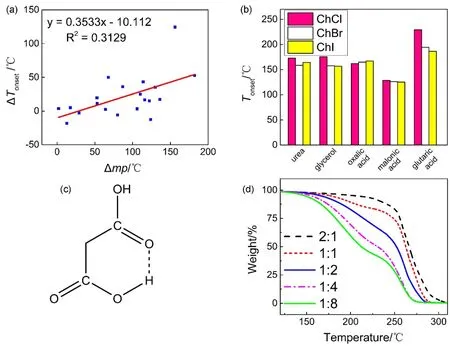
Fig. 3 (a) The overall correlation between ΔTonset and Δmp of 20 DESs; (b) The Tonset values of different DESs with three anions; (c) the structure of intra-molecule hydrogen bond in malonic acid; (d) the TG curves for ChCl-urea with different molar ratio (2 ∶ 1, 1 ∶ 1, 1 ∶ 2, 1 ∶ 4 and 1 ∶ 8).
4 Conclusions
The investigation on the thermal stability of DESs shows that thermal behavior of DESs significantly differs from that of ionic liquids. DESs undergo progressive decomposition during the fast scan TGA. The hydrogen bond plays an important role on the thermostability of DESs. It hinders the “escape” of molecules, which influences the thermal decomposition of DESs. The effects of HBAs, HBDs and molar ratio of HBAs to HBDs on the thermal stability of DESs were analyzed and some rules were obtained. By selecting appropriate HBDs and HBAs, especially the HBDs, DESs with appropriate thermal stability could be prepared. Finally, the long-term stability of DESs was investigated at isothermal mode, and the results further prove that there is an overestimation on the thermal stability of DESs by Tonset. This study would help people to understand the thermal behavior of DESs and apply guidance for preparing DESs with appropriate thermal stability.
杂志排行
物理化学学报的其它文章
- Catalytic Electroreduction of CO2 to C2H4 Using Cu2O Supported on 1-Octyl-3-methylimidazole Functionalized Graphite Sheets
- Green and Cost-Effective Preparation of Small-Sized ZSM-5
- Physicochemical Properties of1-Methoxyethyl-3-Methylimidazolium Glycine
- Study on Solution Enthalpies of Ionic Liquids [Cnmim][H2PO4](n = 3, 4, 5, 6) by Using Pitzer’s Equation
- Influence of External Electric Field on Vibrational Spectrum ofImidazolium-Based Ionic Liquids Probed by Molecular Dynamics Simulation
- Self-Assembly Behavior of Amphiphilic Diblock Copolymer PS-b-P4VP in CO2-Expanded Liquids
