Ultra-High SO2 Capture by Anion-Functionalized Resins through Multiple-Site Adsorption
2018-09-18HEXiXiaoyuFANXiLINWenjunLIHaoranWANGCongmin
HE Xi , LÜ Xiaoyu , FAN Xi , LIN Wenjun , LI Haoran ,2, WANG Congmin ,*
JU-NHU United R&D Center, Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China.
ollege of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, P. R. China.
Abstract: The anion-functionalization strategy has been proposed and applied for the synthesis of macro-porous resins [IRA-900][An], thus realizing anultra-high SO2 adsorption capacity (>10 mmol·g−1) at 101.3 kPa and 20 °C.Compared with the normal azole-based anion-functionalized resins, the poly(imidazolyl)borate anion-functionalized resin [IRA-900][B(Im)4] exhibited an outstanding adsorption capacity at low SO2 partial pressures (10.62 mmol·g−1 at 20 °C and 10.13 kPa). From the results of the IR spectrum investigation and DFT calculations, the multiple-site adsorption mechanism was verified. On account of the unique tetrahedral configuration of [B(im)4], the conjugation and electronic communication between the electronegative nitrogen atoms were disrupted, making them behave as local reactive sites. Therefore, at least four electronegative nitrogen atoms could be provided by one [B(im)4] to react with SO2 without evident adsorption enthalpy deterioration (from −50.6 kJ·mol−1 to −37.2 kJ·mol−1) during the continuous SO2 capture; this was responsible for the ultra-high SO2 adsorption capacity achieved by[IRA-900][B(Im)4] at low partial pressures. Moreover, the thermal stability and reversibility of [IRA-900][B(Im)4] for SO2 capture and desorption were investigated. Six cycles where the adsorption was carried out at 20 °C and 10.13 kPa and the regeneration was performed at 70 °C demonstrated the adequate reversibility of [IRA-900][B(Im)4] for SO2 capture,showing the resin’s great potential for industrial desulfurization. Thus, the anion-functionalization strategy and multiple-site adsorption behavior provide new perspectives to realize effective SO2 capture from flue gas.
Key Words: Anion functionalized; Macro-porous resins; Poly(imidazolyl)borate anion; Multiple-site; SO2 capture
1 Introduction
With the rapid development of society and economy, surging energy consumption demands inevitably result in excessive combustion of fossil fuels including coal, oil and natural gas.Hence, the explosive growth of SO2emission in flue gases from the burning of fossil fuels has drawn serious attention as SO2is a significant source of atmospheric pollution and vegetation deterioration, which has been considered as an intractable project for environment and human health1−3.However, situation is becoming even worse as continuous dependence upon fossil fuels is inevitable for future decades,though the further exploration for renewable and cleaner sources of energy is never ceased. Accordingly, capture and sequestration of SO2has been recognized as the most promising means to reduce the SO2 emissions from fossil fuel combustions and thereby mitigate environmental burdens. For decades, desulfurization technologies have been used to realize SO2separation from flue gases in industrial settings, such as limestone scrubbing4−7, ammonia scrubbing8−10and organic solvent absorbents11−13. Although these technologies have quite high capture efficiency even at low partial pressure (e.g., 0.2%,volume fraction, SO2), several inherent drawbacks make the road to commercial deployment much less certain, including highly exothermic reaction enthalpy, inevitable byproducts(e.g., calcium sulfate) and the volatilization and corrosivity of solvents14,15. Therefore, the development of new materials and novel strategies those could efficiently, reversibly and economically capture of SO2, particularly at relatively low partial pressure, is urgently required, motivating chemists to pursue alternatives with outstanding performance for SO2capture and sequestration.
Considerable progress has been directed towards the design and preparation of porous materials as viable gas separation alternatives because of their relatively weak adsorption interaction, rendering the recovery of the gas and sorbents regeneration less energy intensive16,17. However, contrary to the significant amount of researches on CO2and hydrocarbons adsorption18−20, rare reports paid attention to the capture of SO2, especially related to low partial pressures. Actually, the acidic nature of SO2molecules brings tough challenges to the stability of the adsorbents, especially for metal-organic frameworks (MOFs), though some of which have realized extremely high adsorption capacity21−23. Besides, the desulfurization of flue gas is always carried out at low SO2partial pressure, which means strong interactions between the adsorbents and SO2are required, making the adsorption and release of SO2 by adsorbents hard to balance24. Both cases limit the scale-up application of the SO2sorbents for industrial desulfurization.
During the past decades, ionic liquids (ILs) have been proposed as promising sorbents for exhaust gases capture and sequestration, owing to the unique properties including negligible volatility, non-flammability and tunable properties25.Guaranteed by their virtually unlimited structural tunability,significant amounts of task-specific ILs have been developed as efficient sorbents for SO2capture26−29. In 2004, Han and co-workers reported the pioneering example of SO2chemisorption through employing the base-functionalized ionic liquid 1,1,3,3-tetramethyguanidinium lactate ([TMG][lactate]),realizing the equimolar SO2uptake at 40 °C with 8% SO2partial pressure24. Recently, Wang and co-workers developed azole-based ILs for SO2capture through multiple-site chemical adsorption strategy30−33. As these ILs providing more interaction sites between the anion and guest gas, high adsorption capacity can be achieved successfully. Due to the presence of the conjugated structure of the azole-based anions,the SO2adsorption enthalpy decreased sharply when one anion binding one more SO2molecules, which confined the effective SO2uptake at low partial pressures as a consequence, making this method less potential in practical flue gas desulfurization34.However, the functionalization method and multiple-site adsorption strategy applied by ILs inspire us new insights and solutions to tune SO2adsorption capacity of the sorbents35.Through a simple step of anion exchange, the physical and chemical properties of ILs as well as SO2adsorption capacity can be reasonably tuned.
The commercial available macro-porous anion exchange resin, [IRA-900][Cl], has been widely employed as porous polymeric supports for heterogeneous catalytic reagents or water treatment. While the application as gas sorbents is limited due to the relatively poor porosity as well as the low surface area compared to other porous materials such as zeolites,MOFs, etc.36,37. However, the anion exchangeable characteristic with abundant loading capacity (≥ 3.5 mmol·g−1) promises it easy to be functionalized through specific anion exchange as ionic liquids and possess great potential in the gas adsorption area38. Normally, the high adsorption capacity of SO2by azole-based ILs relies heavily on multiple-site chemical interaction of the anion with SO2, particularly at low pressures.We believe that immobilizing the specific functional anion groups into the porous resin as porous adsorbents can make the SO2 adsorption capacity improved and tuned.
Herein, we report the successful synthesis of various azole-based anion functionalized macro-porous ion exchange resins [IRA-900][An] as the SO2adsorbents, which were simply prepared through neutralizing the corresponding weak donors with the basic hydroxyl resin [IRA-900][OH]. Through the multiple-site interaction of azole-based anions with SO2, the ultra-high SO2adsorption capacity of >10 mmol·g−1could be realized at 20 °C and 101.3 kPa. Furthermore, the poly(imidazolyl) borate anion [B(Im)4] can afford four uniform reactive sites to bind at least four SO2molecules, which has been investigated by IR spectroscopy and density functional theory (DFT) calculations. The ultra-high SO2 uptake performance can still be maintained at low pressure (10.13 kPa)by [IRA-900][B(Im)4] with good reversibility, which is superior to other normal azole-based anion functionalized resins and reported sorbents, providing a promising strategy for desulfurization from flue gas.
2 Experimental
2.1 Materials and reagents
Imidazole (Im), trizole (Triz), tetrazole (Tetz) and sodium borohydride (NaBH4) were all obtained from Aldrich.Macro-porous resin [IRA-900][Cl] was purchased from the Dow Chemical Company. All chemicals were obtained in the highest purity grade and used without further purification. All the anion functionalized adsorbents [IRA-900][An] as well as[IRA-900][Cl] were dried under vacuum at 60 °C for 24 h before used to reduce the possible traces of water.
2.2 Experimental and synthesis procedures
2.2.1 Synthesis of sodium tetrakis(1-imidazolyl)borate
Sodium tetrakis(1-imidazolyl)borate Na[B(Im)4] was prepared by the reported procedure39. In a typical experiment,sodium borohydride NaBH43.78 g was mixed with 54.5 g of imidazole in a three necked flask with nitrogen atmosphere.Firstly, the flask was heated in the oil bath at 90 °C for 1 h and the imidazole was found to be melted. Then the mixture was stirred slowly with rising the temperature to 230 °C until about 2.24 L of hydrogen was evolved. The mixture was cooled and then poured into 200 mL of acetone. The resulting mixture was filtered and recrystallized from ethanol. The solid was dried at 130 °C (2 mm) for 12 h and obtained in 91% yield as white solid.1H NMR (400 MHz, D2O): 7.12 (s, 4H), 6.83 (s, 4H),6.64 (s, 4H). MS (negative ion): 279 m/z (B(Im4)−).
2.2.2 Synthesis of hydrogen tetrakis (1-imidazolyl)borate
The free acid hydrogen tetrakis(1-imidazolyl)borate H[B(Im)4] was prepared by adding an equimolar amount of hydrochloric acid to a fairly concentrated aqueous solution of the appropriate sodium tetrakis(1-imidazolyl)borate. In a typical experiment, Na[B(Im)4] 3.02 g was dissolved in 100 mL H2O at room temperature in a flask. To this solution was slowly added 10 mL of 1.0 mol·L−1HCl with stirring when the pH of the resultant solution reached neutrality. White precipitate was collected by filtration and dried at 130 °C (2 mm) for 12 h,affording the product with 95% yield.1H NMR (400 MHz,D2O): 7.88 (s, 4H), 7.32 (s, 4H), 7.15 (s, 4H).13C NMR (101 MHz, D2O): 138.81, 124.71, 122.45. MS (negative ion): 279 m/z (B(Im4)−).
2.2.3 Preparation of the anion functionalized sorbents[IRA-900][An]
The anion functionalized resins [IRA-900][An] were prepared as the following procedure: 10 mL of the fresh resin[IRA-900][Cl] was washed repeatedly with 1 mol·L−1NaOH in an ion-exchange column until no free chloride can be detected with the aqueous HNO3/AgNO3, followed with the leaching of deionized water until neutral effluent was found. Hence, the hydroxide resin [IRA-900][OH] with strong basicity was obtained. The amount of chloride exchanged was measured via argentometric titration of the leach solution collected. Typically for the synthesis of [IRA-900][B(Im)4], equimolar HB(Im)4was added to the flask with [IRA-900][OH] immersed in ethanol. The mixture was then slowly stirred at room temperature overnight. Subsequently, the solvent and water generated were removed by heating at 60 °C under 100 Pa overnight.
To investigate the effect of the anion on SO2adsorption capacity, other types of azole-based anions [Tetz] and [Triz]were supported on the resin through the neutralizing reaction of[IRA-900][OH] with the corresponding proton donors using the method mentioned above.
2.2.4 Determination of SO2adsorption capability
The SO2adsorption capacities of the anion functionalized resins were measured using the weighing method. In a typical SO2 adsorption experiment, SO2 at atmospheric pressure was pumped into a 5 mL glass container containing 0.3 g of the adsorbent at a flow rate of 60 mL·min−1. The glass container with an inner diameter of 10 mm was partly immersed in an oil bath at the desired temperature such as 20 °C. The amount of SO2adsorbed was determined at regular intervals by the electronic balance with an accuracy of ±0.1 mg until equilibrium. The resin was regenerated by heating at 70 °C under N2atmosphere for 30 min when the whole weight tended to equilibrium.
2.2.5 Computational details
Quantum chemical calculations were performed using Gaussian 09 programs package based on the DFT calculations.We followed the widely used B3LYP/ 6-311++G(d,p) method for configuration optimization and frequency calculations of the functionalized anions [An] and [An]-SO2 complexes, which has been proved to be able to provide results consistent with experimental values40–43. The natural bond orbital (NBO)charges of electronegative nitrogen atoms as multiple reactive sites were also measured to evaluate the binding energy in SO2-anion interactions.
3 Results and discussion
3.1 Design and characterization of anion functionalized resins
The anion functionalized sorbents were prepared on the basis of deprotonation of weak proton donors with the basic resin[IRA-900][OH], which was obtained by the anion-exchange method44. To investigate the effect of the anion on SO2 adsorption capacity, normal azole-based anions [Tetz] and[Triz] as well as poly(azolyl)borates anion [B(Im)4] were selected and supported on the resin, which has been shown in Scheme 1.
The anion functionalization process was monitored by FT-IR spectroscopy as Fig. 1. Compared with [IRA-900][Cl], the adsorption bands at 1242, 1143, 1075 and 683 cm−1, which was attributed to the presence of C=N and N=N bonds, could be found in the azole-based anions functionalized resins.Additionally, elemental analysis results (Table S1) showed evident increase of the nitrogen content in the functionalized resins compared with the initial [IRA-900][Cl], demonstrating the functional anions were immobilized into the resin successfully.
Porosity has been demonstrated as a crucial role in the gas capture of the adsorbent materials. Hence, the anion functionalized resins [IRA-900][An] were characterized by nitrogen adsorption/desorption isotherm measurements on Micromeritics ASAP 2020. The surface area was determined by multipoint BET method. The total pore volume was evaluated at a p/p0 close to 0.995. Table 1 showed the N2 adsorption data of [IRA-900][Cl] and azole-based anion functionalized resins.Contrary to many other adsorbents with abundant surface area,the BET surface area of the fresh resin [IRA-900][Cl] was relatively low (only about 20 m2·g−1). Taking this into consideration, choosing this material as a support for further functionalization may be not a good choice as the poor surface area would limit the physical adsorption. However, contrast to physical impregnation and chemical graft method, obvious decrease of the surface area was not found when chloride was exchanged by other functional anions. Therefore, the anion-exchange process avoid pore channels constricted and blocked to the maximum extent since the size of the functionalized anion was not so large compared with other adsorption groups45. As a result, the surface area and pore volume could be reserved through the functionalization strategy of the tunable anion46.
3.2 SO2 adsorption by azole-based anion functionalized resins
We have demonstrated in our previous work that azole-based ILs possess multiple reactive sites toVia the interaction between multiple electronegative nitrogen atoms of theazole-based anions and the acidic SO2, extremely enhanced SO2capacity can be realized. The similar phenomenon can also be found in the SO2adsorption results by the azole-based anion functionalized resins, which was shown in Table 2. At 101.3 kPa and 20 °C, all the anion functionalized resins displayed the highly capture of SO2 with the adsorption capacity of > 10.0 mmol·g−1. Additionally, at this adsorption condition, the initial resin [IRA-900][Cl] also exhibited a high SO2uptake of 10.75 mmol·g−1due to the interaction between halogen and SO2.Among them, [IRA-900][B(Im)4] has the highest adsorption capacity of 11.18 mmol·g−1.

Scheme 1 Structures of the anion functionalized macro-porous resins for SO2 capture.

Fig. 1 Infrared spectrum of each anion functionalized resin using the ATR method.blue: [IRA-900][B(Im)4]; green: [IRA-900][Triz]; red: [IRA-900][Tetz];grey: [IRA-900][Cl]. Color online.
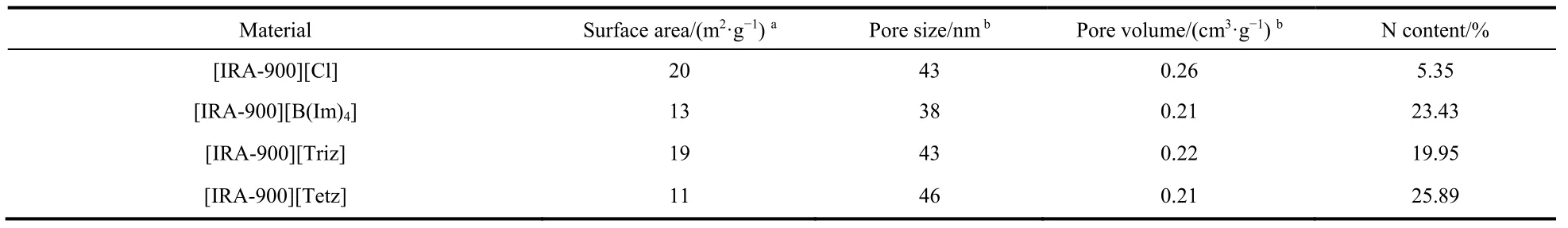
Table 1 Structural features and nitrogen content for the original resin [IRA-900][Cl] and anion functionalized resins [IRA-900][An].
The SO2 adsorption capacities by anion functionalized resins at low pressure were further investigated and the adsorption curves were shown in Fig. 2. At 10.13 kPa and 20 °C, the adsorption capacity above 4 mmol·g−1can still be maintained by these functionalized resins. Contrast to many adsorbents whose adsorption capacity drop drastically as SO2partial pressure decreases47,48, the anion functionalized resins were able to keep the considerable high adsorption capacity even at 10.13 kPa. Moreover, it was worth noting that the poly(imidazolyl)borates functionalized resin [IRA-900][B(Im)4]were capable to maintain the SO2adsorption capacity of 10.62 mmol·g−1at 10.13 kPa and 20 °C, only a little decrease from 11.18·mmol g−1at 101.3 kPa, which was obviously superior to other normal azole-based functionalized resins. Based on this result, we also measured the SO2adsorption capacity of[IRA-900][B(Im)4] at 10.13 kPa and 40 °C, which was relevant to the post-combustion desulfurization condition. Adsorption experiment showed that the adsorption capacity of 8.4 mmol·g−1was achieved by [IRA-900][B(Im)4], which was superior to many other reported SO2sorbents (Table S2).
SO2capture at low partial pressure by these sorbents mainly depends on chemical adsorption of the anion with strong binding energies. Therefore, the SO2adsorption capacity at 10.13 kPa and 20 °C by these anion functionalized resins were also compared as molar ratio, which represented the molar SO2adsorbed by per mol functional anion. The loading capacity of functional anion immobilized on the resin could be estimated by the nitrogen content obtained from elemental analysis. The last column in Table 2 listed the molar ratio of SO2 to[IRA-900][An] at 10.13 kPa and 20 °C. Though the loading capacity of [B(Im)4] was only 1.86 mmol·g−1, much smaller than that of other normal azole-based anions on account of the heavier anion mass, the molar ratio of SO2to [IRA-900][B(Im)4] was 5.71, apparently higher than 1.45 of [IRA-900][Triz], 1.37 of [IRA-900][Tetz] and 1.14 of [IRA-900][Cl].These results indicated that the extremely high SO2 adsorption capacity by [IRA-900][B(Im)4] was originated from the highly efficient multiple-site adsorption of [B(Im)4] even at low SO2partial pressure.
3.3 Verification of SO2 adsorption mechanism
To understand the SO2 adsorption mechanism by anion functionalized resin and investigate the unique SO2adsorption capacity at low partial pressure of [IRA-900][B(Im)4], the adsorption process and regeneration of [IRA-900][B(Im)4] was monitored by IR spectroscopy. Fig. 3 presented the infrared spectrum of the [IRA-900][B(Im)4] sample during the SO2 capture process. Compared with initial [IRA-900][B(Im)4], new peaks at 954, 1176 cm-1emerged evidently after the adsorption of SO2at 10.13 kPa, attributable to the sulfate (S-O) stretch30.However, adsorption band at 1338 cm-1which was always on behalf of the physical adsorption of SO2was unapparent,proving that the highly capture of SO2 at low pressure mostly comes from chemical interaction between [B(Im)4] with SO2.Though chemical adsorption always means a high adsorption enthalpy, which may result in large amount of desorption residual that could not be ignored, it has not been found in the[IRA-900][B(Im)4] regeneration process. As shown in Fig. 3,the infrared adsorption peak at 954, 1176 cm-1related to chemically adsorbed SO2 disappeared and the spectrum of[IRA-900][B(Im)4] recovered to the initial shape,demonstrating the good reversibility of SO2capture by[IRA-900][B(Im)4].

Fig. 2 SO2 adsorption curves by anion functionalized resins at 10.13 kPa and 20 °C.blue:[IRA-900][B(Im)4]; green: [IRA-900][Triz]; red:[IRA-900][Tetz]; black: [IRA-900][Cl]. Color online.

Table 2 SO2 adsorption capacity of the anion functionalized resin.
Multiple-site adsorption methodology has been proven to be an effective mean to realize highly efficient SO2capture in our previous reports32,33. However, the superior adsorption behaviour performed by the [IRA-900][B(Im)4] to other normal azole-based anion functionalized resins at low pressure is worth to be studied. Hence, the DFT calculations was used to further investigate the SO2adsorption mechanism of [IRA-900][B(Im)4], which was performed on the Gaussian 09 programs package. Owing to the SO2capture at low pressures mainly depending on the chemical interaction between anion with SO2,thus we followed the widely used B3LYP/6-311++G(d,p)method for optimization and frequency calculations of the borate anion [B(Im)4] and [B(Im)4]-SO2 complexes. As shown in Fig. 4, contrary to conjugate structures of the normal azole-based anions [Triz] and [Tetz], the initial anion [B(Im)4]exhibited tetrahedral configuration with the central B atom attached to four imidazole rings via the B―N bonds. Upon this tetrahedral configuration, the negative NBO charge focused on the B atom was equally distributed over all the imidazole rings,with the NBO charge on B of [BH4] changed from −0.698 to 1.106 of [B(Im)4] (see Fig. S1). The NBO charges on the four electronegative nitrogen atoms were −0.526, −0.523, −0.525 and −0.524. With continuous SO2attachment, the initial tetrahedral configuration of the [B(Im)4] was still maintained and played a crucial role by breaking the conjugation and disrupting electronic communication among the imidazole rings, as no apparent decrease was found of the NBO charge on unreacted nitrogen atoms. Furthermore, we evaluated each adsorption enthalpy during the attachment of SO2to [B(Im)4]. Contrary to the normal azole-based anions as well as [Cl] which exhibited sharply decrease of the adsorption enthalpy with continuous capture of SO2, the adsorption enthalpy changed only a little from −50.6 kJ·mol-1of the first attachment to −37.2 kJ·mol-1of the fourth attachment. The bond length of the electronegative nitrogen atoms with SO2during the stepwise capture was also maintained. Therefore, we can speculate that the electronegative nitrogen atoms of the imidazole rings behaved as local reactive sites and each can react with SO2 forming the sulphate. Hence, combining the DFT calculations results with IR spectroscopy, the multiple-site adsorption mechanism and ultra-high adsorption capacity of[IRA-900][B(Im)4] at low partial pressures can be depicted.Attributing to one [B(Im)4] anion can provide four reaction sites to attach SO2 without evident adsorption enthalpy decline,at least 4 molar SO2can be adsorbed by per molar [B(Im)4]even at low partial pressures, which was consist with the extremely high SO2adsorption capacity of 10.62 mmol·g-1by[IRA-900][B(Im)4] at 10.13 kPa and 20 °C.
3.4 The study on [IRA-900][B(Im)4] as an ideal adsorbent for SO2 capture
The multiple sites interaction of [B(Im)4] with SO2ensured the ultra-high adsorption capacity of SO2by [IRA-900][B(Im)4]at 10.13 kPa and optimistic application prospects in industrial capture. However, the thermal stability and reversibility of sorbents are also important criteria for an effective capture process. Hence, the stability of the anion functionalized resins[IRA-900][An] was evaluated by the TGA method with the heating rate of 10 °C min-1. Shown in Figs. S2 and S3, all the resins exhibited similar thermogravimetric curves and weight loss was not happened until the temperature increased to 130 °C. Accordingly, 70 °C and N2atmosphere was chosen as the regeneration condition of [IRA-900][B(Im)4] for the desorption of SO2adsorbed at 10.13 kPa and 20 °C. In order to further investigate the stability and reversibility of [IRA-900][B(Im)4] for the SO2capture and regeneration, 6 adsorption/desorption cycles were carried out, which was shown in Fig. 5.It was clear that the high capacity SO2adsorption at low partial pressure by [IRA-900][B(Im)4] was well maintained in spite of a little decline, indicating that the SO2adsorption process by[IRA-900][B(Im)4] was reversible.

Fig. 5 SO2 adsorption/desorption by [IRA-900][B(Im)4] for 6 cycles.SO2 adsorption was carried out at 10.13 kPa and 20 °C,desorption was performed at 70oC under N2 atmosphere.
4 Conclusions
In summary, we have developed the anion functionalized strategy of the macro-porous resins and applied these azole-based resins [IRA-900][An] for SO2capture. At 101.3 kPa and 20 °C, the ultra-high adsorption capacities > 10 mmol·g−1of SO2were achieved successfully through the multiple-site adsorption mechanism by azole-based anions. At low partial pressure adsorption condition (10.13 kPa and 20 °C), the poly(imidazolyl)borate functionalized resin[IRA-900][B(Im)4] exhibited the amazing SO2adsorption capacity of 10.62 mmol·g−1, only a little decrease from 11.18 mmol·g−1at 101.3 kPa, which is obviously superior to other normal azole-based functionalized resins and many other reported sorbents. Combining the IR spectrum investigation and DFT calculations, the multiple-site adsorption mechanism of [B(Im)4] was verified without evident adsorption enthalpy decline during the continuous capture, which was responsible to the ultra-high SO2adsorption capacity achieved by[IRA-900][B(Im)4] at low partial pressures, providing new perspectives to realize effective SO2capture at desulfurization conditions. Above all, the anion functionalized strategy and multiple-site adsorption behavior provide an efficient approach for the design and synthesis of novel porous materials as sorbents.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
杂志排行
物理化学学报的其它文章
- Catalytic Electroreduction of CO2 to C2H4 Using Cu2O Supported on 1-Octyl-3-methylimidazole Functionalized Graphite Sheets
- Green and Cost-Effective Preparation of Small-Sized ZSM-5
- Physicochemical Properties of1-Methoxyethyl-3-Methylimidazolium Glycine
- Study on Solution Enthalpies of Ionic Liquids [Cnmim][H2PO4](n = 3, 4, 5, 6) by Using Pitzer’s Equation
- Influence of External Electric Field on Vibrational Spectrum ofImidazolium-Based Ionic Liquids Probed by Molecular Dynamics Simulation
- Self-Assembly Behavior of Amphiphilic Diblock Copolymer PS-b-P4VP in CO2-Expanded Liquids
