Green and Cost-Effective Preparation of Small-Sized ZSM-5
2018-09-18XUETengDONGLiluZHANGYingWUHaihong
XUE Teng , DONG Lilu , ZHANG Ying ,2, WU Haihong ,*
hanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200062, P. R. China.
hanghai University of Medicine & Health Sciences, Shanghai 200237, P. R. China.
Abstract: Small-sized zeolite ZSM-5 for a wide SiO2/Al2O3 ratio range was prepared using a small amount of colloidal silicalite-1 as the active seeds. The thus-prepared small-sized ZSM-5 samples have been characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM),N2 adsorption-desorption analysis, temperature-programmed ammonium desorption (NH3-TPD) analysis, and adsorbed pyridine infrared spectroscopy (Py-IR). The use of the active silicalite-1 seeds was effective in directing the reaction towards the formation of the MFI phase, avoiding the impure phases and reducing the crystal sizes. The prepared sample exhibited aggregated morphologies when a lower ratio of starting batch SiO2/Al2O3 (SiO2/Al2O3 ratio = 30) was used. The aggregates, with the size of ~500 nm, were formed with nano-sized primary crystals 50 nm in size, possessing large external surface area (84.9 m2·g−1) and secondary pore volume (0.22 cm3·g−1) and relatively regular mesopores. Different morphologies could be observed when the SiO2/Al2O3 ratio was increased (SiO2/Al2O3 ratio = 60–120). ZSM-5 with the size of 200 nm could be prepared, with the external surface area and the secondary pore volume being ~60 m2·g−1 and 0.10 cm3·g−1, respectively. It should be highlighted that all the prepared samples could be directly ion-exchanged to obtain the acidic H-form samples without complete blocking of the micropores due to the low dose of the organic structure-directing agent. The obtained acidic H-form samples exhibited acidic properties similar to the samples ion-exchanged after calcination and the conventional ZSM-5 possessing similar SiO2/Al2O3 ratio, showing catalytic performance comparative to the catalytic conversion of methanol to olefins.Compared with conventional methods, this method largely reduced the use of organic templates and avoided the subsequent combustion before ion-exchange. The method is green and cost-effective, possessing wide potentials in the industrial processes.
Key Words: Zeolite ZSM-5; Small-sized crystallites; Seed-induced method; Green synthesis; Catalytic performance
1 Introduction
Zeolites are crystalline aluminosilicates with three-dimensional framework structures which form uniformly sized pores of molecular dimensions. They are widely used as catalysts in areas of petrochemistry and fine-chemical synthesis due to their unique pore structures and the tunable active sites1. Zeolites are usually prepared by a hydrothermal process with reagents being a silica source, an alumina source, a mineralizing agent such as OH−or F−2. In the early 1960s, an organic cation,tetramethylammonium, was first introduced in zeolite synthesis by Barrer and Denny3. And later on, other quaternary ammonium ions, organic amines and O- and S-containing organic molecules have been tried as templates for the zeolite synthesis4. This led to the burst of many novel high silica zeolites. However, the organic templates were usually occluded in the zeolite micropores during the crystallization, which must be burned out to open the micropores. This would lead to the emission of CO2and NOx, which is contradicting to the strict environmental protection policy. Furthermore, the templates could constitute up to 50% of the production cost during zeolite synthesis5. It might be of great significance both environmentally and economically if the organic templates for the zeolite synthesis and subsequent calcination step could be avoided.
Zeolite ZSM-5 is widely used as solid acid catalyst in chemical and petrochemical processes due to the high thermal/hydrothermal stability, strong acidity, well-defined microporosity and its unique shape selectivity6,7. It was first synthesized by Argauer et al. using TPAOH as a template8.After that, many other organic templates have been used for the preparation of ZSM-5 with various morphologies for different purposes till now9–11. Also, efforts have been made to synthesize ZSM-5 through a template-free method based on the consideration of cost and economy12–15. However, when a template-free method was used, highly crystallized pure ZSM-5 could only be obtained within a relative narrow SiO2/Al2O3ratio range. It was usually accompanied by Mordenite when a lower SiO2/Al2O3 ratio was used while α-quartz could be detected when the SiO2/Al2O3ratio was higher9,16,17.Furthermore, the crystallites of the prepared samples were usually large, which might impose diffusion limitation during catalytic reactions18. Seeding was found to be effective which could direct the synthesis towards a desired phase with consequent reduction in impurities, reduction in the synthesis time and control product crystal size distribution19. They may remain inert, dissolve, act as pure seeds in that mass is deposited upon them and they grow, or give rise to secondary nuclei and hence a new crop of crystals. Sufficient surface area was necessary to achieve an effect, so that the quantity and crystal sizes played an important role when added to the synthesis mixtures20,21. Seeds with small crystallites possessing large surface area were considered to be more effective.Previously, we reported the preparation of hierarchically porous ZSM‑5 aggregates using quite a small amount of active seeds.Quite a small amount of colloidal silicalite-1 was used as seeds for the preparation of hierarchically porous ZSM-5 aggregates with the SiO2/Al2O3ratio of ~35. Trace amount of TPAOH introduced from colloidal seeds did not totally block the micropores and the prepared sample could be ion-exchanged directly without the calcination of the organic templates in advance, showing advantages in reducing the cost and pollution during the zeolite synthesis22. Actually ZSM-5 could be prepared within a wide SiO2/Al2O3ratio range and ZSM-5 with different SiO2/Al2O3ratios possessing different acid properties could find its uses in different reactions23–25.
In this article, we investigated the preparation of ZSM-5 with different SiO2/Al2O3ratios using a small amount of colloidal silicalite-1 as active seeds. The effectiveness of the active seeds on the direction to the desired MFI phase with different SiO2/Al2O3ratios and the influence of the SiO2/Al2O3ratio on the morphologies, acid and textural properties was discussed.The obtained samples were ion-exchanged without previous calcination of the organic templates and characterized and preliminary evaluated using the catalytic methanol conversion as the probe reaction.
2 Experimental section
2.1 Preparation of the active seeds
Active seeds were prepared following a molar composition of SiO2:0.35TPAOH:20H2O:4EtOH with tetraethylorthosilicate(TEOS) as the silica source and TPAOH as the structure-directing agent. The presence of ethanol (EtOH) in the molar composition is a consequence of the hydrolysis of TEOS. TEOS was added into the mixture aqueous solution of TPAOH. After stirring at room temperature for 24 h, the mixture was then hydrothermally treated at 80 °C for 72 h. The obtained gel solution was directly used as the active seeds.
2.2 Preparation of zeolite ZSM-5 with different SiO2/Al2O3 ratios using the active seeds
The molar composition for the synthesis was SiO2: 1/xAl2O3:0.12Na2O: 30H2O. 0.5% of the active seeds were added based on the SiO2in the colloidal seeds to that in the total batch proportion. Water glass (SiO2, 27.10% (w, mass fraction);Na2O, 8.39% (w)) and Al2(SO4)3·18H2O (≥ 99.0% (w)) were used as silica and aluminum source, respectively. H2SO4(98%(w)) was used to adjust the OH−/SiO2ratio. Pre-calculated amount of Al2(SO4)3and H2SO4were dissolved together and then dropped into water glass under vigorous stirring. The pre-synthesized active seeds were then added. The mixture was homogenized for about 2 h and transferred into Teflon-lined stainless steel autoclaves and crystallized at 175 °C for 24 h.After crystallization, the autoclave was quenched in tap water and the resultant solid product was separated from the mother liquor by filtration. The product was washed several times with deionized water, dried at 100 °C overnight. The prepared samples were directly ion-exchanged in aqueous solution of NH4NO3(1.0 mol·L−1) at 80 °C for 2 h without combustion of the occluded templates in advance. H-form zeolite ZSM-5 was obtained after calcination at 550 °C for 6 h and denoted as LTZ-xDH, where x represents the batch SiO2/Al2O3ratio. For comparison, samples were also ion-exchanged after combustion of the organic template following a conventional procedure,and the obtained sample was denoted as LTZ-xCH. To investigate the effective of the active seeds, TPAOH was used as template for the synthesis with the molar composition of SiO2: 1/120Al2O3: 0.12Na2O: 0.00175TPAOH: 30H2O. The amount of the TPAOH was the same with that when 0.5% of active seeds were used. The obtained sample was denoted as Z-TPA-120 after calcination. Well crystallized large ZSM-5 prepared according to our previously reported method was used as the reference sample (Ref-Z5) and the preparing procedures and the morphologies and the properties were presented in the supporting information (Figs. S1–S3)26.
2.3 Characterization
X-ray diffraction (XRD) patterns were recorded on a Rigaku Ultima IV diffractometer using nickel-filtered Cu Kαradiation(λ = 0.15418 nm) operated at 30 kV and 30 mA within a 2θ range of 5°–35°. The degree of crystallinity was calculated by comparing the area of the five peaks between 2θ = 22.5° and 25.0° of each sample with that of Ref-Z5 (Supporting Information). The scanning-electron microscopy (SEM)measurements were performed on a Hitachi Model S 4800 instrument at an operating voltage of 3 kV. The SiO2/Al2O3ratio of the obtained zeolites was quantified by inductively coupled plasma atomic emission spectrometer (ICP-AES) on a Thermo IRIS Intrepid II XSP after dissolving the samples in HF solution. Nitrogen adsorption-desorption isotherms at 77 K of the as-prepared and the H-form samples were carried out on a Micromeritics 2020 M physisorption analyzer. Prior to the measurement, the samples were first outgassed at 300 °C under vacuum for 6 h. Specific surface areas were calculated according to the BET-method using five relative pressure points in the interval of 0.01–0.20, and the external surface area and the micropore volume of the samples were calculated by the t-plot method. The mesopore size distribution was obtained by the BJH model applied to the adsorption branch of the isotherm. Temperature-programmed ammonium desorption(NH3-TPD) was carried out on a Micromeritics Autochem II chemisorption analyzer. 0.1 g of the obtained H-form samples were pretreated at 300 °C for 2 h under helium flow, and NH3adsorption was carried out at 100 °C. After purging in helium for 30 min, the temperature was then raised to 600 °C at a rate of 10 °C·min−1. The TPD profile for chemisorbed ammonium was monitored by a TCD detector. FTIR spectrometry of adsorbed Pyridine was carried out using a Nicolet Nexus 670 spectrometer equipped with an in situ vacuum system containing a secondary vacuum (Xiamen Tops Equipment Development Co., Ltd.). 10–15 mg of the obtained H-form samples was pressed into self-supporting wafers with a diameter of 11 mm and pretreated at 600 °C for 2 h. The samples were then cooled to 50 °C for pyridine adsorption.After that, the samples were subjected to the thermal desorption at 150 °C followed by the IR measurement.
2.4 Catalytic reaction
To evaluate the performances of the prepared samples,methanol-to-olefins (MTO) reaction was performed under atmospheric pressure in a fixed bed reactor. About 2 g of powder catalyst was loaded into the reactor and then pretreated with 100 mL·min−1flow of N2at 500 °C for 3 h followed by adjusting the temperature of the reactor was reduced to the reaction temperature of 460 °C. N2 flow was then turned off and pure methanol was fed by a syringe pump into the reactor with a weight hourly space velocity (WHSV) of 6 h−1, Products were analyzed by on-line gas chromatographs (GC9890A,HP-PLOT/Q capillary column, FID).
3 Results and discussion
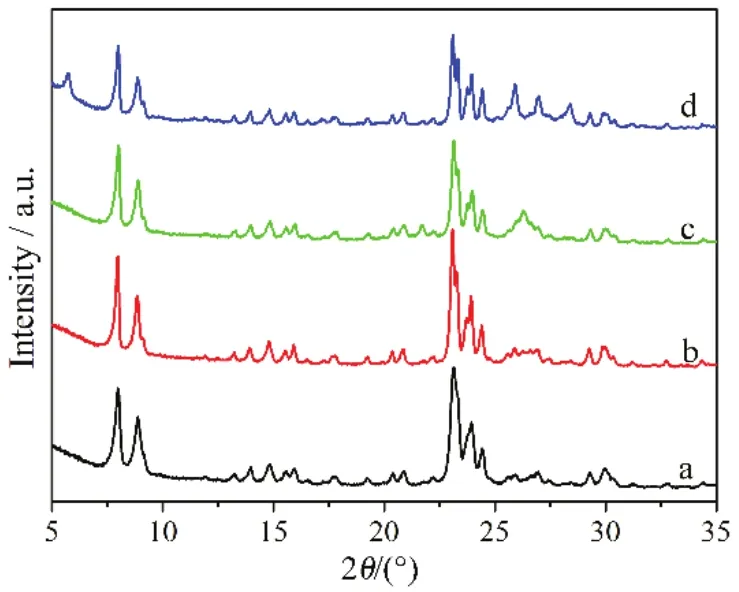
Fig. 1 XRD patterns of the prepared samples.a: LTZ-30DH, b: LTZ-60DH, c: LTZ-120DH, d: Z-TPA-120.
When 0.5% of the active seeds were used, all the samples prepared with different SiO2/A2O3ratios, LTZ-30DH,LTZ-60DH and LTZ-120DH, exhibited well resolved peaks corresponding to the MFI topologies (Fig. 1). It should be noted that when a lower SiO2/Al2O3(SiO2/Al2O3ratio = 30)ratio was used, XRD peaks of the prepared samples was much broader. This might be ascribed to the nanocrystalline nature of the primary particle according to the Scherrer equation, which is similar with that reported previously22,27. Relative crystallinity was calculated by comparing the area of the five peaks between 2θ = 22.5° and 25.0° of the prepared sample with that for a conventional large sample, which were nearly the same (Table 1). With the increase of the batch SiO2/Al2O3 ratio to 60, relative crystallinity of the sample LTZ-60DH remained. But the diffraction peaks became sharp and narrow,which might be ascribed to the increase of the primary size.Further increasing the batch SiO2/Al2O3ratio to 120, a peak centered at 26.3°, which do not belong to the MFI topology,could be observed for sample LTZ-120DH. This leads to the slight decrease of the relative crystallinity (Table 1). It should be pointed out that when the active seeds were replaced by TPAOH (TPAOH/SiO2ratio = 0.00175, which was equal to that when 0.5% of active seeds were used), impurity of obvious Magadiite phase could be observed for sample Z-TPA-120,leading to the further decreasing of the relative crystallinity28.This proved the effectiveness of the active seeds in directing to the synthesis of zeolite ZSM-5. The SiO2/Al2O3ratios of the prepared H-form samples were determined using ICP-AES,which were slightly lower than the starting batch SiO2/Al2O3ratios (Table 1). This could be explained by the lower availability of silica than alumina in alkaline medium during the synthesis.
The morphologies and the crystal sizes of the prepared samples were investigated using SEM and TEM (Fig. 2).LTZ-30DH exhibited aggregated morphologies, with no amorphous or impure phases detected. LTZ-30DH comprised of nanosized primary particles with size of about 50 nm. The size of the ZSM-5 aggregates was smaller than 500 nm (Fig.2a). TEM image in Fig. 2e confirmed aggregated morphologies of LTZ-30DH. Furthermore, interparticle porosity formed by the aggregation of the primary nanosized particles could be observed. This might be helpful in solving the diffusion limitation encountered during catalyzing large molecule involved reactions. Different to sample LTZ-30DH prepared with low SiO2/Al2O3ratio, the size of the primary particles increased and the morphologies changed when the SiO2/Al2O3ratio increased. This is in accordance with the XRD characterization, where the peaks became sharper and narrower.The crystal size for sample LTZ-60DH was about ~200 nm(Fig. 2b, f) and it didn’t further increased when the starting batch SiO2/Al2O3ratio further increased to 120 (Fig. 2c, g).However, some impure nano-sheets different to the conventional ZSM-5 morphologies could be observed for LTZ-120DH, as cycled out in Fig. 2c. This coincided with the XRD results where impure phase was detected and the relative crystallinity decreased for sample LTZ-60DH (Fig. 1 and Table 1). When the active seeds were replaced by TPAOH, the obtained sample Z-TPA-120 possessed two kinds of obviously different morphologies (Fig. 2d). The aggregates of the nano-sheets might be ascribed to Magadiite phase (Fig. 1d)28.The ZSM-5 exhibited conventional coffin-like morphology,with the crystal size of up to ~5 μm. The appearance of impure Magadiite phase and the large crystals of the obtained ZSM-5 when TPAOH was used as the structure-directing agent further proved the effectiveness of the active seeds in directing towards the pure MFI phase and reducing the crystal sizes.
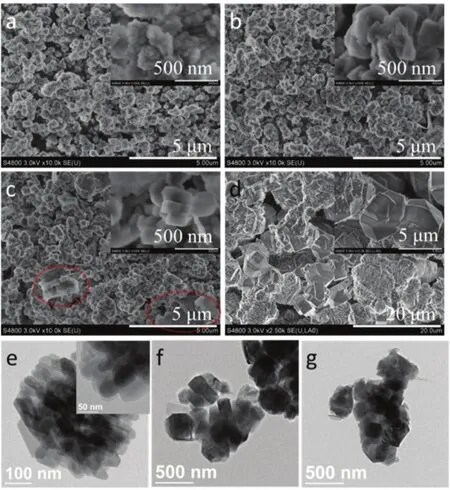
Fig. 2 SEM (a–d) and TEM (e–g) images of the prepared samples.a, e: LTZ-30DH; b, f: LTZ-60DH; c, g: LTZ-120DH, d: Z-TPA-120

Table 1 Textural properties of the ZSM-5 zeolite prepared trace amount of seeds.
Textural properties of the prepared samples have been characterized using nitrogen adsorption-desorption technique and the isotherms and the BJH pore size distributions were presented in Fig. 3. The sample LTZ-30DH exhibited combined type I and type IV isotherm with obvious hysteresis loop and steep uptake of nitrogen in higher p/p0range. This indicated the presence of secondary porosity besides the inherent micropore of the zeolite ZSM-5, which could be ascribed to the interparticle voids formed by the aggregation of the small crystallites. Widely distributed secondary pores could be observed from the BJH pore size distributions. And large external surface area (84.9 m2·g−1) and secondary pore volume(0.22 cm3·g−1) could be found for sample LTZ-30DH, showing the hierarchical porosity. Furthermore, BET surface area,micropore surface area and the micropore volume reached 395.9, 311.0 and 0.13 cm3·g−1, respectively, showing the high crystallinity of the sample. When the starting batch SiO2/Al2O3increased, the isotherm for sample LTZ-60DH approached to type I, with nearly plateau uptake at higher p/p0range. An obvious decrease of the secondary pore volume could be observed, compared with LTZ-30DH. It should be pointed out that the external surface area of LTZ-60DH reached 58.0 m2·g−1, attributing to the small crystallites (~200 nm, as shown in Fig. 2b,e). This was remarkable, much larger than that of the conventional samples with large crystals29. The BET surface area, micropore surface area and the micropore volume were still as large as that of LTZ-30DH, corresponding to the high crystallinity. When the SiO2/Al2O3 ratio further increased, the micropore volume and micropore surface area of LTZ-120DH decreased slightly in accordance with the decrease of the crystallinity. But the mesoporous properties of LTZ-120DH increased slightly, this might be consequence of the formation of some impurities with large external surface area. Compared to the samples prepared with the active seeds, the sample of Z-TPA-120 prepared with TAPOH as the structuring-directing agent exhibited much lower nitrogen uptake, leading to the obviously decreased surface area and pore volume (Fig. 3 and Table 1). This is in accordance with decrease of the crystallinity of sample Z-TPA-120 and the formation of large of amount of Magadiite as the impure phase, as observed in Figs. 1 and 2.

Fig. 3 Nitrogen adsorption-desorption isotherms at -196 °C (A) and BJH pore size distributions (B) for sample LTZ-30DH (a), LTZ-60DH(b), LTZ-120DH (c) and Z-TPA-120 (d).Isotherms for b, c and d have been shifted by 50 cm3·g−1,100 cm3·g−1 and 150 cm3·g−1, respectively.
ZSM-5 is mainly used as catalyst in acid-catalyzed reactions. And thus besides the porosity, the type, strength and the relative amount of the acid sits played an important role during the catalytic reactions, which might influence the conversion, selectivity30,31. The acid properties of the prepared samples were characterized using NH3-TPD and pyridine-adsorbed FTIR (Fig. 4). Both the directly ion-exchanged samples (DH)and the samples ion-exchanged after calcination following the conventional procedures (CH) were investigated and compared together. The density of the acid sites decreased with the increase of the SiO2/Al2O3 ratio, as shown in Table 2.Furthermore, the little amount of TPAOH introduced from the 0.5% of active seeds did not block the micropores totally,irrespective of the SiO2/Al2O3ratios. The occluded TPAOH showed little influence on the ion-exchange and the Na+balancing the negatively charged AlO4−tetrahedrons could readily be ion-exchanged without the necessary of calcination.It was reflected by the amount of the directly ion-exchanged and the sample ion-exchanged after calcination. The acid amount of the directly ion-exchanged samples was slightly lower than that of the calcined-and-ion-exchanged samples and the bulk Ref-Z5 (Table 2 and Fig. S3). This demonstrated that the method herein for the preparation of small-sized acidic H-form ZSM-5 without previous calcination of the organic templates before ion-exchange was effective and could be applied within a wide SiO2/Al2O3ratio range.

Fig. 4 NH3-TPD curves (A) and pyridine-adsorbed FTIR spectra (B)for sample LTZ-30CH (a), LTZ-30DH (b), LTZ-60CH(c), LTZ-60DH(d), LTZ-120CH (e) and LTZ-120DH (f).

Table 2 Acid properties of the ZSM-5 zeolite prepared trace amount of seeds.
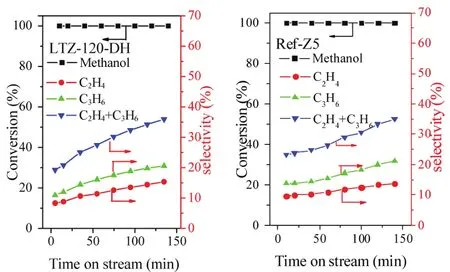
Fig. 5 The catalytic activities of LTZ-120DH catalysts compared with Ref-Z5 for MTO reaction.
The catalytic performance of the prepared sample LTZ-120DH was preliminary evaluated by using methanol-to-olefins (MTO)reaction and compared with that of a conventional sample possessing the similar SiO2/Al2O3ratio (Ref-Z5, Fig. S2 shown in the Supporting Information). Both of the two samples exhibited conversion of higher than 99.9% within the investigated time (Fig. 5). Furthermore, there seemed to be little difference between these two samples on the selectivity of ethylene and propylene. However, it should be noted that the reported method herein was still of great importance,considering the synthesis cost, the reduced crystal sizes and the reduction of the pollution by avoiding the calcination of the templates before ion-exchange. A detailed investigation on the performance, life time and coke formation of the prepared samples is still in progress.
4 Conclusions
High crystallized zeolite ZSM-5 within a wide SiO2/Al2O3ratio range was prepared using quite a small amount of colloidal silicalite-1 as active seeds. The prepared ZSM-5 could be directly ion-exchanged to the acidic H-form samples without calcination of the organic templates in advance. The obtained acidic H-form samples exhibited similar acidic properties with the conventional ZSM-5 possessing the similar SiO2/Al2O3ratio. It should be noted that the morphologies of the prepared zeolites could be influenced by the batch SiO2/Al2O3ratios.Hierarchically porous ZSM-5 aggregates composed of nanosized primary crystallites could be prepared when a lower batch SiO2/Al2O3 ratio was used. The size of the primary particles increased with the increase of the batch SiO2/Al2O3 ratio and ZSM-5 with size of ~200 nm could be prepared when the batch SiO2/Al2O3ratio was higher than 100. When used as catalysts for the methanol conversion, the directly ion-exchanged sample exhibited similar performance with that of the conventional sample prepared with large amount of templates.This method is green and cost-effective by largely reducing the amount of the organic templates and avoiding the subsequent combustion before ion-exchange, possessing wide potentials in the industrial processes.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
杂志排行
物理化学学报的其它文章
- BmmimOAc-Catalyzed Direct Condensation of 2-(Arylamino) Alcohols to Synthesize 3-Arylthiazolidine-2-thiones
- Self-Assembly Behavior of Amphiphilic Diblock Copolymer PS-b-P4VP in CO2-Expanded Liquids
- Catalytic Electroreduction of CO2 to C2H4 Using Cu2O Supported on 1-Octyl-3-methylimidazole Functionalized Graphite Sheets
- Study on Solution Enthalpies of Ionic Liquids [Cnmim][H2PO4](n = 3, 4, 5, 6) by Using Pitzer’s Equation
- Physicochemical Properties of1-Methoxyethyl-3-Methylimidazolium Glycine
- Influence of External Electric Field on Vibrational Spectrum ofImidazolium-Based Ionic Liquids Probed by Molecular Dynamics Simulation
