Green Catalysis for Three-Component Reaction of Carbon Dioxide,Propargylic Alcohols and Nucleophiles
2018-09-18ZHOUZhihuaXIAShumeiHELiangnian
ZHOU Zhihua , XIA Shumei , HE Liangnian ,2,*
tate Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071,P. R. China.
ollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, P. R. China.
Abstract: Carbon dioxide (CO2) is one of the main greenhouse gases that can be utilized as a useful C1 source owing to its abundance, non-toxicity, and renewability. In fact, the transformation of carbon dioxide into valuable organic molecules has attracted considerable attention over the past decades. One-pot multicomponent reactions generally proceed with more than two different raw materials reacting in one pot, thus simplifying the reaction in operation and workup. In this regard, a three-component reaction of CO2, propargylic alcohols, and nucleophiles such as amines, water, and alcohols, to prepare useful carbonyl compounds (e.g., carbamates, oxazolidinones,α-hydroxyl ketones, and organic carbonates) is particularly appealing because of the advantages of step and atom economy. From a mechanistic point of view, the three-component reaction of CO2, a propargylic alcohol, and a nucleophile is a type of cascade reaction, involving the carboxylative cyclization of CO2 and propargylic alcohol, and subsequent reaction of a nucleophile with the in situ formed α-alkylidene cyclic carbonate. On the other hand, reactions involving CO2 are generally thermodynamically unfavorable because of the thermodynamic stability and kinetic inertness of CO2. Cyclic carbonates are widely used in organic synthesis, and their preparation from vicinal diols and CO2 represents a green synthetic method because biomass is utilized as the source of vicinal diols. However, the low yields of cyclic carbonates are obtained in most cases because of thermodynamic limitations and deactivation of the catalyst by water, which is the co-product of cyclic carbonates. The most commonly used method to improve the yields of cyclic carbonates involves the addition of dehydrating agents. However, decreased selectivity is often observed because of the side reaction of vicinal diols with the hydrolysis products of the dehydrating agent. In addition, the reaction of 2-aminoethanols and CO2 to obtain the corresponding 2-oxazolidinones also encounters the analogous thermodynamic limitation. To solve this problem, an efficient three-component reaction of CO2, propargylic alcohols, and nucleophiles was developed to offer thermodynamically favorable ways for converting CO2 into cyclic carbonates and 2-oxazolidinones with vicinal diols or 2-aminoethanols as nucleophiles. In this strategy, water is not generated and the α-alkylidene cyclic carbonate formed from CO2 and propargylic alcohol as the actual carbonyl source reacts with vicinal diol or 2-aminoethanol to give the corresponding cyclic carbonates or 2-oxazolidinones in high yields and selectivity with the simultaneous formation of hydroxyketones. This review aims to summarize and discuss the recent advances in three-component reactions of CO2,propargylic alcohols, and nucleophiles to prepare various carbonyl compounds promoted by both metal catalysts and organocatalysts.
Key Words: Three-component reaction; Carbon dioxide conversion; Propargylic alcohols; Green catalysis;Thermodynamically favorable strategy
1 Introduction
Hitherto, fossil fuels remain the main source of energy.However, burning fossil fuels causes huge amount of waste gases such as carbon dioxide emitted to air. In addition,predictions on energy of fossil fuels would be exhausted in the future have been made. From the viewpoint of environmental protection and resource utilization, it is imperative to reduce the consumption of fossil resources. On the other hand, CO2can be also regarded as a potential C1resource in organic synthesis because of its advantages of ubiquity, abundance,non-toxicity, non-flammability and renewability1–3. Carbonyls are very common backbones in organic chemistry and carbonyl compounds have been widely used in synthetic chemistry4,5.Usage of CO2as a building block to prepare carbonyl compounds is an interesting topic in the field of CO2chemistry6,7.Multicomponent reactions, in which more than two different substrates to react in one-pot, recently raise the chemists’attention due to the advantages of simplicity in operation and workup processes8. In this context, three-component reaction of CO2, propargylic alcohol and a nucleophile with step and atom economy provides an attractive strategy to convert CO2into various carbonyl compounds including carbamates,oxazolidinones, α-hydroxyl ketones and organic carbonates(Fig. 1a–d). Typically, this kind of cascade reaction goes sequentially through carboxylative cyclization of CO2with propargylic alcohol, followed by nucleophilic reaction of a nucleophile, e.g., amine, with the in situ generated α-alkylidene cyclic carbonate from CO2and propargylic alcohol.
Cyclic carbonates9,10and oxazolidinones11,12are important carbonyl compounds in organic chemistry. The preparation from vicinal diols or 2-aminoethanols with CO2represents a green synthetic process featuring with high atom efficiency and generation of water as only by-product13,14. To date, great progress has been gained and dehydration has been found to be a critical issue for the synthesis of cyclic carbonates or oxazolidinones using vicinal diols or 2-oxazolidinones and CO2as raw material due to the thermodynamic restriction15. With the development of various dehydration strategies, side reactions caused by hydrolysis of dehydrating agents or generation of a large amount of waste become major problems.The interesting thing could be such a three-component reaction involving bi-nucleophiles, namely nucleophiles with two nucleophilic groups such as vicinal diols (Fig. 1e) and 2-aminoethanols (Fig. 1f), could proceed through a sequence of carboxylative cyclization, nucleophilic attack and intramolecular cyclization, producing cyclic carbonates and 2-oxazolidinones efficiently in a thermodynamically favorable way without the formation of water.
2 Three-component reaction with N-nucleophiles
N-nucleophiles including secondary and primary amines react with CO2and propargylic alcohols in domino model to afford carbamates and oxazolidinones, which are appealing compounds with pharmacological activities. This cascade reaction covers the carboxylative cyclization of propargylic alcohols with CO2 and subsequent nucleophilic addition of amines to the in situ generated α-alkylidene cyclic carbonates from propargylic alcohols and CO2. The step of nucleophilic addition is influenced by the type of secondary and primary amine which may be due to the difference in steric hindrance on nitrogen atom, resulting in the formation of different products. In general, β-oxopropylcarbamate 1 is obtained when using secondary amine as N-nucleophile, through the sequential steps of carboxylative cyclization, ring-opening and tautomerization, as shown in Fig. 2a. However, oxazolidinone is gained with primary amine as N-nucleophile. In detail,β-oxopropylcarbamate intermediate 2 is formed through nucleophilic addition of primary amine to the in situ generated α-alkylidene cyclic carbonate form CO2and propargylic alcohol. Further intramolecular cyclization of the formed β-oxopropylcarbamate intermediate 2 furnishes the 4-hydroxy 2-oxazolidinone intermediate 3, the reaction intermediate which could afford 4-methylene-2-oxazolidinone 4 (Fig. 2, Eq.b) or 4-methyloxazol-2-one 5 (Fig. 2, Eq. c) after an alternative elimination of hydroxyl with hydrogen atom of 4-methyl or with hydrogen atom in the 5-position. The regioselectivity of 4 or 5 is controlled by the type of propargylic alcohol.Specifically, 4 is given with the usage of tertiary and secondary propargylic alcohol and 5 is generated when providing primary propargylic alcohol.
Seminal work on the reaction of CO2, propargylic alcohols and secondary amines is focused on ruthenium catalysis16,17. In the presence of ruthenium compounds such as Ru3(CO)1216and RuCl2(NBD)n(I)17, β-oxoalkylcarbamates are generated with yields up to 64% under 5 MPa CO2pressure. Until 1997, the copper complex [Cu(L)]PF6(Fig. 3) was developed as an effective catalyst for the formation of β-oxoalkylcarbamates through the three-component reaction18. Almost quantitative yields are given in most cases with 1% (molar fraction)[Cu(I)]PF6and 10% (molar fraction) 2,2’-bipyridine, even though a high CO2pressure (3.5 MPa) is needed. Interestingly,further investigation shows that β-oxopropylcarbamates could also be efficiently attained even without any additional catalyst and solvent in compressed CO2(14 MPa)19. It is believed that secondary amine in this system is used not only as a reagent but also as a catalyst.
Recent progress on the reaction of CO2, propargylic alcohols and secondary amines is the development of catalysts for promoting this conversion under mild conditions especially under low CO2pressure in view of economy and safety. In this regard, the silver (I)20–22and copper (I)23,24catalysts show high catalytic activity. Especially, by providing [(PPh3)2Ag]2CO3as the catalyst, a series of β-oxopropylcarbamates are formed in 68%–98% yields at atmospheric CO2pressure22.
Compared with three-component coupling of CO2,propargylic alcohols and secondary amines, primary amines as N-nucleophiles give N-substituted 4-methylene-2-oxazolidinones.A pioneering work is reported by Sasaki in which the ruthenium complex is employed as the catalyst16. In the presence of Ru3(CO)12, n-propylamine reacts with prop-2-yn-1-ol or but-3-yn-2-ol under 5 MPa CO2 pressure to afford the corresponding 2-oxazolidinone in 13% or 28% yield. Later,cheap copper (I) compounds are developed as efficient catalysts with the co-operation of ionic liquids (ILs)23. For example, the reaction of CO2, propargylic alcohols and primary amines can give 4-methylene-2-oxazolidinones in 78%–95% yields under 2.5 MPa CO2 pressure in the presence of CuCl and [BMIm]BF423.In particular, products in this system could be easily separated through extraction and CuCl/[BMIm]BF4can be reused at least for three times without significant decrease of activity. Deng et al.25have reported that IL for example [DMIm]BF4, used as both catalyst and reaction medium, could promote the formation of N-substituted 4-methylene-2-oxazolidinone in the absence of metal compounds. However, a high CO2pressure (5 MPa) is necessary.
Supercritical CO2has advantages of nonflammability,availability, low cost and easy separation, having been used as green reaction medium and charming raw material26. In 2011,Xu et al.27reported 4-methylene-2-oxazolidinones were obtained under supercritical conditions. A series of 4-methylene-2-oxazolidinones can be produced in 65%–88% yields from the corresponding propargylic alcohols and primary amines under 12 MPa CO2pressure at 120 °C with no additional catalyst and solvent. Even though, the addition of metal catalysts such as copper (I)28and silver (I)29into the reaction could obviously reduce the pressure of supercritical CO2to 8 MPa. This may be the excellent performance of metal catalysts for thecarboxylative cyclization of CO2with propargylic alcohols30,31,thus rendering the three-component reaction performing under relatively mild condition. Further study reveals that without any additional solvent, 4-methylene-2-oxazolidinones are obtained under atmospheric CO2pressure when providing CuCl as the catalyst32. In this system, excess amount of primary amines (2 equiv. relative to propargylic alcohols) are required.

Fig. 2 Three-component reaction of CO2,propargylic alcohols and amines.
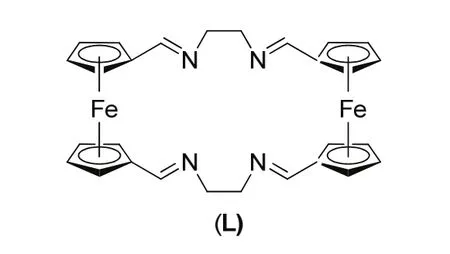
Fig. 3 Ligand (L) in copper complex [Cu(L)]PF6.
3 Three-component reaction with O-nucleophiles
O-Nucleophiles such as water and monohydric alcohols with weaker nucleophilicity than amines are also found to react with CO2and propargylic alcohols. These reactions are also cascade reactions, in which O-nucleophile attack the in situ formed α-alkylidene cyclic carbonates from CO2and propargylic alcohols, followed by ring-opening and tautomerization. With water as O-nucleophile, α-hydroxyl ketones are generated after CO2molecule elimination; while β-oxopropylcarbonates are obtained by using monohydric alcohols as O-nucleophiles.
Three-component reaction of CO2, propargylic alcohols and water catalyzed by AgOAc/DBU33or ionic liquid (IL)[Bu4P+][Im−]34has been reported for the synthesis of α-hydroxyl ketones (Fig. 4), which are important building block in bioactive molecules5. The reaction proceeds through a sequence of CO2incorporation into propargylic alcohol,hydrolysis of in situ generated α-alkylidene cyclic carbonate,keto-enol tautomerization and CO2elimination from alkylcarbonic acid intermediate (Fig. 5). Considering direct hydration of propargylic alcohols can also give α-hydroxyl ketones35,36, the reaction of propargylic alcohol and water in the presence of [Bu4P+][Im−] is conducted34. However, no reaction occurs, meaning CO2plays an important role in the three-component reaction.
With monohydric alcohols as nucleophiles, the threecomponent reaction produces β-oxoalkylcarbonates, a kind of valuable dissymmetric carbonates, as products37,38. Costa et al.39have firstly achieved this conversion. Monohydric alcohols including MeOH, n-BuOH, and butan-2-ol react efficiently with 2-methylbut-3-yn-2-ol, 1-ethynylcyclohexanol or 2-phenylbut-3-yn-2-ol respectively under supercritical CO2 with bicyclic guanidine such as TBD (2,3,4,6,7,8-hexaydro-1H-pyrimido[1,2-a]pyrimidine), MTBD (1,3,4,6,7,8- hexaydro-1-methyl-2H-pyrimido[1,2-a]pyrimidine) and DBU (2,3,4,6,7,8,9,10-octahydropyrimido[1,2-a]azepine) as the catalyst, giving the corresponding β-oxopropylcarbonates in 22%–87% yields(Fig. 6). A proposed pathway involves carboxylative cyclization of CO2 with propargylic alcohol and subsequent transesterification of monohydric alcohol with initially formed α-alkylidene cyclic carbonates as depicted in Fig. 7. Recently,the silver compound combined with phosphine40or IL41is successfully applied to this reaction under mild conditions. In particular, high catalytic efficiency and wide substrate scope are observed under 1 MPa CO2 pressure using Ag2CO3/PPh3 as the catalyst40. Further decreasing CO2pressure to 0.1 MPa is achieved when employing AgCl/[BMIM]OAc system41. In addition, the metal salt/IL system could be conveniently recycled and reused at least for five times.
4 Vicinal alcohols and 2-aminoethanols as bi-nucleophiles: thermodynamically favorable process for CO2 conversion
Preparation of cyclic carbonates by using CO2as building block is a flourishing research in CO2conversion because of wide applications of cyclic carbonates42. Especially, synthesizing cyclic carbonates from CO2 and vicinal diols13is attractive in view of the biomass source of vicinal diols43and water formed as the only by-product. In this regard, main progresses focusing on the metal-based catalysts such as Ce44–46, Sn47and Mg48have been made. Dehydrating agents for example nitriles49,50are commonly utilized to improve the yields of cyclic carbonates due to the thermodynamic limitation and deactivation of catalyst caused by water, while decreased selectivity of cyclic carbonates is observed in most cases presumably due to side reactions of vicinal diols with hydrolysis products of nitriles51. Based on these, assumptions of avoiding generation of water and developing thermodynamically favorable process for synthesizing cyclic carbonates from CO2 and vicinal diols is proposed. In view of the fact that β-oxoalkylcarbonates are products of three-component reaction of CO2, propargylic alcohols and monohydric alcohols, we envisage that hydroxyl functionalized β-oxoalkylcarbonates derived from reaction of CO2, propargylic alcohols and vicinal diols may give cyclic carbonates with the co-products of α-hydroxyl ketones via an intramolecular cyclization (Fig. 8).
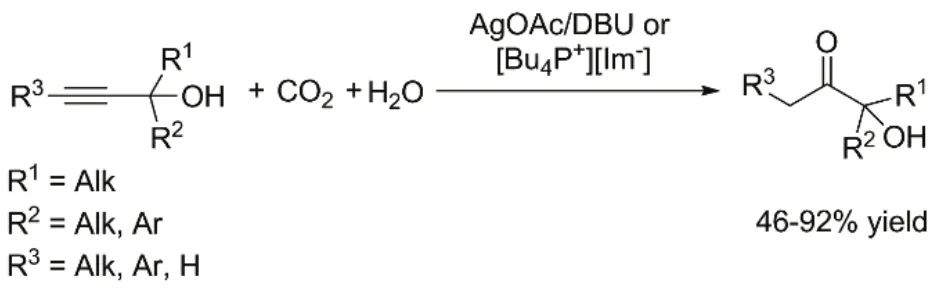
Fig. 4 Reaction of CO2, propargylic alcohols and water.

Fig. 5 Possible reaction pathway.
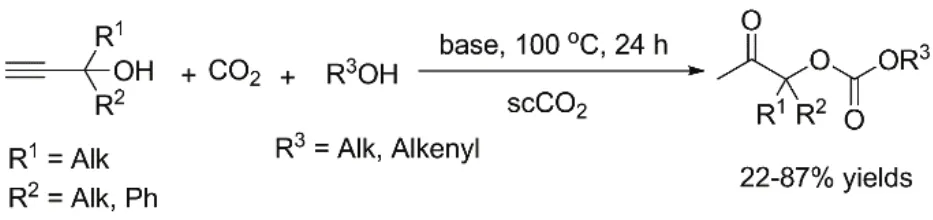
Fig. 6 Organic base-catalyzed synthesis of β-oxoalkylcarbonates.
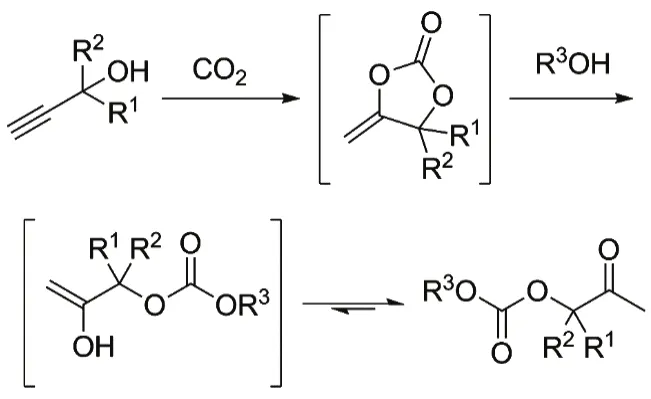
Fig. 7 Proposed mechanism.
Following this hypothesis, we have recently developed an efficient silver (I)-catalyzed three-component reaction of CO2,propargylic alcohols and vicinal diols to synthesize cyclic carbonates52. Initially, the reaction of CO2, propane-1,2-diol(1a) and 2-methylbut-3-yn-2-ol (2a) to generate propylene carbonate (3a) and 3-hydroxy-3-methylbutan-2-one (4a) is studied by density functional theory (DFT) calculation, giving a ΔrG (298.15 K, in vacuo) value of −170.0 kJ·mol−1. In other words, this is a thermodynamically favorable reaction. As shown in Fig. 9, various vicinal diols with/without substituents are treated with CO2 and 2-methylbut-3-yn-2-ol, furnishing the corresponding cyclic carbonates (3a–3f) in good to excellent yields (65%–91%) with Ag2CO3/Xantphos as the optimal system. Notably, glycerol carbonate as a versatile building block53is produced with a yield of 80% in this protocol. In addition, this strategy is also effective for the synthesis of several relatively bulky cyclic carbonates such as 3h and 3i and six-membered cyclic carbonates 3j.
Direct dehydration of CO2and 2-aminoethanols to form 2-oxazolidinones is another reaction suffering from thermodynamic limitation. This limitation could be well circumvented by employing three-component strategy, which is thermodynamically feasible supported by DFT study54. With Ag2CO3/Xantphos as the optimal catalytic system, various electron-donating and electron-withdrawing groups substituted 2-(benzylamino)ethanols as well as 2,2′-iminodiethanol could now be transformed into the corresponding 2-oxazolidinones with appreciable yields by reacting with CO2and 2-methylbut-3-yn-2-ol (Fig. 10). Further investigation reveals that Ag2O/TMG (1,1,3,3-tetramethylguanidine) could promote the synthesis of 2-oxazolidinones from CO2and 2-aminoethanols with a turnover of number (TON) up to 126055.
Preliminary mechanistic study shows that carbonyls of cyclic carbonate and 2-oxazolidinones originate from α-alkylidene cyclic carbonates52,54,55. Therefore, the proposed pathway is drawn in Fig. 11. α-Alkylidene cyclic carbonate intermediate is initially afforded from the reaction of CO2with propargylic alcohol. Subsequent nucleophilic attack of vicinal diol/2-aminoalcohol to α-alkylidene cyclic carbonate gives cyclic carbonates/2-oxazolidinone, through a sequence of ringopening, tautomerization and intramolecular cyclization.
5 Conclusions
In conclusion, we have summarized the main progress on the three-component reaction of CO2, propargylic alcohols and nucleophiles to prepare valuable carbonyl compounds and particularly updated the recent advances on using vicinal diols and 2-aminoethanols as bi-nucleophiles to react with CO2and propargylic alcohols. The three-component cascade reaction of CO2, propargylic alcohols and vicinal diols/2-aminoethanols can be regarded as a thermodynamically favorable method for CO2 conversion to offer an alternative way to prepare cyclic carbonates/2-oxazolidinones from CO2and vicinal diols/2-aminoethanols. Further investigation on this field would be focusing on the design of efficient catalytic systems and new reactions.

Fig. 8 Synthesis of cyclic carbonates from CO2, propargylic alcohols and vicinal diols.

Fig. 9 Substrate scope with respect to vicinal diols.
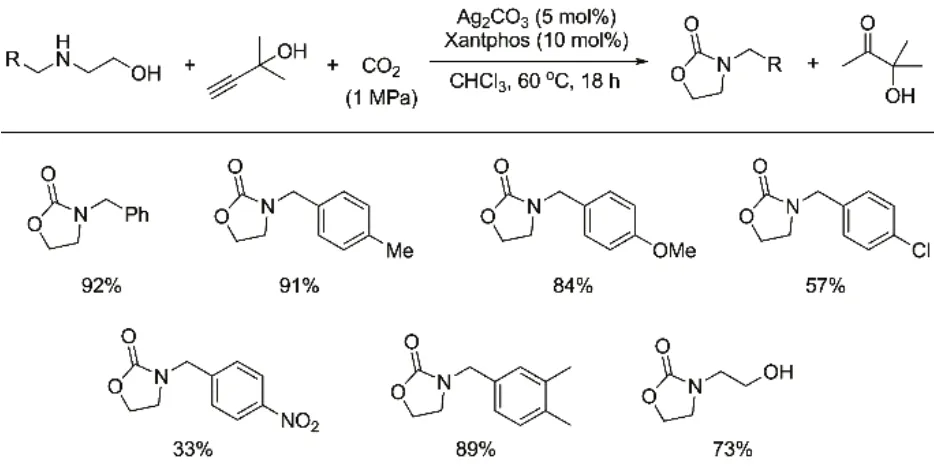
Fig. 10 Substrate scope with respect to 2-aminoethanols.

Fig. 11 Proposed reaction pathway.
杂志排行
物理化学学报的其它文章
- Catalytic Electroreduction of CO2 to C2H4 Using Cu2O Supported on 1-Octyl-3-methylimidazole Functionalized Graphite Sheets
- Green and Cost-Effective Preparation of Small-Sized ZSM-5
- Physicochemical Properties of1-Methoxyethyl-3-Methylimidazolium Glycine
- Study on Solution Enthalpies of Ionic Liquids [Cnmim][H2PO4](n = 3, 4, 5, 6) by Using Pitzer’s Equation
- Influence of External Electric Field on Vibrational Spectrum ofImidazolium-Based Ionic Liquids Probed by Molecular Dynamics Simulation
- Self-Assembly Behavior of Amphiphilic Diblock Copolymer PS-b-P4VP in CO2-Expanded Liquids
