Catalytic Synthesis of Formamides with Carbon Dioxide and Amines
2018-09-18ZHANGYujingDAIXingchaoWANGHongliSHIFeng
ZHANG Yujing , DAI Xingchao , WANG Hongli , SHI Feng ,*
tate Key Laboratory for Oxo Synthesis and Selective Oxidation, Center for Green Chemistry and Catalysis, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, P. R. China.
niversity of Chinese Academy of Sciences, Beijing 100049, P. R. China.
Abstract: Carbon dioxide is a green C1 resource that can be efficiently recycled by catalytic transformation into value-added chemicals. Formamides are important intermediates and solvents that are used extensively in pharmaceutical, daily-chemical, and petrochemical industry. Therefore, it is worthwhile to synthesize formamides with CO2 and amines. In this review, the main advancements in the synthesis of formamides by using CO2 as the C1 feedstock with noble metal catalysts (Ir, Pd, Pt, Ru, Rh, etc.), non-noble metal catalysts (Ni, Mo, Cu, Fe, Co, Zn, Al, etc.), organocatalysts, and catalyst-free systems have been summarized. In addition, the role of the reducing agents such as H2, silanes, and boranes involved in these transformations has also been reviewed. In addition, the reaction mechanisms with the different catalyst systems have been discussed.
Key Words: Carbon dioxide; Amines; Formamides; Carbonylation
1 Introduction
Current research shows that human activities produce an excess of 3.9% CO2 with respect to the natural ‘carbon cycle’1,2.Such a drastic increase in CO2 emissions causes the remarkable rise of atmospheric temperature and the abnormal change of the global climate3–7. The main sources of excess CO2emitters come from power generation, public electricity and heat production from fossil fuel combustion. The fact that only 30%–35% of the chemical energy content associated with the carbon used in anthropic activities is used as various forms of energy, while the remaining 65%–70% is lost as heat that directly affects the atmosphere deserves attention8,9. CO2conversion and utilization are considered with much attention as it would promote carbon recycle, thus preserving resources,and creating a promising solution to the storage of renewable energy10,11. However, only a handful of industrial processes utilizing CO2as a C1building block because CO2is the most oxidized state of carbon, and the biggest obstacle for establishing industrial processes based on CO2is its low energy level. In other words, a large energy input is required to transform CO2into value-added fine chemicals. Therefore, a lot of explorations have been carried out to catalytically transform CO2 into useful chemicals under relatively mild conditions10–14.
Formamides are an important class of compounds that used as intermediates in fungicide and pharmaceutical syntheses,isocyanate, formamidine, and nitrile formation15–22.Formamides are also appeared as Lewis base organocatalysts in hydrosilylation reactions23and other transformations24,25.Moreover, the formyl group is a helpful protecting group of the amine functionality in peptide synthesis26. So it would be highly valuable if formamides synthesis can be realized with the reaction of amine s and CO2.
2 Formamides synthesis from CO2 and amines
2.1 Iridium catalyst system
In 1970, the first Ir based homogeneous catalyst was reported27. Several complexes were tested for the synthesis of DMF and the most active catalysts were (dppe)2CoH, (PPh3)2(CO)IrCl and (PPh3)3CuCl (dppe: 1,2-bis (diphenylphosphino)ethane) with turnover numbers (TONs) reaching 1200. The reactions were carried out at 125 °C, with an equivalent mixture of H2and CO2at a total pressure of 5.4 MPa (Table 1,entries 1 and 2). However, Rh or Pt based catalysts showed less activity in the N-formylation of amines with CO2/H2 under similar reaction conditions. It is a good start for homogeneous catalysts in the N-formylation of amines and CO2with H2as reductant.
The use of NH3as a substrate presents a further challenge for N-H bonds are potentially active in the formylation with CO2and H2. The iridium complex [IrCl(CO)(PPh3)2] was an effective catalyst for the synthesis of N-formylamine from NH3,CO2and H2, in toluene or methanol solutions at 125 °C(Scheme 1)28,29. After 10 days, a TON of 1145 was obtained.The methylated byproducts monomethylformamide (MMF)were also obtained, but the methyl origination from either the hydrogenation of CO2 or the MeOH solvent was unclear.
Several noble-metal-based catalysts prepared by impregnation methods were studied with dimethylamine as starting material at 140 °C for 10 h under 6 MPa of an equimolar mixture of CO2/H2by the group of Cao30. It was found that only catalysts based on Ir exhibit notable activity with appreciable DMF yields. Several Ir based heterogeneous catalysts with different treatment methods were tested in the synthesis of DMF (Table 2). It was found that 60.6% yield of DMF was achieved if the catalyst Ir/HAS-TiO2-A was applied.When extending the reaction time to 16 h, 93.5% yield was obtained and the catalyst still maintained excellent activity after reused for five times.
2.2 Palladium catalyst system
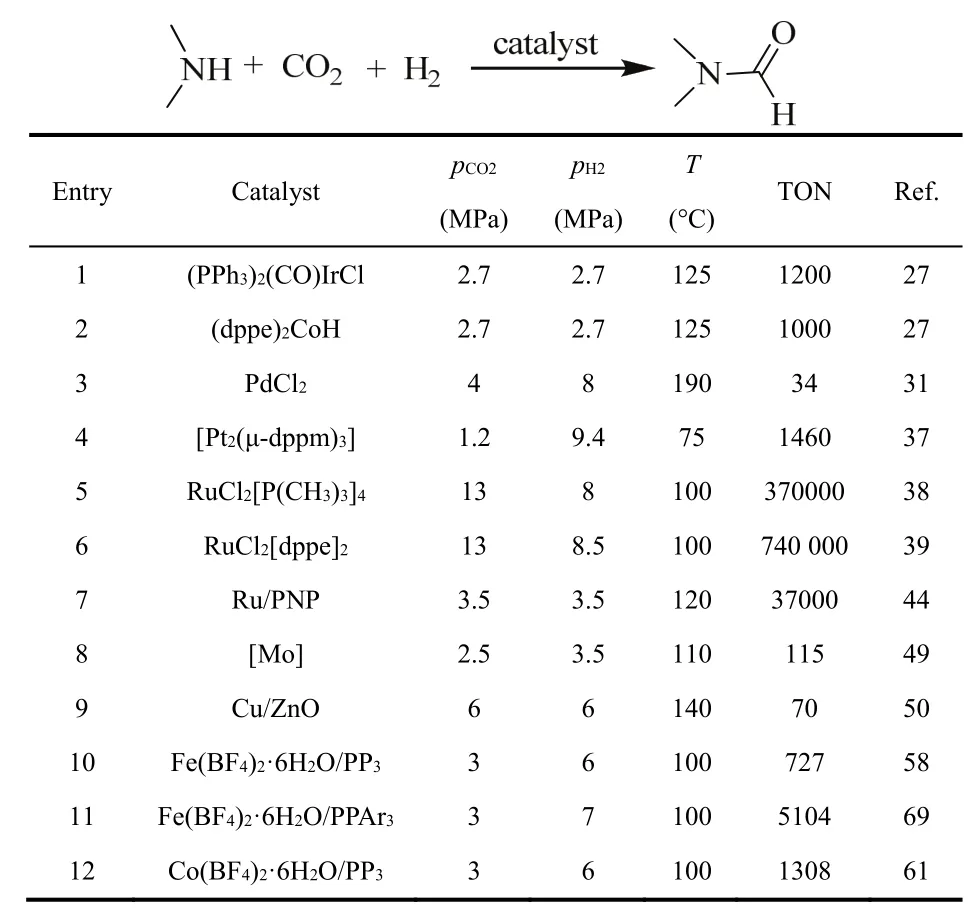
Table 1 List of catalysts for DMF synthesis.

Scheme 1 Formylation of ammonia promoted by [IrCl(CO)(PPh3)2] 29.
Kudo et al. demonstrated that simple PdCl2salt with a catalyst loading of about 3% (molar fraction) is an effective catalyst for the synthesis of DMF31. The reaction was performed under the condition of 4 MPa of CO2, 8 MPa of H2,170 °C with potassium bicarbonate as the base and the best result was obtained at 190 °C (Table 1, entry 3).
Based on above study, in 1986, Morimoto et al. developed a new palladium based catalyst system for the synthesis of diethylformamide32. An optimized ratio of sodium formate and 2-methoxyethanol (0.05 mmol·mL−1) in the presence of a catalytic amount of PdCl2(MeCN)2(2.5%, molar fraction)under an atmospheric pressure of CO2 was sufficient to obtain 18.7% diethylformamide without hydrogen. However it is unclear whether CO2or sodium formate is the source of the formyl group in this transformation, because formate reagent can act as a formyl donor or a reducing agent for CO2. They further demonstrated that in the presence of a well-defined ratio of PPh3/MeCN/CCl4, tetraethylurea could also be obtained without any reducing agent.
Our group prepared Pd/Al2O3-NR-RD, Pd/α-Al2O3-RD and Pd/γ-Al2O3-RD by the reductive deposition of nano-Pd particles on Al2O3-NRs and the Al2O3-NRs was prepared by a modified hydrothermal method using AlCl3as a precursor,1-butyl-3-methyl imidazolium chloride (BMImCl) as a template and (NH4)2CO3as a precipitant33. There is no reaction occurred under catalyst free conditions or in the presence of Al2O3-NRs (Table 3, entries 1 and 2). Pt, Ru or Rh/Al2O3-NR-RD, Pd/α-Al2O3-RD and Pd/γ-Al2O3-RD exhibited poor catalytic performance (Table 3, entries 3–10).Pd/Al2O3-NR-DP catalyst was prepared by deposition precipitation (DP) method and Pd/Al2O3-NR-IP was prepared by impregnation (IP) method. The pressure ratio of CO2to H2,solvents and reaction temperatures were screened. They found that under the same conditions of CO2/H2(1/2 MPa) at 130 °C with octane as solvent, Pd/Al2O3-NR-RD showed highest activity in the catalytic formylation of secondary amines but it was not fit for the formylation of primary amines or aniline.The redox properties of the catalysts were investigated by temperature-programmed-reduction (TPR) and the results showed that the amount of reducible Pd species in these catalysts was consistent with the catalytic performance.
Pd NPs decorated over hypercrosslinked microporous polymer (Pd@HMP-1) was prepared as a catalyst for formylation of amines with CO2, and diphenylmethylsilane or H2as reducing agent, and the best catalytic activity was achieved at 60 °C in dioxane and H2O mixed solvents, CO2(1 MPa) with diphenylmethylsilane as reducing agent reagent34.The yield of N-methylformanilide was increased with augmenting CO2pressure and maximum yield was achieved at 1 MPa pressure, since then yield remained almost constant. Up to now, the reaction conditions in this catalyst system are the mildest among the reported heterogeneous catalysts for N-formylation of CO2and amines with H2as reducing agent.
Following, our group prepared the Pd(NH3)xCly/C catalyst,which possesses rich hydroxyl groups on carbon surface, can be a nice catalyst for the reaction of amine with CO2/H235. The hydroxyl group density was determined by Boehm titration analysis, and it was found that the yield for N-formyl piperidinewas improved with the increasing of hydroxyl group density.After detailed study, the results suggested that in the N-formylation reaction, the carbamate was first formed from amine and CO2 in the presence of Pd/C-3 and KOH and then the in situ generated carbamate was hydrogenated to give the formylation product.

Table 2 DMF synthesis from CO2, H2 and aqueous NHMe2 over different DP-derived TiO2 supported Ir catalysts a 30.

Table 3 Reaction conditions optimization with 1-methylpiperazine formylation by CO2/H2 as model reaction a 33.
Pd-Au bimetallic supported on polyaniline-functionalized carbon nanotubes (Pd-Au/PANI-CNT ) was used as catalyst for N-formylation of amines with CO2/H2 (3.5 MPa/3.5 MPa)36. It was found that after mixing Pd and Au, N-formylpyrrolidine yield increased as a decrease of Pd/Au molar ratio in Pd-Au/PANI-CNT, producing a maximum of 98.3% yield at Pd/Au molar ratio of 1 : 1 at 125 °C. It was found that Pd atoms should be the true active sites for the hydrogenation reaction and the N-formylation reaction might occur mainly over Pd atoms or over the interface between Pd atoms and Au atoms for the bimetallic Pd-Au/PANI-CNT catalyst.
2.3 Platinum catalyst system
[Pt2(µ-dppm)3] (dppm: bis(diphenylphosphino)methane)was proved to be an active catalyst for the synthesis of DMF starting from DMA and carbon dioxide (1–1.2 MPa) in the presence of molecular hydrogen (6.7–9.4 MPa) at 75 °C in toluene (Table 1, entry 4)37. Importantly, the authors demonstrated that DMF could be achieved under very mild reaction conditions, i.e., 0.1 MPa of each of the three gas reactants, even at room temperature. Moreover, they also demonstrated that the formation of DMF is readily reversible by heating DMF at 150 °C in the presence of [Pt2(µ-dppm)3]under low pressure of H2(0.1 MPa).
2.4 Ruthenium catalyst system
An superduper procedure was developed for the synthesis of DMF with TONs up to 370000, and with overall rates up to 10000 h−1by Noyori et al. in 1994 (Table 1, entry 5)38. The reaction was performed under 8 MPa H2 and 13 MPa supercritical CO2(scCO2), and the applying of a well-defined homogeneous ruthenium catalyst, namely (RuCl2[P(CH3)3]4),was the key success of this system. Subsequent studies by the group of Baiker et al. in 1997 found that the best catalyst system among a series of ruthenium complexes of the formula[RuCl2L2] (L: dppm, dppe or dppp (1,3-bis(diphenylphosphino)propane)) was RuCl2(dppe)239. The reaction was performed at 100 °C in the presence of 13 MPa CO2and 8.5 MPa H2with very high TONs of up to 740000 (Table 1, entry 6). Under these conditions, 530 kg of DMF could be produced within 2 h.Formylation of morpholine with ‘supercritical’ CO2 using the bidentate ruthenium catalyst RuCl2(dppe)2 afforded high N-formylmorpholine production rate at almost 100%selectivity40.
Up to now, highly efficient homogeneous catalysts have been identified for the synthesis of dialkylformamides from CO2and amines, but no catalysts have been found for the preparation of formanilide from CO2 and anilines. Jessop et al. observed the homogeneously catalyzed conversion of aniline, CO2and H2into formanilide, and under the optimized conditions, i.e., 5 mmol aniline, 3 μmol RuCl2(PMe3)4, 12 MPa CO2, 8 MPa H2and 10 mmol DBU (1,8-diazabicyclo-[5.4.0]undec-7-ene),100 °C, 23 h, 85% yield (1,400 TON) of formanilide was obtained (Scheme 2)41. Interestingly, in the absence of H2,carbanilide is formed. Further study revealed that aniline by itself does not react with CO2to form a carbamate, whereas it does so in the presence of DBU or a guanidine base.
In 2005, the highly active in situ formed Ru catalysts by adding a suitable phosphine ligand, 1,2-bis(diphenylphosphino)ethane (dppe) or triphenylphosphine(PPh3), to the reaction mixture containing RuCl3as pre-catalyst was developed42. The formylation of 3-methoxypropylamine as a test reaction was investigated. It is shown that the catalytic performance of the in situ generated homogeneous catalysts is comparable to that of RuCl2(dppe)2 and RuCl2(PPh3)3, and high activity and virtually 100% selectivity were obtained.Afterwards, a 5% (mass fraction) Ru/Al2O3catalyst modified with a phosphine (dppe or PPh3) was used to catalyze the formylation of 3-methoxypropylamine, and it showed good activity and 100% selectivity43. The catalytically active species were found to be a homogeneous chlorine free Ru-dppe complex that formed under reaction conditions not the phosphinemodified ruthenium particles. Besides, it is found that the presence of dppe led to dissolution of ruthenium and formation of a highly active homogeneous Ru(dppe)2X2complex, which was even sufficiently active to achieve high conversion at very small concentrations (50 × 10-6Ru in the reaction mixture).
The group of Ding developed an elegant Ru/PNP pincer complex catalyst (Scheme 3), which showed good catalytic performance for N-formylation of different amines with CO2and H244.
They disclosed that the reaction progresses well in THF at 120 °C under a CO2/H2partial pressure of 3.5/3.5 MPa/MPa, to afford formamides in good to excellent yields with 0.01%(molar fraction) 1a. It is worth noting here that the robust catalyst 2a at a loading of 0.002% (molar fraction) (S/C =50000) was used to synthesize DMF, and after being recycled for 12 runs without significant loss in activity (TONs ranging from 22400 to 37000) (Table 1, entry 7).

Scheme 2 Ru-promoted synthesis of formanilide 41.
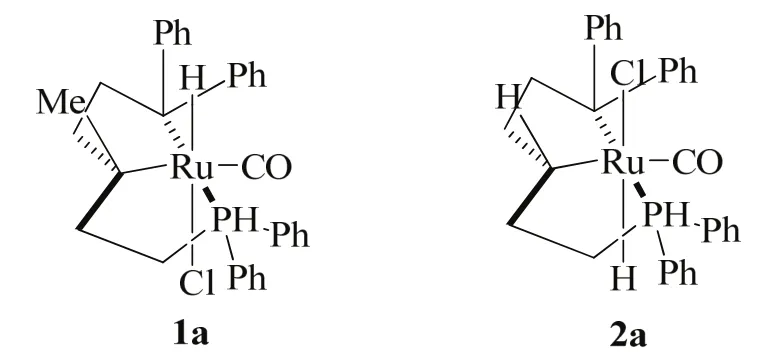
Scheme 3 The catalysts employed in the of N-formylation of amines 44.
The selective formation of dialkyl formamides through photochemical CO2reduction catalyzed by a [Ru(bpy)2(CO)2]2+(bpy: 2,2′-bipyridyl)/[Ru(bpy)3]2+/Me2NH/Me2NH2+system in CH3CN was reported45. In this process, a ruthenium carbamoyl complex ([Ru(bpy)2(CO)−(COMe2)]+) formed by the nucleophilic attack of Me2NH to [Ru(bpy)2(CO)2]2+worked as the intermediate to DMF. The ESI-MS spectrum of a CH3CN solution of [Ru(bpy)2(CO)2]2+containing 0.5 mol·L−1Me2NH indicates the generation of [Ru(bpy)2(CO)(CONMe2)]+(m/z 514.11), and also contains small peaks owing to[Ru(bpy)2(CO)(COOH)]+(m/z 487.11). Similar photochemical CO2 reductions with R2NH and R2NH2+(R = Et, nPr, or nBu)also afford the corresponding dialkyl formamides (R2NCHO)together with HCOOH as a by-product.
The time-dependent electronic absorption spectra of a CH3CN solution of [Ru(bpy)2(CO)2]2+(0.50 mmol·L−1) were monitored by rapid scanning spectroscopy upon the addition of excess amounts of R2NH (R = Me and Et) (Fig. 1a,b). The rate constants Kobs determined by analysis of the time course data at 400 nm were 165 and 4.4 s−1for the reactions of[Ru(bpy)2(CO)2]2+with Me2NH and Et2NH, respectively (Fig.1c,d).
Similarly, the pseudo first-order rate constants for the reaction of [Ru(bpy)2(CO)2]2+with n-Pr2NH and n-Bu2NH were also determined, and the results are summarized in Table 4. The rate constant of the reaction of [Ru(bpy)2(CO)2]2+with Me2NH is close to two orders of magnitude larger than those obtained with Et2NH, nPr2NH, and nBu2NH. The selectivity between R2NCHO and HCOOH was found to depend on the rate of[Ru(bpy)2(CO)(CONR2)]+formation.
2.5 Rhodium catalyst system
A Rh catalyst system combination of Rh2(OAc)4(OAc =acetate) and inorganic O-donor bases such as K2CO3and Cs2CO3, was buildup by the group of Mizuno. It was found that various kinds of hydrosilanes such as diphenylmethylsilane triphenylsilane, and triethylsilane could be utilized for the hydrosilylation of CO2in 0.1 MPa of CO2using simple Rh2(OAc)4and inorganic bases (Scheme 4). Following, the reaction of the silyl formate with amines (including aniline)produced the corresponding formamides and formic acid. Water and the Grignard reagent could also be utilized as nucleophilic reagents to react with the silyl formate, producing formic acid and the corresponding secondary alcohol, respectively46.
2.6 Nickel catalyst system
The first catalyst reported in the literature for formamides synthesis was Raney nickel in 193547. A variety of amines including secondary amines (piperidine, phenyl- ethylamine) as well as primary amines (n-amylamine) were converted to their corresponding formamides in low to moderate yields under quite harsh reaction conditions (6 MPa of CO2, up to 16 MPa of H2and temperatures up to 250 °C).
In another catalyst system, Ni(II) acetate or acetylacetonate salts combined in situ with ligands were evaluated as potential catalysts for CO2 hydrogenation to prepare formamides,including N-formylmorpholine, 2-ethylhexylformamide, and dimethylformamide from the corresponding amines48. From these initial screening results, the most active catalyst system for these reactions was found to be Ni(acac)2/dmpe (dmpe =1,2-bis(dimethylphosphino)ethane) in DMSO. The optimal reaction conditions were found to be amines (3.05 mmol),DMSO (0.5 mL), T = 135 °C, t = 21 h, CO2(6 MPa), and H2(10 MPa). Morpholine was formylated with up to 18000 TONs,which was the highest TON for the hydrogenation of CO2to formamides using any non-noble-metal-phosphine complex.With an appropriate selection of catalyst and reaction conditions, > 90%–98% conversion of amine to formamide could be achieved. Following, Garcıa et al. reported[(dippe)Ni(μ-H)]2(1) catalyzed CO2hydrosilylation promoted by Et3B to selectively give silyl formate (Et3SiOC(O)H) in high yields (85%–89%) in 1 h at 80 °C, and the silyl formate can be further used to produce formic acid, formamides, and alkyl formates (Scheme 5)48.

Fig. 1 Spectral changes of a) [Ru(bpy)2(CO)2]2+ solution upon addition of a) 0.5 mol·L-1 Me2NH and b) Et2NH, and variations in the absorption at 400 nm over time after the mixing of[Ru(bpy)2(CO)2]2+ and c) Me2NH or d) Et2NH 45.

Table 4 Redox potentials of [Ru(bpy)2(CO)2]2+ in the presence of various amines and the reaction rate constants between[Ru(bpy)2(CO)2]2+ (0.5 mmol·L-1) and various alkyl amines (0.5 mol·L-1) 45.
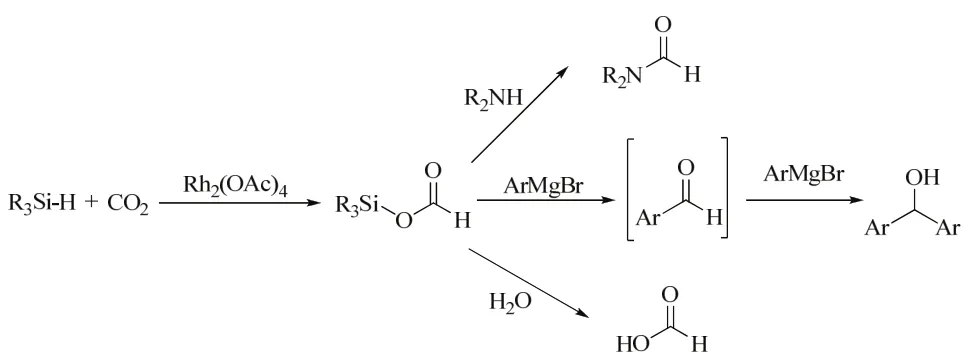
Scheme 4 Synthesis of silyl formates and their utilization46.
2.7 Molybdenum catalyst system
In 2001, the group of Ito reported a new class of well-defined molybdenum-silyl/ phosphorus catalyst for DMF synthesis (Table 1, entry 8)49. The reaction was performed under CO2/H2: 2.5/3.5 MPa at 110 °C. Even though the catalytic productivity is lower than those of noble metal catalysts reported, the introduction of a noble metal-free system is highly desirable for such transformations. It was found that the molybdenum complex 1 reacts under 1.7 MPa of CO2in toluene at room temperature to yield the formate complex 2 and complex 2 at 60 °C in benzene reverted to complex 1 (Scheme 6). Furthermore, complex 2 was proved to be active in the catalytic formation of DMF, suggesting that it could be a potential intermediate in the catalytic reaction pathway.
2.8 Copper catalyst system
Dimethylammonium dimethylcarbamate (DIMCARB) is often used as a source of dimethylamine as it could dissociate into dimethylamine and CO2above 333 K38,50. The group of Han used it as starting material to synthesize DMF catalyzed by Cu/ZnO. They discovered that the mole ratio between Cu and Zn has a significant effect on the catalytic capacity (Table 5).When the reaction was undertaken at 140 °C for 6 h under 12 MPa of an equimolar mixture of CO2/H2, 97% yield of DMF was obtained with a mole ratio of 3 : 2 (Table 1, entry 9)50.

Scheme 5 Synthesis of silyl formates and their transformations 48.

Scheme 6 Reversible insertion of CO2 in the Mo―H bond of complex 1 49.
On the basis of the knowledge in the literature and the results of the work, they proposed a tentative reaction mechanism for the synthesis of DMF from CO2, H2, and dimethylamine. In the reaction, H2is first activated by the copper and then reacts with CO2 to form the formate species on the copper surface. Following, the formate species could transform into DMF by two possible routes. One pathway is that the formate species on Cu are directly hydrogenated to formic acid and formic acid then reacts with dimethylamine to form dimethylamine formate which can be easily converted into DMF by releasing a molecule of H2O. The other one is that the formate species on Cu are first migrated onto the surface of ZnO, and then reacts with hydrogen activated by cooper to provide formic acid. Finally, the generated acid is combined with dimethylamine to form dimethylamine formate that dehydrates to afford DMF.
N-formylation of amines containing unsaturated groups using CO2 and H2 was achieved for the first time with a Cu(OAc)2-4-dimethylaminopyridine (DMAP) catalyst system51. The substrates with unsaturated groups, such as C=O, C=C, C≡N and ester groups, were converted to the desired formamides under the condition of P(CO2) = P(H2) = 4 MPa,metal precursor 10% (molar fraction) based on the substrate,THF 1.5 mL, 90 °C, and the unsaturated groups remained unchanged. For example, 1-cinnamylpiperazine was almost quantitatively transformed to the target product. The authors considered that the main reason for the excellent selectivity of the Cu(OAc)2-DMAP catalyst system was that it was very active for the N-formylation reaction, but was not active for the hydrogenation of the unsaturated groups.
The combination of Cu(OAc)2and bis(diphenylphosphino)ethane (dppe) was very efficient for the N-formylation of amines at room temperature and 0.1 MPa CO2, with only 0.1%(molar fraction) of catalyst loading and PhSiH3as a reducing agent in toluene52. It was found that solvent affected the reaction significantly. Toluene is the best solvent for the reaction and the yield of N-formylaniline was as high as 96%.In THF and 1,4-dioxane, the yields of N-formylaniline were 76% and 84%, respectively If the reactions were performed in other solvents such as dichloromethane, hexane and CH3CN,the reaction did not happen. The reducing agents such as Ph2SiH2 and PMHS did not afford any desired product at the reaction conditions.
Baba et al. reported the Cu-diphosphine complexes asefficient catalysts for hydrosilylation of CO253,54. Then in 2013, they demonstrated that Cu-diphosphine complexes were active for N-formylation of various amines under 0.1 MPa of CO2with PMHS as reducing agent (Scheme 7)55. It was found that the reactivity of amines was strongly affected by the steric hindrance, which induced regioselective N-formylation. In the copper-catalyzed reaction, the highest TON and TOF values were observed, as compared with previously reported catalyst systems using 0.1 MPa of CO2.
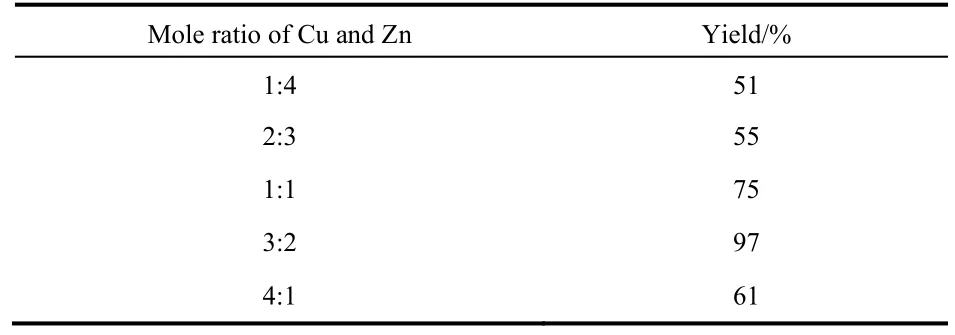
Table 5 Effect of the molar ratio of Cu to Zn in Cu/ZnO onthe yield of DMF a 50.

Scheme 7 Formylation of amines using CO2 and hydrosilane reducing agents and diphosphine ligand 55.
After detailed exploration, the proposed reaction mechanism for the N-formylation of amines with CO2 and hydrosilane is shown in Scheme 856. The initial step involves the formation of a Cu-hydrideeediphosphine complex from the Cu precursor,ligand, and hydrosilane (i). After the formation of the Cu-hydride complex, the catalytic N-formylation of amines is initiated. The Cu-hydride complex reacts with CO2or piperidino carbamic acid/carbamate to afford a Cu-formate (ii).Then, the Cu-formate reacts with piperidine in the presence of hydrosilane (iii), giving the N-formylpiperidine product and Cu-hydride complex (iv). The Cu-hydrideediphosphine catalyst was found to be highly active for the reduction of CO2to the Cu-formate intermediate.

Scheme 8 Proposed reaction mechanism of Cu catalyzed formylation of amines with CO2/hydrosilane 56.
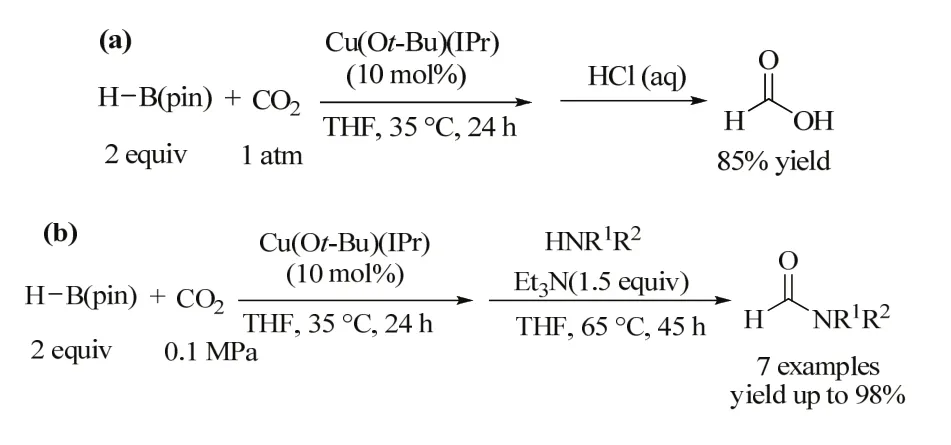
Scheme 9 Synthesis of formic acid and formamide derivatives from CO2 and amines using H-B(pin) 57.
Nozaki et al. tested the catalytic performance of Cu(Ot-Bu)(IPr) in the reaction of pinacolborane and CO2 because copper/NHC complexes are reported to be effective catalysts for carboxylation of various carbon nucleophiles by using CO257. After investigation, they found that Cu(Ot-Bu)(IPr) can catalyze the reaction of pinacolborane and CO2(0.1 MPa) in THF under mild conditions to give formic acid in 85% yield (Scheme 9a). Further investigation showed that H-B(pin) produced in the present catalyst system can be directly used as a formylation reagent of various amines(Scheme 9b).
2.9 Iron catalyst system
Recently, the formylation of dimethylamine to DMF was performed by the group of Beller by using Fe(BF4)2·6H2O as a catalyst and RP3 (tris[2-(diphenylphosphino)ethyl]-phosphine)as the ligand. In the condition of CO2/H2= 3/6 at 100 °C, a yield of 75% was obtained with a TON of 727 (Table 1, entry 10)58. Better result was observed when tetradentate P(PAr)3(tris(2-(diphenylphosphino)phenyl)phosphine) was used as the ligand, and DMF was obtained with TONs up to 5104 (Table 1,entry 11)59.
Another iron catalyst, i.e., Fe(acac)2, was also found to be active towards the formylation of amines and CO260. The reaction can occur in the presence of an atmospheric pressure of CO2(0.1 MPa) using PHSiH3as a reducing agent at room temperature (Scheme 10). Primary and secondary amines could be converted to the corresponding formamides in moderate to good yields. This was the first example of iron catalysts able to promote the reductive functionalization of CO2using hydrosilanes as reductants. It should be noted that the reaction is chemo-selective and tolerant to ketone and ester groups. At 100 °C, the catalytic system is also active for the methylation of aniline derivatives.
2.1 0 Cobalt catalyst system
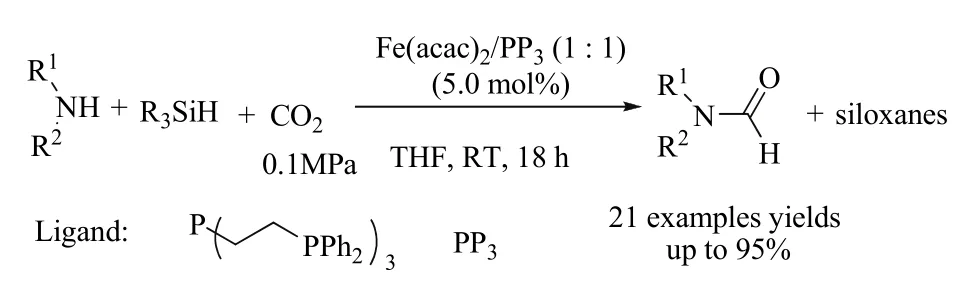
Scheme 10 Formylation of amines using CO2 and hydrosilane reducing agents 60.
A novel cobalt catalyst, i.e., Co(BF4)2·6H2O/PP3, exhibited nice catalytic activity with a TON of 1308 for the production of DMF (Table 1, entry 12)61. Lately, the novel paramagnetic Co(II) chloride complex Co(iPrPNHP)Cl2 1 was used to synthesize N-formamides in the presence of CO2 (3 MPa) and H2(3 MPa) using NaHBEt3(5%, molar fraction), tBuOK (5%,molar fraction) and complex 1 (5%, molar fraction) as catalyst in toluene (Scheme 11)62. Temperature is an important factor for the formylation reaction. For example, N-hexylformamide was afforded in 60% yield at 120 °C and in 99% yield at 150 °C after 36 h.
A plausible mechanism is proposed in Scheme 11. First, 1-Cl is generated from the treatment of complex 1 with 1 equiv., of NaBEt3H. A highly reactive unsaturated intermediate (A) is expected to be formed via the reaction of 1-Cl with tert-BuOK,which is reduced into the Co(I) hydride (B) under H2. Then, the insertion of CO2into Co―H bond gives the η1-formato complex (C). The presence of excess amine results in formate salt formation, likely by deprotonation of the coordinated amine of the pincer ligand. The formate salt liberates water and forms the formamide as well as regenerates the active intermediate A. However, direct nucleophilic attack on the format complex C by amine, following liberation of H2O from the metal center still cannot be excluded at this stage.
2.1 1 Zinc catalyst system
Liu et al. designed a fluoro-functionalized polymeric NHC-Zn complex (F-PNHC-Zn) via taking fluorous imidazolium salts as precursors through a two-step alkylation63.The resultant F-PNHC-Zn was applied as catalyst in the formylation and methylation of amines with CO2as a C1building block in the presence of organosilane. It was found that a broad range of amines could be converted to the corresponding formamides and methylamines with good yields,and the catalyst F-PNHC-Zn showed nice stability and easy recyclability.
Another Zn catalyst, i.e., Zn(salen) complex, together with quaternary ammonium salts was successfully developed for N-formylation reaction of amines using CO2 and hydrosilanes under solvent-free conditions (Scheme 12)64.
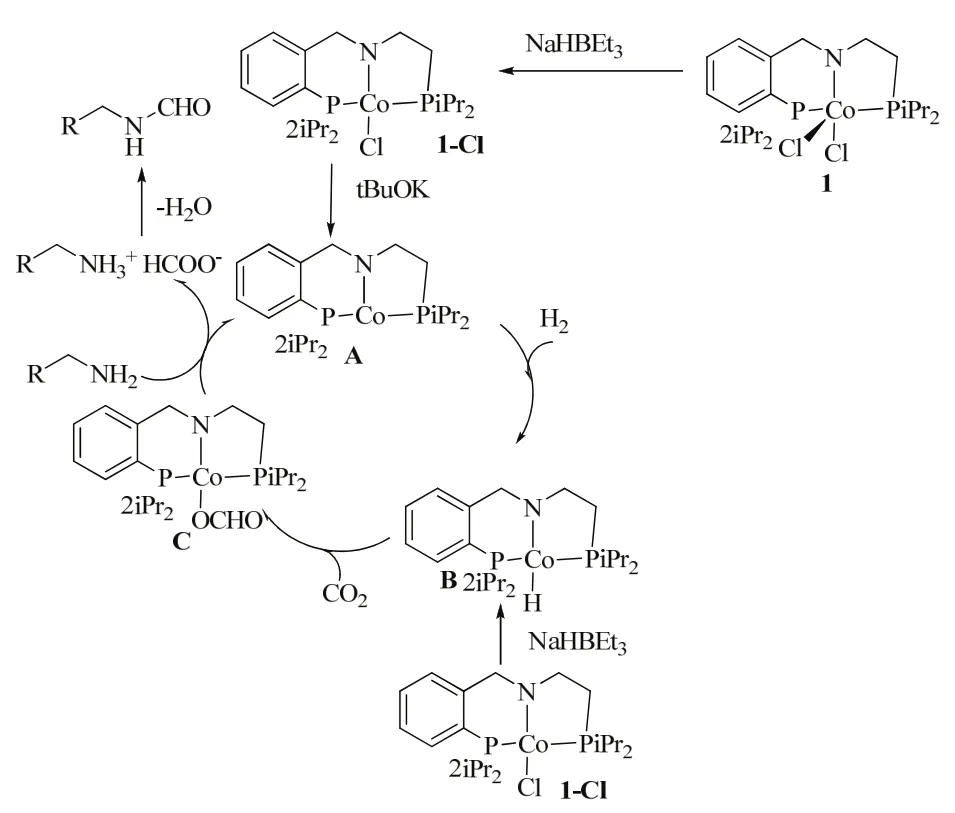
Scheme 11 Proposed mechanism for the N-formylation of amines using CO2 and H2 catalyzed by a Co-PNP complex 62.

Scheme 12 Zn(salen) complex 64.
They further envisioned the heterogeneous bifunctional porous organic polymers (POPs)-based catalysts, which were capable of easily recycling and presented high catalytic activity and selectivity. It was demonstrated that different kinds of amines including aliphatic, cyclic and aromatic amines were converted to the corresponding formamides in excellent yields at 25 °C within 15 h with 0.5% (molar fraction) Zn(salen)catalyst in the presence of 0.5 MPa CO2and PhSiH3. Also, this catalyst showed excellent substrate generality and tolerance to various functionalities.
2.1 2 Aluminium and alkali-metal carbonates catalyst systems
Recently, Bhanage et al. prepared a mesoporous Al2O3@MCM-41 for the formylation of amines and CO2with dimethylamine-borane (DMAB) as reducing agent, and up to 99% yield of the desired product was obtained (Scheme 13)65.It was the first aluminum catalyst able to catalytic the formamide synthesis from CO2 and amines with dimethylamine-borane as reductants.
K2CO3has also been used as an efficient catalyst for the formylation of amines by hydrosilylation of CO2using PMHS66.Under the optimized reaction conditions, i.e., 1 mmol amine,10% (molar fraction) K2CO3, 4 mmol PMHS (4.0mmol), 5 mL THF, 3 MPa CO2, 100 °C for 12 h, the formylation of morpholine was achieved in 99% yield. Cs2CO3 was another alkali-metal carbonate that was proved to be active for the formation of N-formylamines and N-methylamines utilizing amines and CO2(0.1 MPa) under mild conditions . The high selectivity can be easily controlled by varying the reaction temperature and the type of silanes67. A “cesium effect” on both reactions was observed by comparing the catalytic activity of various alkali-metal carbonates such as Li2CO3, Na2CO3,K2CO3and Rb2CO3. Combining experimental and computational studies, the probable reaction mechanism was proposed: (i) activation of Si―H by Cs2CO3, (ii) insertion of CO2 into Si―H, (iii) formylation of amines by silyl formate,and (iv) reduction of formamides to methylamines.
2.1 3 Metal free catalyst system
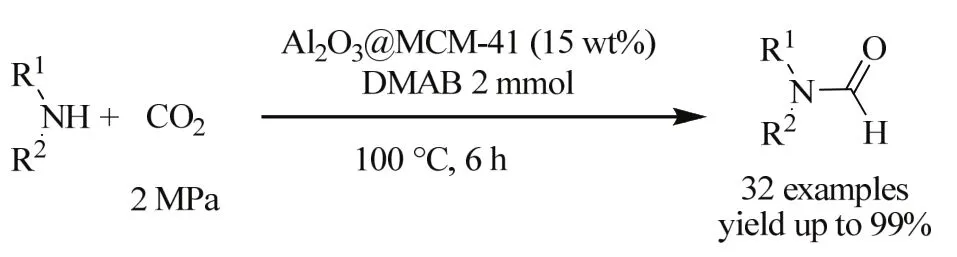
Scheme 13 Synthesis of formamide derivatives from amines and CO2 with DMAB as reducing agent 65.
In 2011, Cantat et al. for the first time reported the organocatalytic synthesis of formamides with CO2as a carbon source and hydrosilanes as reducing agents catalyzed by 1,5,7-triazabicyclo-[7.4.0]dec-5-ene (TBD) (Scheme 14, I)68,69.In fact, it is able to convert secondary amines to the corresponding formamides with good to excellent yields when the reactions are performed in THF at 100 °C with PhSiH3.Aromatic amines are proved to be active substrates as well,with yields ranging from 20 (for PhNH2) to 39% (MePhNH).However, no formamide was observed in the case of primary aliphatic amines such as n-hexylamine. Notably, the increased polarity of the solvent has a positive influence on the rate of the reaction and the best result was obtained in the absence of the solvent, where the polarity of the medium is increased by the formation of the formamide product.
From a mechanistic perspective, preliminary stoichiometric transformations suggested that TBD is able to activate the CO2 molecule to yield adduct 3 (Scheme 15)70. In the presence of morpholine, 3 is transformed into salt 4 in which the carbamate anion is efficiently stabilized by the guanidinium cation TBDH+through H-bonds. The reaction of 4 with one equivalent of phenylsilane affords the formamide product, the silanol as a by-product and the active TBD.

Scheme 14 Formylation of amines and CO2 using hydrosilane reducing agents 68,72,74,76.
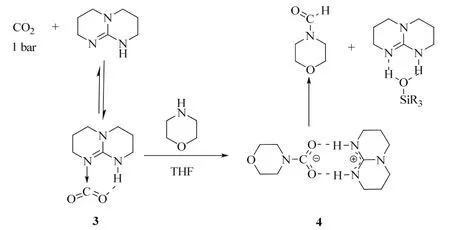
Scheme 15 Mechanistic investigation for the TBD-catalyzed formylation of morpholine 70.
Inspired by the seminal work of N-heterocyclic carbenes(NHCs) as efficient catalysts in the hydrosilylation of CO2to methoxysilanes71, Cantat et al. found that NHCs are able to promote the formylation of a wide scope of amines with CO2and hydrosilanes (Scheme 14, II)72. In the presence of IPr as the most reactive organocatalyst, primary and secondary amines were quantitatively converted to the corresponding formamides at room temperature. Notably, IPr is about 2000 times more reactive than TBD and this increased catalytic activity enabled the use of less reactive hydrosilanes as reducing agent.
Formylation of amines with another N-heterocyclic carbene catalyst and CO2as carbon source was achieved73. As the results shown in Scheme 16, formylation of several amino acid ester derivatives with NSC1 as the catalyst can be realized with high selectivity.
Poly-N-heterocyclic carbene (poly-NHC) materials were used as heterogeneous organocatalysts for the reduction of CO2to methanol and the formylation of N―H bonds with hydrosilanes as reducing agent73. Except the ease recycling and reusability property of the catalyst, the yields of products with the heterogeneous catalysts were lower than those with the homogeneous counterparts.
Recently, a 1,3,2-diazaphospholene catalyst system has been developed by the group of Kinjo74. By applying this catalyst,an atmospheric pressure of CO2 (0.1 MPa) was sufficient for the formylation of amines using PH2SiH2 as a reducing agent at room temperature (Scheme 14, III). Primary and secondary amines could be converted to the corresponding formamides in moderate to very good yields.
γ-Valerolactone (GVL), which is a natural chemical in fruits,can also be produced from carbohydrate-based biomass75. Due to the excellent properties of low toxicity, biodegradability and low vapor pressure, GVL has been recognized as a sustainable dipolar aprotic solvent for different reactions. Han et al. found that the formamides could be synthesized from amines, CO2(3 MPa) and organosilanes (2 equiv.,) at 80 °C in GVL without any additional catalysts (Scheme 14, IV)76.
Lactone compounds including γ-butyrolactone,δ-valerolactone and ε-caprolactone also showed the similar performance as GVL, while ethyl acetate and ethyl levulinate gave no formamides, indicating the crucial role of the lactonestructure (Table 6). The polarity of solvents might be responsible for the different results. The lactone structure might generate a dipolar aprotic solvent with strong polarity. In contrast, ethyl acetate and ethyl levulinate have weaker polarity than GVL.

Scheme 16 N-formylation and N-methylation of amines using NSC1 and PMHS 73.General conditions: amine, 0.5 mmol; NSC1,7.5% (molar fraction); PMHS, 20–300 μL; 50–100 °C; 24–48 h.
Liu et al. reported the initial work to use ILs as the functional solvents for the N-formylation reaction of different amines with CO2and phenylsilane. It was discovered that imidazolium-based ILs could catalyze the reaction at room temperature to produce the formamides in excellent yields(Scheme 17)77.
Unsymmetrical N,N-disubstituted formamides (NNFAs) was synthesized from primary amine, CO2and aldehyde promoted by ionic liquid 1-butyl-3-methylimidazolium chloride at room temperature. This approach features wide scopes of amines and aldehydes, and various unsymmetrical NNFAs could be obtained in good to excellent yields (Scheme 18). The ionic liquid can be reused for at least five runs without deactivation.
Recently, Gao et al. described the synthesis of two-dimensional (2D) covalent organic frameworks (COFs) by using a three-component, i.e., 4,4′,4′,4′′-(pyrene-1,3,6,8-tetrayl) tetraaniline (PyTTA), 2,5-dihydroxyterephthalaldehyde (DHPA) and 1,4-phthalaldehyde(PA). The COFs can be a nice support to immobilize IL(2-bromoethyl)-triethylammonium bromide via the formation of ether bond. This immobilized IL exhibited nice catalyticperformance in the formylation of amines with CO2and organosilanes. N-methylanilines with electron donating or electron-withdrawing groups were well tolerated, and up to 94% yields could be obtained78.
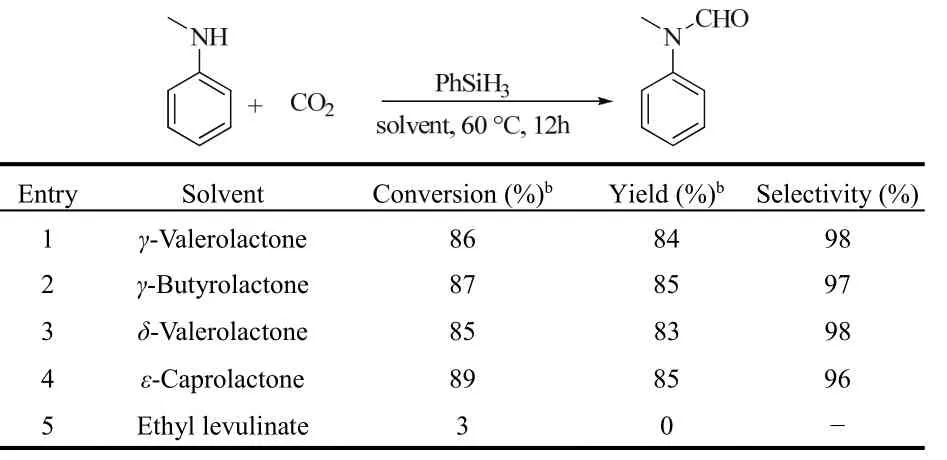
Table 6 Formylation of N-methylaniline using CO2 as the carbon source in various lactone-containing solvents a 76.

Scheme 17 Formylation of amines using CO2 and hydrosilane reducing agent 77.
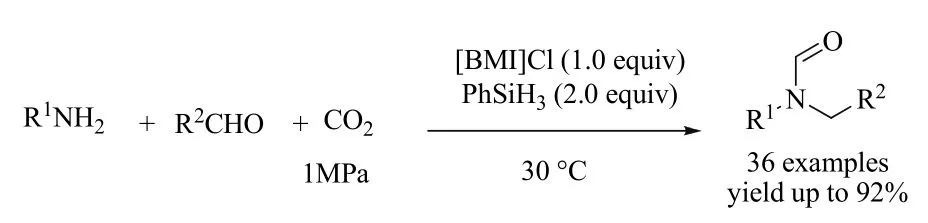
Scheme 18 One-step synthesis of unsymmetrical NNFAs through three-component coupling of primary amine, aldehyde and CO2 77.
Glycine betaine, which was widely observable in plants, is a kind of quaternary ammonium alkaloid possessing a zwitterionic structure (Scheme 19). As a biodegradable,harmless, cheap raw material, glycine betaine has been employed as a catalyst for the N-formylation reaction using CO2 as the carbon resource with organosilanes as the reducing agent79,80. A broad range of amine substrates could be converted to the desired product in 15%–98% yields under mild conditions.
Another organocatalyst tetrabutylammonium fluoride(TBAF) was developed for the reductive functionalization of CO2with amines to selectively afford formamides or methylamines with hydrosilanes81. Hydrosilanes show discriminatory reducing activity with different substituents.Thus, the formation of formamides and methylamines could be controlled by tuning hydrosilane types. Formamides were obtained exclusively with triethoxysilane under an atmospheric pressure of CO2.
2.1 4 Catalyst free system
An catalyst-free formylation of amines using CO2and hydrosilanes was developed with DMSO as solvent82. In order to clarify the mechanism of the reaction, in situ NMR (1H and13C) analyses were conducted and the formation of the intermediates A and B were confirmed by in situ29Si NMR analysis. Based on the investigations, a possible reaction pathway was proposed (Scheme 20).
Following, the catalyst-free N-formylation of amines using CO2as the C1 source and BH3NH3as the reducing agent was developed by the group of Wu83. The corresponding formylated products of both primary and secondary amines were obtained in good to excellent yields (up to 96% of isolated yield) under mild conditions. Furthermore, the BH3NH3by-product is water soluble simplifying the separation of the products.

Scheme 19 Chemical structure of glycine betaine 79, 80.
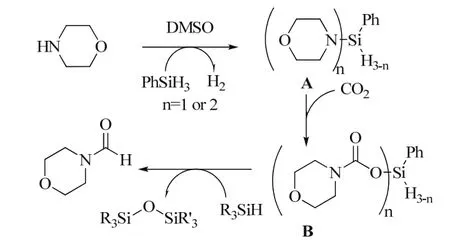
Scheme 20 Proposed mechanism for the N-formylation of amines using CO2 and hydrosilanes 82.
3 Conclusions
This review has covered the results of N-formylation of amines with CO2 as the carbonyl source. Although great progress has been made in this field, some challenges still remain to be addressed. First, although H2, silanes or boranes as reducing agents over different catalysts were discovered,normally, the reactions can progress under mild conditions with silanes or boranes as reducing agents but harsh reaction conditions are needed in the case of H2 as reducing agent. As H2 is currently the economic and green reducing agent in industry, it should be important to build efficient catalyst system with H2as reducing agent under mild reaction conditions. Moreover, detailed mechanistic investigations on the basis of in situ characterization techniques in the N-formylation of amines with CO2 will continue to play an important role in order to realize rational design of more effective catalysts. Finally, based on the results of mechanistic study, the development of multi-functional nano-catalytic materials with the ability of co-adsorption, activation and transformation of amines, CO2and H2under mild conditions is highly desirable.
杂志排行
物理化学学报的其它文章
- Catalytic Electroreduction of CO2 to C2H4 Using Cu2O Supported on 1-Octyl-3-methylimidazole Functionalized Graphite Sheets
- Green and Cost-Effective Preparation of Small-Sized ZSM-5
- Physicochemical Properties of1-Methoxyethyl-3-Methylimidazolium Glycine
- Study on Solution Enthalpies of Ionic Liquids [Cnmim][H2PO4](n = 3, 4, 5, 6) by Using Pitzer’s Equation
- Influence of External Electric Field on Vibrational Spectrum ofImidazolium-Based Ionic Liquids Probed by Molecular Dynamics Simulation
- Self-Assembly Behavior of Amphiphilic Diblock Copolymer PS-b-P4VP in CO2-Expanded Liquids
