Catalytic Electroreduction of CO2 to C2H4 Using Cu2O Supported on 1-Octyl-3-methylimidazole Functionalized Graphite Sheets
2018-09-18NINGHuiWANGWenhangMAOQinhuZHENGShiruiYANGZhongxueZHAOQingshanWUMingbo
NING Hui , WANG Wenhang , MAO Qinhu , ZHENG Shirui , YANG Zhongxue ,ZHAO Qingshan , WU Mingbo ,*
tate Key Laboratory of Heavy Oil Processing, College of Chemical Engineering, China University of Petroleum, Qingdao 266580,Shandong Province, P. R. China.
AS Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001,P. R. China.
Abstract: The electrocatalytic reduction of CO2 to C2H4 is a topic of great interest. It is known that the preparation of efficient catalysts for this transformation is the key factor that determines the yield of C2H4. In this study,we prepared 1-octyl-3-methylimidazole functionalized graphite sheets (ILGS) in a facile manner by the electro-exfoliation of pure graphite rod in an aqueous solution of 1-octyl-3-methylimidazolium chloride (OmimCl : H2O = 1 : 5, V/V) at 10 V. They were then dispersed in an aqueous solution of copper chloride and sodium citrate. Subsequent reduction with sodium borohydride led to the formation of a composite comprised of cuprous oxide supported on Omimfunctionalized graphite sheets (Cu2O/ILGS). This composite was found to be an efficient catalyst for the electroreduction of carbon dioxide to ethylene. The as-made materials were characterized by transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and X-ray diffraction (XRD). The TEM images showed that the ILGS were composed of multiple layers of graphene. The XRD pattern and Raman spectrum indicated that the surface of the ILGS possessed several defects. In the electro-exfoliation process,the defects in the ILGS were modified in situ by covalent bonding with Omim groups, which was also confirmed by XPS.The Cu2O nanoparticles with an average diameter of 5 nm were uniformly distributed on the surface of the ILGS because the Omim groups grafted to the graphite sheets acted as anchors and prevented their aggregation by the steric effect. The electrocatalytic activities of Cu2O/ILGS for CO2 reduction were measured at different voltages in 0.1 mol L–1 KHCO3 aqueous solution under ambient temperature and pressure. These experiments showed that the catalytic performance of the Cu2O/ILGS composite was determined by cuprous oxide, while the ILGS displayed nearly no catalytic activity in the electroreduction of carbon dioxide. The faradaic efficiency of hydrogen and carbon dioxide reduction products changed with the reaction time because of the reduction of Cu2O to Cu under the electroreduction conditions. The faradaic efficiency of ethylene was ~14.8% at –1.3 V (versus reversible hydrogen electrode). The performance of Cu2O/ILGS in the catalytic electroreduction of carbon dioxide was attributed to the stabilization of the Cu2O nanoparticles by the nest-like microstructures in the Cu2O/ILGS composite.
Key Words: Cu2O; Graphite sheets; CO2 reduction; Ethylene; Ionic liquids
1 Introduction
The electrochemical reduction of CO2to value-added chemicals is one of the best ways to recycle the CO2as C1 compounds resource and store electrical energy in chemical bonds1–3. Ethylene is a widely used chemical raw material in the world. The electrochemical reduction of CO2 to ethylene under atmospheric conditions is a clean and promising route to replace the traditional ethylene production methods. Several new materials have been identified as electrocatalysts for the CO2reduction, with copper exhibiting the highest energetic efficiency and selectivity toward the formation of hydrocarbons,especially for producing ethylene.
In the recent years, numbers of strategies have been studied to increase the performance of Cu catalysts, including crystal surface4,5and morphology control6–8, alloying9, surface modification10, composite construction11and oxidation treatment12. Among them, Cu2O has gotten much attention due to its unique catalytic properties in CO2 electroreduction. Above all, Cu2O is considered as a promising precursor of cube metallic copper13. When used directly as a catalyst, the Cu2O is firstly reduction to metallic Cu during the electrolysis while the CO2is reduced to C2H4selectively on the Cu(100) surface14. The morphology of Cu can be controlled by the morphology of Cu2O.Yeo et al.15prepared Cu2O films of different thicknesses by galvanostatic deposition method on Cu disks and investigated the electrochemical reduction of CO2on Cu2O films in aqueous 0.1 mol·L−1KHCO3electrolytes. Through ex situ scanning electron microscopy, X-ray diffraction and in situ Raman spectroscopy, it is revealed that Cu2O reduced rapidly and remained as metallic Cu0particles during the CO2reduction. In addition, they found the thin Cu films derived from Cu2O have better selectivity than the thick ones, which indicates the thickness of Cu2O precursor is closely related to the morphology of Cu12. In fact, it cannot be so sure that all the Cu2O are reduced to Cu0during the electrolysis, some reports argue that there is remain some Cu(I) species on the surface of Cu. These Cu(I)species promote the selectivity of C2H4and the bi-phase of Cu2O-Cu is considered to be the active site for CO2electroreduction to ethylene16. The oxygen-vacant structures derived from the decrease in the oxygen concentration of the Cu2O electrode during electrolysis has been confirmed by using bulk and surface-sensitive analytical techniques and the in situ formed structures play a critical role in maintaining the high reactivity of the electrode for C2H4production17. Since most of the reports studied the catalytic performance of Cu2O using metallic Cu as substrates, the catalytic effect of Cu and Cu2O-Cu interface cannot be excluded. As a result, there is an ongoing debate whether the oxide layer contributes to catalysis, or whether it only acts as a promoter of the well-structured metal catalysts.
Ionic liquids (ILs) have aroused great interest in the past decades due to their unique properties, such as negligible vapor pressure, wide potential window and high conductivity.Recently, Luo et al.18reported that the natural graphite was electrochemical exfoliated in ionic liquid/water solution to prepare ionic-liquid-functionalized graphene sheets, which can be further gratified to obtain nanocomposites with high structural homogeneity and excellent electrical conductivity.Herein, we propose a facial strategy to prepare graphite sheets by electro-exfoliation of pure graphite rod assisted by an ionic liquid, with further supporting the Cu2O nanoparticles (Cu2O NPs) on the graphite sheets. Because the graphite sheets as prepared are nearly inert for CO2electrocatalytic reduction theoretically, it offers a new strategy to study the catalytic performance of Cu2O independently for CO2electroreduction.
2 Experimental
2.1 Materials
1-Octyl-3-methylimidazolium chloride (OmimCl, purity >99.0%) was purchased from the Centre of Green Chemistry and Catalysis, LICP, CAS, P. R. China. Copper chloride dihydrate(purity ≥ 99.0%), trisodium citrate dehydrate (purity ≥ 99.0%),sodium borohydride (purity ≥ 98.0%), potassium hydrogen carbonate (purity ≥ 99.5%), and ethanol anhydrous (purity ≥99.7%) were provided by Sinopharm Chemical Reagent Co.,Ltd., P. R. China. Nafion N-117 membranes (0.180 mm thick, ≥0.90 meq·g−1exchange capacity) were purchased from Alfa Aesar China Co., Ltd. High purity graphite rod (ø: 3 mm) was purchased from Beijing Crystal Dragon Carbon Technology Co.,Ltd., P. R. China. All the reagents were used as received. The water used in all experiments was purified by a Millipore system.
2.2 Preparation of 1-octyl-3-methylimidazole functionalized graphite sheets (ILGS)
The details process of ILGS preparation was described as follows: Two high-purity graphite rods were placed parallel as electrodes in the OmimCl/water (1 : 5, volume ratio) solution with a separation of 2.0 cm. A MS-605D model potentiostat(Shenzhen MST Technology Co., Ltd, P. R. China) was used to provide the potential. Static potentials of 10 V were applied to the two electrodes. After electrolysis for 6 h, a dark red solution appeared. The resulting powder was isolated from the solution by centrifugation, washed with deionized water and ethanol thoroughly, and dried under vacuum at 60 °C for 6 h. Finally, the ILGS was obtained.
As a control experiment, the electrolyte was replaced by 0.1 mol·L−1K2SO4aqueous solution and the un-functionalized graphite sheets (GS) were obtained using the same procedure with ILGS.
2.3 Preparation of Cu2O/1-octyl-3-methylimidazole functionalized graphite sheets(Cu2O//ILGS)
The details process of Cu2O/ILGS preparation were described as follows: 0.0978 g copper chloride dihydrate and 0.0429 g sodium citrate were dissolved in 50 mL deionized water and stirred for 30 min under Ar atmosphere to get a sky-blue solution. 0.06 g ILGS were added to 10 mL deionized water and sonicated for 30 min, an ILGS dispersion solution was obtained.The above two solutions were mixed together and stirred for 30 min under Ar atmosphere. Then, 15 mg sodium borohydride dissolved in water was dropwise added to the mixture under stirring for 20 min vigorously and a dark yellow solution was formed. Finally, the powder product was filtered, following by washed with water and ethanol thoroughly, and dried under vacuum at 60 °C for 12 h. Finally, the Cu2O/ILGS was obtained.
As a control group, Cu2O-loaded graphite sheets (Cu2O/GS)was obtained with the same procedure, except that the ILGS were replaced by GS.
2.4 Materials characterization
The morphology of materials as made was characterized by transmission electron microscopy (TEM, JEM-2010, 220 kV,Japan). X-ray diffraction (XRD, X'Pert PRO MPD, Netherlands)operated at 40 kV and 40 mA (Cu Kαradiation, λ = 0.15406 nm)was used to observe the crystal structure of cuprous oxide and graphite. Raman spectroscopy (Renishaw RM2000, UK) were used to examine the graphitization degree of the obtained samples. The elemental composition and chemical bonding were studied by X-ray photoelectron spectroscopy (XPS, Thermo Scientific Escalab 250XI, USA) with Mg Kα radiation. Typically,the hydrocarbon C 1s line at 284.8 eV from adventitious carbon was used for energy referencing.
2.5 CO2 reduction electrolysis and product analysis
The working electrode was prepared as follows: 1 mg Cu2O/ILGS was added to 200 µL of ethanol anhydrous. With further adding 5 µL of 5% Nafion solution and sonicated for 20 min to obtain a uniformly dispersed suspension, which was dropped on a L-type glassy carbon electrode (ø: 10 mm) and dried with N2.
The apparatus and procedures were similar with those reported previously for electrochemical reduction of CO219–22.Briefly, the reduction electrolysis was performed under room temperature (25 °C) in an H-type cell with an Ag/AgCl reference electrode (saturated KCl) and a Pt plate (1 cm × 1 cm) counter electrode. 0.1 mol·L−1KHCO3aqueous solution was used as electrolytes and the cathode and anode compartments were separated by Nafion 117 proton exchange membrane.
Before CO2eletrocatalysis, the working electrode (cathode)side was bubbled with Ar for 30 min to discharge the air in the electrolyte. Then CO2was bubbled for another 30 min at a flow rate of 20 mL·min−1to saturate the electrolyte.
An electrochemical workstation (CHI 760, Shanghai CH Instruments Co., P. R. China) was used for the potentiostat CO2reduction eletrocatalysis experiments. All the measured potentials in this work were cited with respect to the reversible hydrogen electrode (RHE) using the following conversion: ERHE(V) = EAg/AgCl(V) + 0.197 V + (0.059 V × pH). The pH value of the electrolyte is 6.8 in CO2atmosphere. The CO2reduction products were detected on a gas chromatograph (North Rayleigh 7890A, P. R. China) with a methanator using flame ionization detector (FID), while the H2was tested on the gas chromatography (Shimadzu GC-2014, Japan) using thermal conductivity detector (TCD). The carrier gases of the FID and TCD are nitrogen and argon respectively. The liquid product was tested by UV method23. The faradaic efficiency (FE) of all the products was calculated according to the methods established by Yeo et al.15.

Fig. 1 XRD patterns of (a) ILGS and GS; (b) Cu2O/ILGS and Cu2O/GS.color online.
3 Results and discussion
3.1 Materials characterization
The crystal structure of the materials as made was characterized by XRD, as shown in Fig. 1.
The XRD patterns of GS (Fig.1a, black line) and ILGS(Fig.1a, red line) both exhibit the (002), (101), and (004)graphite diffraction peaks, indicating the structure of graphite is preserved after the electro-exfoliation process18,24. However, the(002) peak of ILGS is broader than GS, which hints that ILGS have more defects than GS due to the 1-octyl-3-methylimidazole grafted to the graphite sheets in the electro-exfoliation process18.
Raman spectroscopy is one of the key analytical techniques used in the characterization of graphite sheets. Fig. 2 shows typical Raman spectra of natural graphite, GS and ILGS. In the Raman spectrum of natural graphite, the G peak at ~1593 cm−1corresponds to an E2gmode of graphite, which is related to the vibration of sp2-bonded carbon atoms in a 2D hexagonal lattice,such as in a graphite layer. The D peak at ~1353 cm−1is associated with vibrations of carbon atoms with dangling bonds in plane terminations of disordered graphite. The intensity contrast of raw material (Fig. 2, grey line) shows that the intensity of the G peak is ten times that of D peak (mean ID/IGratio = 0.10), indicating an ideal degree of graphite. The ID/IGratio of GS (Fig. 2, black line) is 0.30, indicating a low degree of defects. On the contrary, the ID/IG ratio of ILGS ((Fig. 2, red line)is 0.93, indicating a high degree of defects, which is due to the functionalization of graphite sheets by 1-octyl-3-methylimidazole through the electro-exfoliation process.
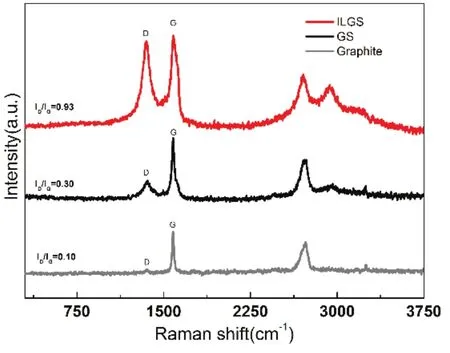
Fig. 2 Raman spectra of ILGS, GS, and graphite raw material.color online.
Except for the characteristic peaks of graphite sheets, the XRD patterns of Cu2O/GS and Cu2O/ILGS both exhibit the(110), (111), (200), (220), and (311) diffraction peaks, as labelled in Fig. 1b, which is consistent with polycrystalline Cu2O (JCPDS 77-0199)15and there is no characteristic peak for impurities. The XRD results indicate that pure phase Cu2O are formed on GS and ILGS.
To further confirm our conclusion, survey XPS spectra of Cu2O/ILGS were recorded, as displayed in Fig. 3. The total XPS spectrum mainly consists of C, N, O and Cu core-elements,indicating successful deposition of N on ILGS. More specially,the high-resolution C 1s spectrum of Cu2O/ILGS (Fig. 3b) can be deconvoluted to four Gaussian peaks located at 284.76,285.60, 286.35, 287.35, and 288.70 eV, corresponding to the C=C, C―C/C―H, C―N, C=O, and O―C=O bonds,respectively. Consistently, the high resolution N 1s spectrum of Cu2O/ILGS, as shown in Fig. 3c, can be deconvoluted predominantly into two peaks at 400.42 and 402.15 eV, which corresponds to di-substituted imidazole nitrogen atoms18. The result indicates 1-octyl-3-methylimidazole is successfully grafted to the defects of graphite sheets during the electroexfoliation of graphite rod in the OmimCl/water solution, as shown in Scheme 1.
In addition, the high-resolution Cu 2p spectrum (Fig. 3d) for the composite heterostructure shows binding energies of 932.5 and 934.5 eV, corresponding to the Cu 2p3/2spin-orbit peaks of Cu+and Cu2+, separately. Despite few Cu+ions having been oxidized into Cu2+, the XRD patterns indicate that the copper in Cu2O/ILGS still remain in the pure Cu2O phase25.
According to the XPS spectra, the contents of elements in all the materials as made are presented in Table 1. Predictably, the main element in GS and ILGS is carbon, while the oxygen comes from the oxidation of graphite in the electro-exfoliation of graphite rod. There is no N in GS while the N content in ILGS is 3.76%, indicating the N completely comes from the 1-octyl-3-methylimidazole grated to the graphite sheets. Finally, the Cu content of Cu2O/ILGS and Cu2O/GS is 12.46% and 12.42%,respectively.

Fig. 3 XPS patterns of Cu2O/ILGS.

Scheme 1 Synthesis process of Cu2O/ILGS.
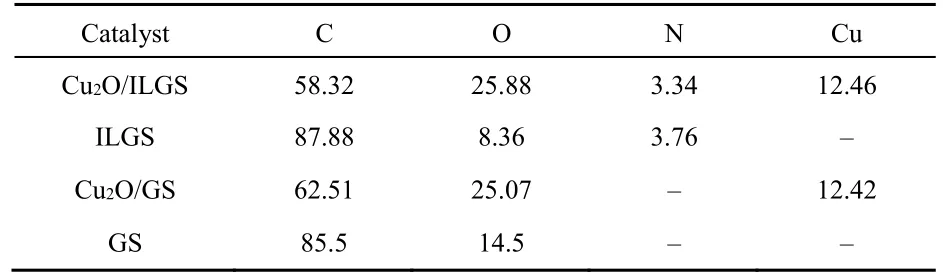
Table 1 Relative contents (atomic fraction, %) of all elements from XPS spectra.
The TEM images (Fig. 4a) depict that GS and ILGS are composed of multilayer graphene sheets. The TEM image of Cu2O/ILGS (Fig. 4c) shows that Cu2O possess uniform size ranging from 4 to 8 nm with an average diameter of 5 nm (inset of Fig. 4c), while in the Cu2O/GS (Fig. 4d), the Cu2O exists obvious aggregation (average diameter of 38 nm), indicating the 1-octyl-3-methylimidazole functionalized graphite sheets can fix the position of Cu2O by offering anchor positions and inhibit the aggregation of Cu2O through steric effect, as depicted in Scheme 1.
3.2 CO2 electrochemical reduction performance
To explore the electrocatalytic performance of Cu2O/ILGS,the potential electrolysis experiments were carried out at different voltages from −1.0 to −1.4 V (vs RHE), the gas in the headspace was collected and analysed by GC, and the liquid mixture was analysed by UV method. It was found that gas product contains CO, CH4 and C2H4, while H2 is the only byproduct. HCOOH is the only product observed in liquid mixtures. The FE of gas products as a function of applied potential using Cu2O/ILGS as electrode was shown in Fig. 5a.As the voltage increases, the FE of H2decreases first and then increases with applied potentials, indicating the hydrogen evolution is a competitive reaction to CO2 reduction26. All the reduction products from CO2 exists a maximum value when rising the applied potentials, while the FE of ethylene (FEethylene)reached the maximum 14.8% at −1.3 V (vs RHE), with 70.9%selectivity in the gas phase products of CO2reduction.

Fig. 4 TEM images for (a) ILGS; (b) GS; (c) Cu2O/ILGS; (d) Cu2O/GS.

Fig. 5 (a) Fractional faradaic efficiency of gas products; (b, c) Faradaic efficiencies and selectivity of gas products at -1.3 V (vs RHE);(d) Faradaic efficiency of gas products and total current density with respect to electrolysis time at -1.3 V (vs RHE).
To further study the catalytic mechanism of Cu2O/ILGS, the controlled potential electrolysis experiments were carried out at−1.3 V (vs RHE) using GS, ILGS and Cu2O/GS as electrodes,respectively.
When GS was used as catalyst, none of carbon-containing compounds was detected. As for ILGS, only trace amounts of CO and CH4were detected but no ethylene. Therefore, Cu2O is believed to be the main active sites for CO2reduction. When using Cu2O/GS as electrode, the FEethylene is 6.4%, corresponding to 17.6% selectivity, indicating the activity and selectivity of ethylene are closely related to the size and aggregation of Cu2O NPs. The 1-octyl-3-methylimidazole grated to graphite may form many special nestle-like structures, which offered anchoring sites for Cu2O NPs. Meanwhile, the steric effect of 1-octyl-3-methylimidazole prevents the aggregation of Cu2O NPs during the deposition of Cu2O. Because of the stabilization effects of ILGS, the Cu2O NPs are much smaller and more uniformly dispersed on ILGS, leading to much better catalytic performance than those on GS.
At last, the long-time electrocatalytic performance of Cu2O/ILGS was studied, as shown in Fig. 5d. During the 6 h electrolysis, the FE of H2increased while the FE of CO, CH4and C2H4decreased gradually. The decline of FEethylenemay arise from the reduction of Cu2O to Cu0during the electrolysis of CO2,which was confirmed by XRD pattern (Supporting Information,Fig. S1). The details of structure changes of Cu2O/ILGS during the CO2 electroreduction will be further investigated in our future work.
4 Conclusions
In summary, we have successfully developed a facile method to achieve Cu2O/ILGS composites, which can be used as efficient electrocatalyst for electrochemical reduction of CO2 to C2H4. Under −1.3 V (vs RHE), the FE of ethylene is up to 14.8%,which is more than two times of Cu2O/GS. The high efficient is due to the 1-octyl-3-methylimidazole grafted to graphite sheets by electro-exfoliation method, which can form anchored position for Cu2O NPs and prevent the aggregation of Cu2O by steric effect. Our work offers a new strategy to prepare imidazole functionalized graphite sheets as new versatile supports to facilitate the dispersion of metal oxides as catalyst in many other applications.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
杂志排行
物理化学学报的其它文章
- BmmimOAc-Catalyzed Direct Condensation of 2-(Arylamino) Alcohols to Synthesize 3-Arylthiazolidine-2-thiones
- Self-Assembly Behavior of Amphiphilic Diblock Copolymer PS-b-P4VP in CO2-Expanded Liquids
- Study on Solution Enthalpies of Ionic Liquids [Cnmim][H2PO4](n = 3, 4, 5, 6) by Using Pitzer’s Equation
- Physicochemical Properties of1-Methoxyethyl-3-Methylimidazolium Glycine
- Green and Cost-Effective Preparation of Small-Sized ZSM-5
- Influence of External Electric Field on Vibrational Spectrum ofImidazolium-Based Ionic Liquids Probed by Molecular Dynamics Simulation
