Study on Solution Enthalpies of Ionic Liquids [Cnmim][H2PO4](n = 3, 4, 5, 6) by Using Pitzer’s Equation
2018-09-18HUZhinanZUOJiantaoXIAMeichenFANGDaweiZANGShuliang
HU Zhinan, ZUO Jiantao, XIA Meichen, FANG Dawei , ZANG Shuliang
Key Laboratory of Rare and Scattered Elements, College of Chemistry, Liaoning University, Shenyang 110036, P. R. China.
Abstract: Ionic liquids (ILs) have become one of the most rapidly growing new research areas of ionic liquid and have attracted considerable attention from industry and the academic community since they are derived from natural ions and are heralded a new “natural ILs” or“bio-ILs”. ILs can be expected to find application in all of the biological,medical, and pharmaceutical sciences. However, the fundamental physical properties of an IL are extremely important in determining if a particular IL is appropriate for a given application. Recently, there is a developing trend in the literature towards estimation of thermodynamic properties for IL’s,which is to be commended because it provides valuable insight into the origins of the behaviour of ILs. Acidic ionic liquids have been applied in desulfuration process widely. Phosphoric acid IL is one kind of acidic ILs, which are also stable and economical efficiency.ILs [Cnmim][H2PO4] (n = 3, 4, 5, 6; 1-alkyl-3-methylimidazolium phosphonate) were synthesized and characterized. An on-line solution-reaction isoperibol calorimeter was constructed. It consists of a water thermostat, a 200 mL pyrex-glass plated silver Dewar, a 4 mL glass sample cell, a calibration heater, a glass-sheathed thermistor probe, an amplifier, a circuit used as an A/D converter and a personal computer for data acquisition and processing. The performance and accuracy of the calorimetric system was tested by measuring the molar enthalpy of solution of KCl in water and THAM[tris-(hydroxymethyl) aminomethane] in 0.1 mol·dm−3 HCl(aq) at 298.15 K. The molar enthalpies of solutions of the ILs[Cnmim][H2PO4] (n = 3, 4, 5, 6) at various molalities in water were measured by using a solution-reaction isoperibol calorimeter at 298.15 K respectively. According to the Pitzer’s electrolyte solution theory, the molar standard enthalpy ofand deduced. The standard molar enthalpies of solution of [Cnmim][H2PO4] (n = 3, 4, 5, 6) decreases with increasing alkyl chain length. When we make a plot with the standard molar enthalpy of solution vs carbon number in alkyl chains of the ionic liquid, a straight line was obtained with a correlation coefficient 0.99 and a slope of 1.54. The mean contribution per methylene (―CH2―) group to the standard molar enthalpies of the solution of [Cnmim][H2PO4] (n = 3, 4, 5, 6) was then obtained, which will give reliable estimation of other IL properties.
Key Words: Ionic liquid; Isoperibol calorimeter; Solution enthalpy; Pitzer’s equation
1 Introduction
Ionic liquids (ILs) are composed of ions and their melting temperatures are below 100 °C1–5. ILs can be expected to find application in all of the biological, medical, and pharmaceutical sciences. According to their unique properties, acidic ILs have been applied in desulfuration process widely. Some companies have applied the technology in industry. Phosphoric acid IL is one kind of acidic ILs, which are also stable and economical efficiency. In recent research on its desulfuration process, the heat bring force from its solution in system damaged the mass balance. So there must have been some detailed problems in the dissolution system. The solution heat of the progress caused pipeline and reaction still exothermic or heating, which are big trouble in technological design. Detailed knowledge of the solution enthalpy of ILs in water is very important for many technological processes6–10. Moreover, the fundamental physical properties of an IL are extremely important in determining if a particular IL is appropriate for a given application.
In this paper, we report the molar enthalpies of solution of ionic liquids (ILs) [Cnmim][H2PO4] (n = 3, 4, 5, 6)(1-alkyl-3-methylimidazolium phosphonate), which are good catalysis for desulfurization, at various molalities in water determined by solution-reaction isoperibol calorimeter11,12at 298.15 K. The standard molar enthalpies of solution of ILs,obtained. Comparing the experimental data in literature, the mean contribution per methylene (―CH2―) group to the standard molar solution enthalpies of ILs is discussed.
2 Experimental
2.1 Chemicals
1-Methylimidazole (99.5%) was obtained from ACROS and was distilled at reduced pressure prior to be used. Ethyl acetate(99.5%), acetone (99.5%) and acetonitrile (99.5%), all from Shanghai Reagent Co. Ltd., were distilled and then stored over molecular sieves in tightly sealed glass bottles, respectively.1-bromopropane (99.5%), 1-bromobutane (99.5%),1-bromopentane (99.5%), 1-bromohexane (99.5%), all from Shanghai Reagent Co. Ltd., were refined before use. KCl, with a purity more than 99.99% was dried in a vacuum oven at 408 K for 6 h, THAM (tris-(hydroxymethyl)aminomethane)(99.9%) was dried in a vacuum oven before use.Double-distilled water was used.
2.2 Preparation of [Cnmim]X
According to literature13, [Cnmim]X was synthesized by refluxing the 1-methylimidazole with a large excess of the haloalkane at 60 °C for 48 h. The excess haloalkane were removed by evaporation and the crude product was recystallized triply from acetonitrile and ethyl acetate. The product was a slightly yellow liquid and then dried in vacuo for 24 h. The yield was approximately 80%.
Then aqueous 1-alkyl-3-methylimidazolium hydroxide([Cnmim][OH]) were prepared from [Cnmim]X using activated anion-exchange resin over a 100 cm column. The onium hydroxide aqueous solution was added dropwise to a slightly excess phosphoric acid solution. The mixture was stirred under cooling for 12 h. Then water and reactant were evaporated under reduced pressure at 75–80 °C and 0.01 MPa. The mixture was added some acetate then separated excess phosphoric acid.Filtrate was evaporated to remove solvents. The products[Cnmim][H2PO4] (n = 3, 4, 5, 6) were dried in vacuo for 2 days at 80 °C (see Fig. 1) . Structures of the resulting products were conf i rmed by1H NMR (see Figs. S1–S4 in Supporting Information). The anion was verified by an ion chromatography, and remained Br−was determined by dripping the silver nitrate solution, which confirmed the structure of synthesized ILs. The water content were determined by a Karl Fischer moisture titrator (ZSD-2 type), which all showed under 2000 × 10−6.
2.3 Determination of the molar enthalpies of solution

Fig. 1 Preparation of ionic liquids by the neutralization method.
On the basis of other calorimetric apparatus11,12, an on-line solution-reaction isoperibol calorimeter was constructed. It consists of a water thermostat, a 200 mL pyrex-glass plated silver Dewar, a 4 mL glass sample cell, a calibration heater, a glass-sheathed thermistor probe, an amplifier, a circuit used as an A/D converter and a personal computer for data acquisition and processing. 150 g of solvent (water) was placed in the Dewar and (0.1 to 2) g of solute (KCl, THAM or[Cnmim][H2PO4]) in the sample cell. The inevitable heat transfer and heat generations owing to friction were compensated and the corrected temperature change (the adiabatic temperature change) ΔT*was obtained according to conventional method (the equal area method)6. The enthalpies of solution were calculated from the equation:

where Qs is the enthalpy of solution of the sample, ΔT*s is the adiabatic temperature change of the solution process, QEis the electric energy calibration, andis the adiabatic temperature change of electric calibration.
The performance and accuracy of the calorimetric system was tested by measuring the molar enthalpy of solution of KCl in water and THAM [tris-(hydroxymethyl) aminomethane] in 0.1 mol·dm−3HCl(aq) at 298.15 K. The results are listed in Tables 1 and 2.
From both tables, the mean molar solution enthalpies are ΔsolHm= (17542 ± 31) J·mol−1for KCl and (−29794 ± 28)J·mol−1for THAM, which are in good agreement with the published data (17536 ± 9) J·mol−1for KCl14,15and (−29766 ±31.5) J·mol−1for THAM16. Then, the molar enthalpies of solution in water of the ionic liquid [Cnmim][H2PO4](n = 3, 4,5, 6) was measured at different molalities. The values of molar enthalpies of solution in water of [Cnmim][H2PO4](n = 3, 4, 5,6) with various molalities at 298.15 K are listed in Table 3.
3 Results and discussion
3.1 Review of Pitzer’s equation
From Table 3, the solution processes of the ionic liquids are exothermic, also the solution enthalpy of [Cnmim][H2PO4](n =3, 4, 5, 6) increased with decreasing molality.
The molar solution enthalpy of [Cnmim][H2PO4] (n = 3, 4, 5,6) is expressed:

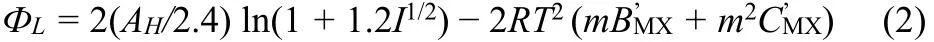
where I means ionic strength, m is molality, R is gas constant,other symbols are defined by following equations:
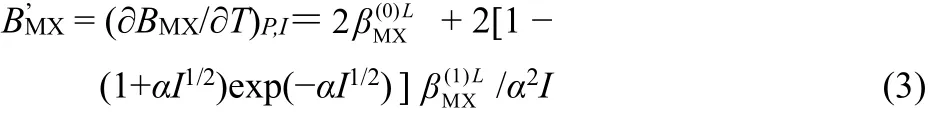
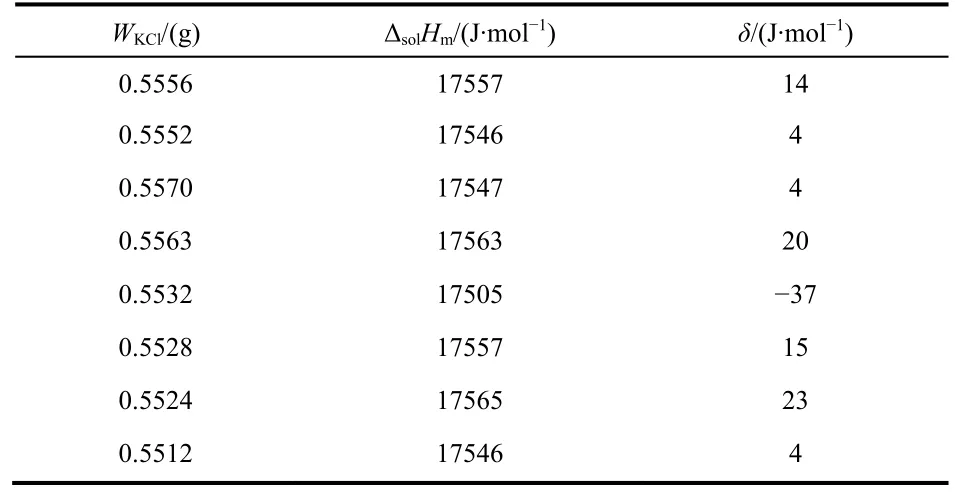
Table 1 Values of molar enthalpy of solution in water of KCl, ΔsolHm,at 298.15 K.
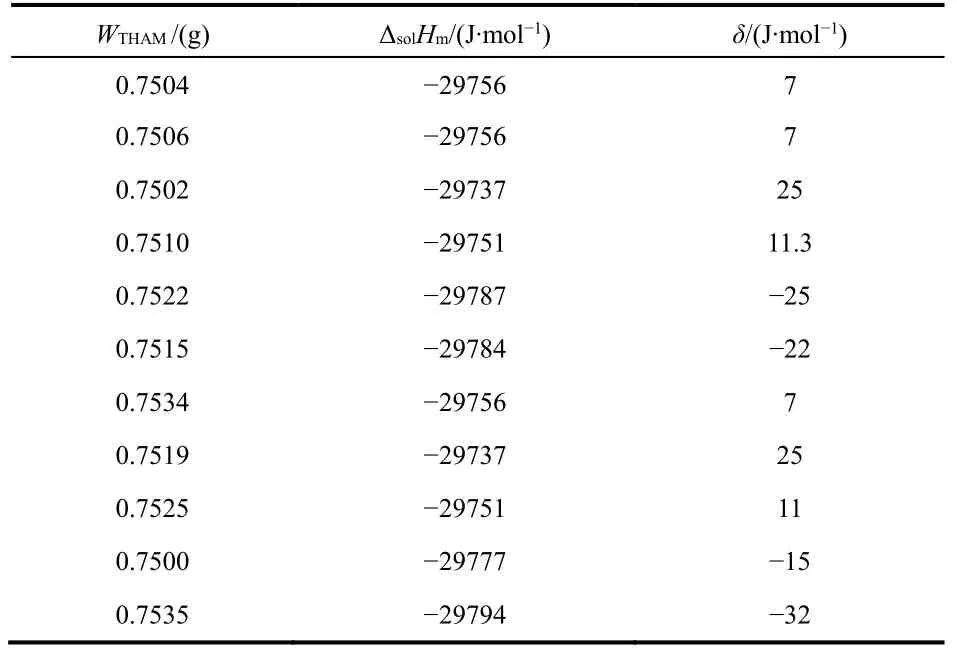
Table 2 Enthalpy of the reaction of THAM in 0.1 mol∙dm-3 of HCl(aq), at 298.15 K.

where subscripts M and X mean cation and anion, respectively,νMand νXare the numbers of cation and anion in the formulashort-distance among ions,is triple-ionic action term,which indicates the important interaction in higher molalities;for [Cnmim][H2PO4](n = 3, 4, 5, 6), α = 2.0. The values offrom the experimental data of osmotic coefficient by the least-squares method. The value of Debye-Hückel parameter of enthalpy, AH, was taken from literature17.
3.2 The molar solution enthalpy of [Cnmim][H2PO4]at infinite dilution
From Eqs. (3 and 4), the working equation to determine the standard molar solution enthalpy and Pitzer’s parameters was obtained18:
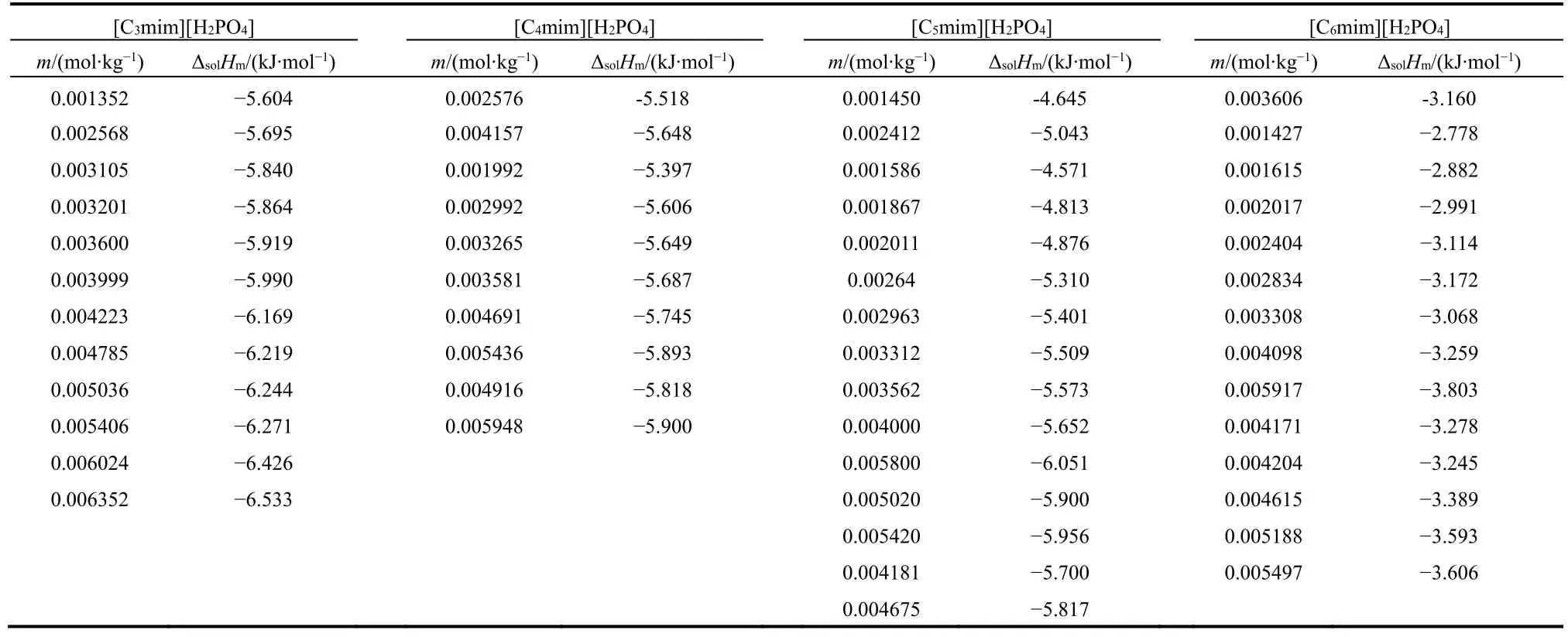
Table 3 Values of molar enthalpy of solution in water of [Cnmim][H2PO4](n = 3, 4, 5, 6) with various molalities at 298.15 K.
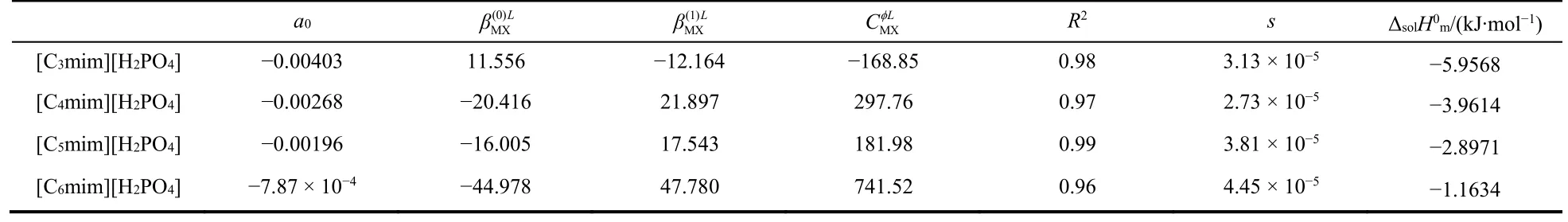
Table 4 Values of standard molar enthalpy of solution and Pitzer equation parameters of [Cnmim][H2PO4] (n = 3, 4, 5, 6).
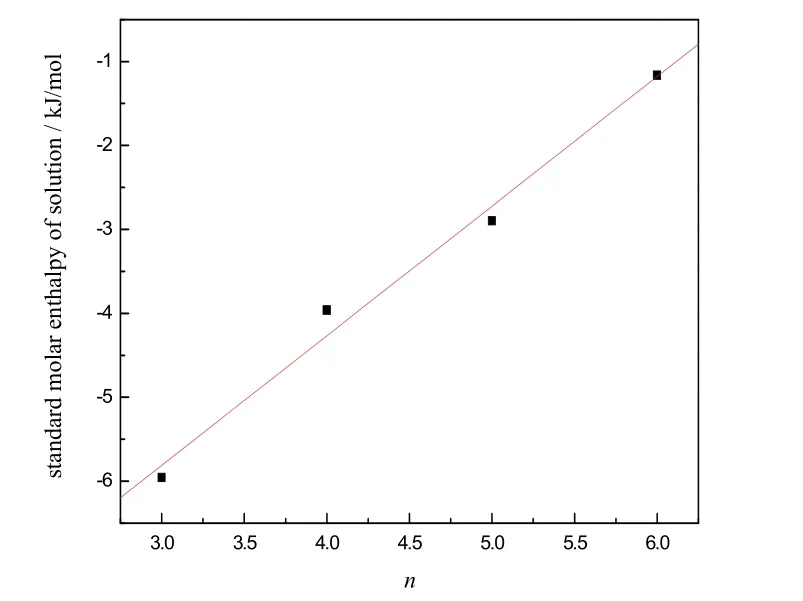
Fig. 2 Plot of standard molar enthalpy vs number of carbons (n)in the alkyl chains of [Cnmim][H2PO4] (n= 3, 4, 5, 6).y = 1.54445n − 10.44467, with s = 0.26947 and r = 0.99.
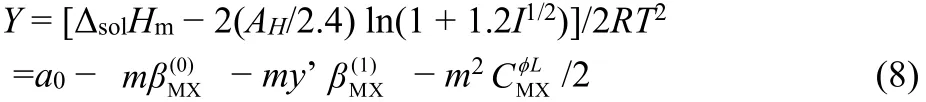
where Y is the extrapolating function, which can be obtained with the experimental data. Parameters a0and y’ in the Eq. (8)are defined by following equations, respectively:
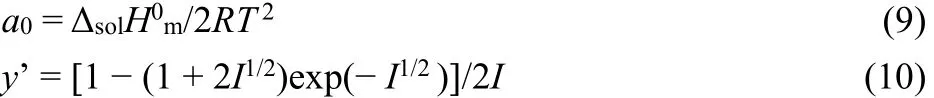
Molar enthalpies of solution of [Cnmim][H2PO4] (n = 3, 4, 5,6) were fit to Eq. (8). The values of the parameters are listed in Table 4.
The standardmolar enthalpy of solution of [Cnmim][H2PO4](n = 3, 4, 5, 6) were calculated from the parameter a0, and also listed in Table 4. We can see that the standard molar enthalpies of solution of [Cnmim][H2PO4] (n = 3, 4, 5, 6) decreases with increasing alkyl chain length. When we make a plot with the standard molar enthalpy of solution vs. carbon number in alkyl chains of the ionic liquid, a straight line was obtained with a correlation coefficient 0.99 and a slope of 1.54, see Fig. 2. We can draw a conclusion that each mole of methylene group contributes to the standard molar enthalpy of solution of[Cnmim][H2PO4] (n = 3, 4, 5, 6) is about 1.54 kJ·mol–1.
4 Conclusions
In order to investigate the heat caused by solution process of acidic ILs in desulfurization technology, a series of molar enthalpies of solution of ILs [Cnmim][H2PO4] (n = 3, 4, 5, 6) at various molalities in water were measured by a solution-reaction isoperibol calorimeter at 298.15 K. The molar standard enthalpy of solution of [Cnmim][H2PO4] (n = 3, 4, 5,6), ΔsolH0m, were calculated by Pitzer’s electrolyte solution theory. The mean contribution per methylene (―CH2―) group to the standard molar enthalpies of solution of [Cnmim][H2PO4](n = 3, 4, 5, 6) is also obtained as 1.54 kJ·mol−1, which will give reliable estimation of other IL properties19–25.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
杂志排行
物理化学学报的其它文章
- BmmimOAc-Catalyzed Direct Condensation of 2-(Arylamino) Alcohols to Synthesize 3-Arylthiazolidine-2-thiones
- Self-Assembly Behavior of Amphiphilic Diblock Copolymer PS-b-P4VP in CO2-Expanded Liquids
- Catalytic Electroreduction of CO2 to C2H4 Using Cu2O Supported on 1-Octyl-3-methylimidazole Functionalized Graphite Sheets
- Physicochemical Properties of1-Methoxyethyl-3-Methylimidazolium Glycine
- Green and Cost-Effective Preparation of Small-Sized ZSM-5
- Influence of External Electric Field on Vibrational Spectrum ofImidazolium-Based Ionic Liquids Probed by Molecular Dynamics Simulation
