Self-Assembly Behavior of Amphiphilic Diblock Copolymer PS-b-P4VP in CO2-Expanded Liquids
2018-09-18CHENGXiaomengJIAODongxiaLIANGZhihaoWEIJinjinLIHongpingYANGJunjiao
CHENG Xiaomeng , JIAO Dongxia , LIANG Zhihao , WEI Jinjin , LI Hongping ,*, YANG Junjiao
ollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, P. R. China.
eijing Key Laboratory of Environmentally Harmful Chemical Analysis, Beijing University of Chemical Technology, Beijing 100029, P. R. China.
Abstract: The self-assembly behavior of block copolymers and their assembled micellar morphologies have attracted considerable attention because of their potential applications in biomedicine, drug delivery, and catalysis. Herein we report that CO2-expanded liquids (CXLs) facilitate the morphology control of the self-assembled aggregates (SAAs) of polystyrene-block-poly(4-vinylpyridine) (PS-b-P4VP) formed in CO2-expanded toluene. It is found that the anti-solvent effect of CXLs can successfully regulate the self-assembly behavior of the copolymer PS-b-P4VP. The difference in amphiphilicity between PS and P4VP block is reduced with increasing pressure of CO2-expanded toluene owing to the anti-solvent effect of CO2. In addition, this diminished difference may influence the interfacial tension at the P4VP core-PS corona interface, which triggers a morphological change of the aggregate. The SAA structures are dependent on both CXL pressure and copolymer composition under the experimental conditions implemented in this work. The morphological evolution of the SAAs in CXLs exhibits remarkable pressure dependence. As the pressure increases, the SAA structure of PS168-b-P4VP420 transits from primarily spheres (0.1 MPa)to mostly interconnected rods (6.35 MPa), the SAA of PS790-b-P4VP263 evolves from small vesicles (0.1 MPa) to large compound vesicles (LCVs, 6.35 MPa), whereas the PS153-b-P4VP1530 counterpart switches from large compound micelles(LCMs, 0.1 MPa ) to mainly large compound vesicles (LCVs, 6.35 MPa). Moreover, transmission electron microscopy (TEM)data on constant copolymer composition implies that the packing parameter p of the SAAs increases with the CXLs pressure. Especially, under the experimental conditions employed in this work, we find that the major factor controlling the SAA shape in conventional toluene is the copolymer composition, while in CO2-expanded toluene, the dominant factor controlling the SAA morphology might be the varying contact area between shell-forming segment PS and the CXLs with increasing pressure. This work proves that the CXL method facilitates the modulation of morphology of the SAAs, and opens a green route for the development of new nano-functional materials.
Key Words: CO2-expanded liquids; Colloidal aggregates; Morphology control; PS-b-P4VP; Self-assembly
1 Introduction
The self-assembly of molecules is ubiquitous in our daily life.The desire to understand molecular self-assembly and to explore the potential applications spurs the studies on molecular selfassembly mechanisms, structures and properties of the assembled clusters1. In comparison with those small-molecule clusters, polymer aggregates generally present higher durability and stability owing to their mechanical and physical properties.So the self-assembly of polymer has attracted considerable interest, not only from academic attention but also out of their potential applications in many fields1. The self-assembly of block copolymers has been the focus of the scientific world due to their ability to self-assemble into various nanostructures such as cylindrical, spherical and vesicular micelles2. The morphologies of the self-assembled aggregates (SAAs) in solution could be regulated by three main effects, which are the core stretching, inter-coronal interactions, and the interfacial tension between solvent and the micelle core3. Among these SAA, the structural advantages of vesicles have made them so appealing in catalysis4, drug delivery5, and the controlled incorporation of hydrophobic units into vesicle walls6or hydrophilic species into its cavities7. Many works have been reported on the self-assembly of poly(styrene-b-4-vinylpyridine)(PS-b-P4VP) with an addition of small molecules via hydrogen bonds with the P4VP block2,8–11. Chang and coworkers2found that the morphology of the PS-b-P4VP and octyl gallate (OG)complex can be regulated due to different hydrogen bonding abilities between PS-b-P4VP and OG in different common solvents. Park and coworkers11examined the fluorescence (FL)of Disperse Red 1 (DR1) in different PS-b-P4VP micellar morphology. They illustrated that DR1 in the PS-b-P4VP micellar core in toluene exhibited stronger emission than that in the soft corona due to the high confinement degree of DR1 in the core.
As we know, solvent also plays a vital role to control the formed SAA structures. There are many self-assembly studies carried out in conventional solvent with the focus on varying the polymer concentration and composition12,13, solvent composition14,pH15–17, the type and amount of the adding species (e.g., acids,bases, carbohydrates12or salts)9,18–21. Up to now, few works were reported using CO2-expanded liquids (CXLs) as solvent system to obtain the self-assembled aggregates (SAA). As is known, CXLs exhibited many beneficial properties in materials processing compared with conventional media owing to their tunable solvating power, and numerous other properties that can be regulated in response to pressure changes22,23. CXLs are a type of ideal solvents for many applications while simultaneously reducing the environmental burden through considerable replacement of organic solvents with eco-friendly CO224. It should be emphasized that CXLs processes can be operated in a milder pressure in contrast with the supercritical antisolvent (SAS) processes. Polymer processes in CXLs have been utilized to pressure tune the particle sizes and morphologies of crystalline polymers25–27, and modulate the structure and emission performance of the self-assembled fluorescent composite (SAFC) between PS-b-P4VP and the dye unit24,28.
In this work, the self-assembly behavior of PS-b-P4VP in CO2-expanded toluene was examined. We presume that the solubility change of copolymer with increasing CXLs pressure will certainly affect the core stretching, the inter-coronal interactions, and the interfacial tension between CXLs and the core, along with the SAA morphology transition in CXLs. Thus,the pressure tuning of CXLs might be an effective way to realize the SAA structure control. Under such guidance, the present work will focus on regulating the SAA morphology through the modular tunability offered by the CXLs pressure and copolymer composition, and try to uncover what is the possible SAA morphology tuning mechanism under CXLs condition. This work illustrates that CXLs method facilitates the modulation of the SAA morphology (Fig. 1), which offers a promising way of developing new nano-functional materials. We believe that our studies will not only contribute to the basic knowledge of the role of pressure response of various intermolecular interactions between copolymer and CXLs, but also help reveal the underlying SAA structure tuning mechanisms.
2 Experimental section
2.1 Materials
Dimethyl formamide (DMF, Shanghai Chemical Reagent,99.5%) was dried before use. Tetrahydrofuran (THF, Tianjin Chemical Reagent, 98%) was distilled in the presence of sodium.The monomer 4-vinylpyridine (4VP, Alfa Aesar, 96%) and styrene (St, Tianjin Chemical Reagent, 98%) were distilled under reduced pressure and stored in a refrigerator. 2,2-Azobisisobutyronitrile (AIBN, Shanghai Chemical Reagent,98%) was purified by recrystallization from ethanol. Benzyl dithiobenzoate (BDTB) was prepared according to the procedure described by Chiefari et al.29. CO2with a purity of 99.95% was provided by Henan Keyi Gas Company (Zhengzhou, China).The other reagents were used as received. The synthesis route for PS-b-P4VP is illustrated in Scheme 1.

Fig. 1 Morphology control of the PS-b-P4VP self-assembled aggregate using CO2 expanded liquids (CXLs).Red, blue and gray refer to PS, P4VP and toluene moieties, respectively.Color online.
2.2 General Information
The average molecular weight (Mw and Mn) and polydispersity index (PDI, Mw/Mn) were obtained by Gel Permeation Chromatography (GPC) (Waters 515 HPLC pump, Waters 2414 Refractive index detector, Waters Corporation, USA). The morphologies of the SAA were obtained by transmission electron microscopy (TEM), which was performed by JEOL JEM-2100 microscope with an accelerating voltage of 200 KV(JEOL Ltd., Tokyo, Japan).
2.3 Preparation of PS (or P4VP)
In a typical polymerization procedure, an appropriate amount of styrene (or 4VP), AIBN, BDTB were added into a dry glass tube with a magnetic bar, followed by three freeze-vacuum-thaw cycles. The tube was sealed under vacuum and then immersed in a water bath thermostated at 353.2 K. After the prescribed time,the tube was cooled down to room temperature immediately. The polymer was dissolved with certain amount of THF, and then precipitated by dropping the solution into anhydrous methanol,followed by filtration to obtain the dithiobenzoate-terminated polystyrene [PS-SC(S)Ph] (or the dithiobenzoate-terminated poly(4-vinylpyridine) [P4VP-SC(S)Ph]. Repeat the dissolvingprecipitation procedure three times, afterwards the product was dried in a vacuum oven for 24 h to achieve the PS-SC(S)Ph (or P4VP-SC(S)Ph).
2.4 Preparation of PS-b-P4VP
The synthesis route for PS-b-P4VP by reversible additionfragmentation chain transfer (RAFT) polymerization is summarized in Scheme 1. A prescribed amount of AIBN, PSSC(S)Ph (or P4VP-SC(S)Ph, as chains transfer agent, CTA),4VP (or St) were successively added into a dry glass tube with a magnetic bar. After three freeze-vacuum-thaw cycles, the tube was sealed under vacuum and then the sealed tube was immersed in a water bath at 353.2 K. After the prescribed time, the tube was rapidly cooled down to room temperature. The copolymer was dissolved with certain amount of DMF, and then the copolymer was precipitated by adding the polymer solution into a mixture of petroleum ether and absolute diethyl ether (1 : 1,volume ratio). After filtration, the precipitation was extracted with toluene and hydrochloric acidic water (pH = 2) successively for 24 h in the Soxhlet extractor to remove the unreacted homopolymers. After being dried in a vacuum oven for 24 h, the block copolymer polystyrene-block-poly (4-vinylpyridine) (PS-b-P4VP) was obtained. The number-average molecular weight(Mn) and polydispersity index (Mw/Mn) of PS and PS-b-P4VP(with relative block length of PS/P4VP based on1H NMR analysis) are tabulated in Table 1. The prepared diblock copolymer is denoted as PS153-b-P4VP1530, PS168-b-P4VP420,PS790-b-P4VP263, respectively, with the subscript indicating the number of repeating unit.
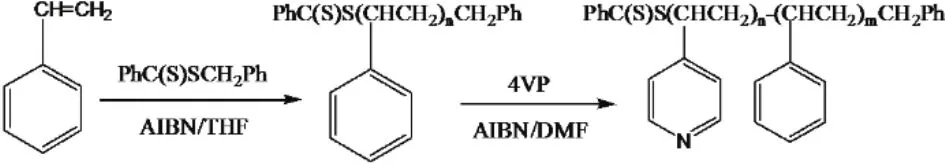
Scheme 1 Preparation of PS-b-P4VP by RAFT polymerization.

Table 1 Polymerization of PS (or P4VP) and PS-b-P4VP at 353.2 K and different reaction conditions.
2.5 Preparation of self-assembled aggregates(SAAs) in CO2-expanded liquids
The experimental apparatus was described in our previous work25. Typically, a certain amount of PS-b-P4VP (5 mg·mL−1)were dispersed in toluene in a glass tube at a suitable temperature, the mixture was then ultrasonicated for 10 min in order to disperse the block copolymer homogeneously in the solution. After equilibration, the tube containing the mixture was quickly transferred into a stainless-steel autoclave at the temperature of 313.2 K. CO2was then charged into the autoclave to achieve the desired pressure within a short time, and the reaction mixture was stirred at the same time. After 72 h treatment in CO2-expanded liquids, the system was cooled down and slowly depressurized, afterwards the SAA sample was collected and labeled.
A droplet of the micellar solution was dropped on a carboncoated copper grid placed on a filter paper, followed by airdrying. The carbon-coated copper grids were then exposed in staining agent iodine for 6 h to make the P4VP block visible so that the contrast is enhanced.
To examine the polymer composition or the molar ratio of 4VP/St (the ratio of 4VP/St is named as R) dependence on morphology change of the SAA, three different copolymer composition as PS153-b-P4VP1530, PS168-b-P4VP420and PS790-b-P4VP263were used. The reaction mixtures were examined with R selected to be 10 (i.e., PS153-b-P4VP1530in toluene at concentration of 5 mg·mL−1), 2.5 (PS168-b-P4VP420at concentration of 5 mg·mL−1) and 0.33 (PS790-b-P4VP263at concentration of 5 mg·mL−1), respectively. The CXLs pressures were selected to be 5.40 and 6.35 MPa, and the SAA samples free of CO2(0.1 MPa) were prepared for comparison.
3 Results and discussion
3.1 The morphology of SAA in conventional solvent toluene (0.1 MPa, free of CO2)
In conventional selective solvent, the factor controlling the morphology of self-assembled amphiphilic structures is the size of the hydrophobic moiety. It is primarily the packing parameter,p = V/al30, that determines the shape of the aggregates, where V is the volume of the hydrophobic moiety, a the contact area related to the size of shell-forming segment, and l the chain length normal to the interface (or the length of the hydrophobic segment). As the packing parameter p increases, the micellar morphology evolves from spheres through rods to vesicles, and then to large compound micelles (LCMs) to large compound vesicles (LCVs). Although it is difficult to obtain the p values experimentally, we can make a qualitative estimation of them from the TEM data. Accordingly, from the TEM images shown in Fig. 2a (spheres, with R = 2.5), Fig. 3a (vesicles, with R =0.33) and Fig. 4a (LCMs, with R = 10) of SAA formed in toluene, we could speculate that in conventional solvent toluene,the p value of SAA (PS168-b-P4VP420with R = 2.5) < p of SAA(PS790-b-P4VP263with R = 0.33) < p of SAA (PS153-b-P4VP1530with R = 10). That is, in conventional solvent or media, when the factors such as selective solvent, temperature and copolymer concentration are fixed, the copolymer composition or R value is the major factor to determine the SAA morphology. As R value varies, the amphiphilic nature difference between PS and P4VP block changes too in toluene. This varying difference induces the interfacial tension at the core-corona interface changing continuously, which induces the aggregate morphology transforming from spherical to nonspherical micelles progressively. However, the amphiphilic nature difference between PS and P4VP block is getting reduced with increasing pressure of CO2-expanded toluene owing to the antisolvent effect of CO2. And this diminished difference may influence the interfacial tension at the P4VP core-PS corona interface, which may trigger a morphological change of the aggregate. In the following section, we will illustrate that pressure is another effective factor to regulate the SAA shape under CXLs condition.

Fig. 2 TEM image of the SAA.SAA (with R = 2.5) were obtained from the copolymer PS168-b-P4VP420 (5 mg·mL−1) in CO2-expanded toluene (313.2 K/varied pressures for 72 h). (a) 0.1 MPa; (b) 5.40 MPa; (c) 6.35 MPa.

Fig. 3 TEM image of the SAA.SAA (with R = 0.33) were obtained from the copolymer PS790-b-P4VP263 (5 mg·mL−1) in CO2-expanded toluene (313.2 K/varied pressures for 72 h). (a) 0.1 MPa; (b) 5.40 MPa; (c) 6.35 MPa.

Fig. 4 TEM image of the SAA.SAA (with R = 10) were obtained from the copolymer PS153-b-P4VP1530 (5 mg·mL−1) in CO2-expanded toluene (313.2 K/varied pressures for 72 h). (a) 0.1 MPa; (b) 5.40 MPa; (c) 6.35 MPa.
3.2 The morphology control of SAA using CXLs method
Firstly we will focus on the evolution of the SAA structure with CXLs pressure at constant R value, suppose the major factors that may influence the morphology of the SAA, and present the possible reasons for their effectiveness. For SAA of PS168-b-P4VP420(with R = 2.5), the SAA shape (Fig. 2) switches from primarily spheres plus small amount of longer rods (0.1 MPa), through spheres plus more amount of short rods (5.40 MPa), to mostly interconnected rods plus less amount of spheres(6.35 MPa) with increasing CXLs pressure. While for SAA of PS790-b-P4VP263(with R = 0.33), the SAA structure (Fig. 3)evolves from spheres and small vesicles with large wall thickness (0.1 MPa), through mainly smaller vesicles with thinner wall thickness (5.40 MPa), to mostly large compound vesicles (LCVs, 6.35 MPa). Whereas for SAA of PS153-b-P4VP1530(with R = 10), the SAA morphology (Fig. 4) transits from large compound micelles (LCMs, 0.1 MPa), through larger size LCMs (5.40 MPa), to primarily LCVs (6.35 MPa) as pressure rises. So it is clear that the SAA morphology is remarkably pressure tunable from the TEM images shown in Figs. 2–4.
Then, what is the possible SAA morphology tuning mechanism under CXLs condition? At constant R value of 2.5,as toluene is PS selective, the observed SAA spherical micelles(Fig. 2a) in toluene are composed of a spherical P4VP cores surrounded by PS coronal chains. The spherical aggregates are generally the start clusters to form, which are usually regarded as the beginning morphology for other upcoming micelles1.Likewise, rod-like aggregates consist of cylindrical P4VP cores and PS coronas surrounding the cores, which represents the next step in the arrangement of copolymer chains in response to the variation in block or solvent media parameters. Besides, the interconnected rods at 6.35 MPa (Fig. 2c) are composed of threedimensional networks of branched rods. Apparently from the morphological change of SAA (PS168-b-P4VP420with R = 2.5)shown in Fig. 2, the value of packing parameter p of SAA in toluene seems smaller than that in CXLs as the SAA structure changes from mainly spheres (0.1 MPa) to more short rods (5.40 MPa), and then to primarily interconnected rods (6.35 MPa).That is, at constant copolymer composition, the packing parameter p of SAA (PS168-b-P4VP420with R = 2.5) keeps increasing as the CXLs pressure rises. And the similar pressure dependence of packing parameter p could be deduced for SAA with R = 0.33 (Fig. 3) and R = 10 series (Fig. 4). In other words,the CXLs method can easily regulate the SAA morphology change by a simple pressure tuning, yet we wonder the plausible reasons for the pressure dependence of the packing parameter p.As pressure increases, more CO2can be dissolved in toluene, the copolymer solubility becomes worse due to the anti-solvent effect of CO2, and the solvated state of both PS and P4VP chains is getting more shrunk with pressure rise, which may result in a simultaneous decrease in both the value of V (volume of the hydrophobic segment P4VP) and the l (the length of the hydrophobic segment P4VP), together with a value decline in a(the contact area between shell-forming segment PS and the solvent CXLs). From the expression of the packing parameter p = V/al, we think that the joint reduction in both V and l cannot guarantee an increase in the packing parameter p with the pressure rise, instead, the continuous decrease in the value of a could be the major factor to induce a steady enhancement of the packing parameter p with increasing CXLs pressure. So at the experimental conditions carried out in this work, an important conclusion can be drawn, that is, in conventional selective solvent, the major drive to control the SAA morphology comes mainly from the copolymer composition. Whereas in CO2-expanded toluene, the dominant factor to determine the SAA structure could be the change in the contact area between shellforming segment PS and the CXLs as the pressure rises, which agrees well with the mean-field theory31that a structural evolution from spheres to rods could possibly occur as the length of corona-forming block reduces. It is true that the coronaforming PS chains are becoming more folded with increasing CXLs pressure. Therefore for the SAA of PS168-b-P4VP420(with R = 2.5) series, the primary SAA structure exhibits mostly spheres (0.1 MPa, Fig. 2a), then the SAA forms more amount of short rods (5.40 MPa, Fig. 2b) owing to the enhanced folding degree of PS chains resulted from the anti-solvent effect of CXLs. With the more shrinking of the corona-forming PS chains at 6.35 MPa, the SAA presents mainly interconnected rods (Fig.2c), accompanied by a continuously reducing contact area between PS and the CXLs. Moreover, the morphological transition with increasing pressure also decreases the total interfacial free energy due to the reduced contact area between PS and the solvent1. And the above analysis on the pressure dependence of morphological transition also works for the SAA of PS790-b-P4VP263(with R = 0.33) and PS153-b-P4VP1530(with R = 10) series.
4 Conclusions
This work reports that CXLs facilitates the morphology control of the SAA of PS-b-P4VP formed in CO2-expanded toluene. It is found that the morphology and self-assembly behaviors of the SAA were strongly dependent on the CXLs pressure and the copolymer composition. At constant copolymer composition, the packing parameter p of SAA keeps increasing as the CXLs pressure rises. And we find that at the experimental conditions carried out in this work, the major drive to control the SAA shape in conventional toluene comes mainly from the copolymer composition. While in CO2-expanded toluene, the dominant factor to determine the SAA morphology might be the varying contact area between shell-forming segment PS and the CXLs as the pressure rises. This work illustrates that CXLs method helps the modulation of morphology of the SAA, and opens a green route for the development of new supra-molecular assembly materials.
杂志排行
物理化学学报的其它文章
- BmmimOAc-Catalyzed Direct Condensation of 2-(Arylamino) Alcohols to Synthesize 3-Arylthiazolidine-2-thiones
- Catalytic Electroreduction of CO2 to C2H4 Using Cu2O Supported on 1-Octyl-3-methylimidazole Functionalized Graphite Sheets
- Study on Solution Enthalpies of Ionic Liquids [Cnmim][H2PO4](n = 3, 4, 5, 6) by Using Pitzer’s Equation
- Physicochemical Properties of1-Methoxyethyl-3-Methylimidazolium Glycine
- Green and Cost-Effective Preparation of Small-Sized ZSM-5
- Influence of External Electric Field on Vibrational Spectrum ofImidazolium-Based Ionic Liquids Probed by Molecular Dynamics Simulation
