Comparison of forage yield, silage fermentative quality, anthocyanin stability, antioxidant activity, and in vitro rumen fermentation of anthocyanin-rich purple corn (Zea mays L.) stover and sticky corn stover
2018-08-06TIANXingzhouPramotePaengkoumSiwapornPaengkoumSorasakThongpeaBANChao
TIAN Xing-zhou, Pramote Paengkoum, Siwaporn Paengkoum, Sorasak Thongpea, BAN Chao
1 Schoolof Animal Production Technology, Institute of Agricultural Technology, Suranaree University of Technology, Nakhon Ratchasima 30000, Thailand
2 Program in Agriculture, Faculty of Science and Technology, Nakhon Ratchasima Rajabhat University, Nakhon Ratchasima 30000,Thailand
Abstract The objective of this study was to observe the forage yield, silage fermentative quality, anthocyanin stability, and antioxidant activity during the storage period and in vitro rumen fermentation of anthocyanin-rich purple corn (Zea mays L.) stover (PS)and sticky corn stover (SS). Forage yield of corn stover was weighed and ensiled with two treatments: (1) hybrid sticky waxy corn stover (control), and (2) hybrid purple waxy corn stover (treatment). Samples were stored in mini-silos for periods of 0, 7, 14, 21, 42, 63, 84, and 105 d. The results showed that PS had significantly higher (P<0.05) yields of dry matter (DM),organic matter (OM), gross energy (GE), crude protein (CP), neutral detergentfiber (NDF), acid detergentfiber (ADF), and totalanthocyanins than that of the SS. Anthocyanin-rich purple corn stover silage (PSS) showed higher (P<0.05) levels of DM and CP relative to the sticky corn stover silage (SSS). Although anthocyanin-rich PSS displayed a lower (P<0.05)levelof pelargonidin-3-glucoside (P3G), it had higher (P<0.05) levels of peonidin (Peo) and pelargonidin (Pel) compared to the control. Delphinidin (Del) and malvidin (Mal) were not detected in SSS during the ensilage period; in PSS, Del was no longer detected after 7 d of ensilage. Specifically, totalanthocyanins in anthocyanin-rich PSS decreased rapidly (P<0.05)prior to 7 d of ensilage, and then remained at relatively stable (P>0.05) constants. Compared to the anthocyanin-rich PSS,SSS displayed significantly higher (P<0.05) pH value and ammonia nitrogen (NH3-N) content. Propionic acid (PA) at 0 d and butyric acid (BA) during the entire study period were not detected, whereas anthocyanin-rich PSS showed a higher(P<0.05) levelof lactic acid (LA) than that of the SSS. Compared with the SSS extract, anthocyanin-rich PSS extract showed a higher (P<0.05) levelof 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity and displayed a lower (P<0.05) half maximal inhibitory concentration (IC50) value. Moreover, anthocyanin-rich PSS reduced (P<0.05) gas production (GP), and displayed lower levels of immediately soluble fraction and ratio of acetic acid (AA) to PA at 12 h, but the other parameters were unaffected (P>0.05) relative to the control. Taken to gether, the results indicated that: (1) anthocyanins could be stable in silage; (2) anthocyanin-rich PSS showed better silage fermentative quality and stronger antioxidant activity; and(3) anthocyanin-rich PSS had no negative effect on rumen fermentation parameters.
Keywords: anthocyanin-rich purple corn stover silage, anthocyanin stability, silage fermentative quality, antioxidant activity,rumen fermentation
1. Introduction
Anthocyanins, a group offlavonoid compounds that are naturally occurring plant pigments responsible for color change from red through purple to blue, are present in a wide variety of plants, such as blackcurrant, wild blueberry,concord grape, and purple corn (Zhao et al. 2009; Silva et al.2016; Zhang et al. 2016). Typically, anthocyanins have been reported to exhibit potent antioxidant activity (Owoade et al.2015). Thus, they may remove excessive free radicals to alleviate oxidative stress in ruminants when the animal’s antioxidant system balance is disrupted during metabolic disorder (Jomova and Valko 2011; Georgiev et al. 2014).
Thailand is a special tropical country with a different climate than other countries and areas due to its long dry season from November to March (Hashimoto et al. 2004).Thus, roughage must be provided to ruminants since it is not available during that period. The process of making silage has become common; silage has been an increasingly important source of ruminant forage feedstuff (Lounglawan et al. 2011; Lukkananukool et al. 2013).
Purple corn is a rich and economical source of anthocyanin colorants for human consumption (Jing and Giusti 2007). Previous studies demonstrated that purple corn by-products, such as the husk, cob, silk and tassels also had abundant anthocyanins (Cevallos-Casals and Cisneros-Zevallos 2004; Dykes and Rooney 2007; Khampas et al. 2013). Consequently, purple corn by-products seem to be a suitable functional ingredient for ruminants (Hosoda et al. 2012c). However, those by-products are usually buried or burned after the corn kernels are harvested,which pollute the environment, it is not in line with the goalof sustainable development. In addition, purple corn is a type offield crop that is widely grown in Thailand (Harakotr et al. 2014; Phinjaturus et al. 2016). Recently, farmers and researchers in Thailand are looking for new ways to improve ruminant health from the perspective of animal nutrition in terms of antioxidant activity. There has been little information reported about anthocyanin in purple corn stover silage (PSS) on forage yield, silage fermentative quality, anthocyanin stability, and antioxidant activity or on rumen fermentation in ruminants. Accordingly, in order to provide a preliminarily understanding of the potentialof anthocyanin-rich PSS as a roughage for ruminants, we investigated its chemical composition, silage fermentative parameters, anthocyanin composition, antioxidant activity during different lengths of ensilage and in vitro incubation with ruminal fluid.
2. Materials and methods
2.1. Plant management and silage making
Afield experiment was conducted from June 16, 2016 to September 1, 2016, during the rainy season at the Suranaree University of Technology (SUT) farm, Nakhon Ratchasima,Thailand (14°53´37.9´´N, 102°01´22.0´´E). F1hybrid purple waxy corn and F1hybrid sticky waxy corn were cultivated in 0.5 m distance under the same fertilizer conditions(N-P2O5-K2O, 50:50:50 kg ha-1; Hydro Thai Limited, Bang Kruai, Thailand) using a completely randomized design with three duplicates per treatment. Corn grain was harvested at the yellow ripe stage, and then corn stover (without the corn grains) was cut into pieces approximately 6-8 cm in length above the soil surface by a cutting machine (SCB-2800, Fermier Engineers Private Limited, Tamil Nadu,India). The fresh materials were immediately transferred to the laboratory and chopped into pieces 2-3 cm in length by an electric automation grinder (Model 5222, Mitsubishi,Tokyo, Japan). Next, the materials were placed into 0.5 L mini-silos, which were kept in the dark at 15-25°C ambient temperature for a period of 0, 7, 14, 21, 42, 63, 84, and 105 d,respectively.
2.2. Chemicalanalysis
After ensilage, 20 g offresh silage samples were placed into a 150-mL beaker, covered with 100-mlof distilled water,and blended for 30 min at room temperature before beingfiltered throughfilter paper (Whatman™ No. 1441-125, GE Healthcare Life Sciences, Marlborough, MA, USA), then pH determination was done immediately by a portable pH meter. Meanwhile, thefiltrate solution was stored at -20°C until measure. Lactic acid (LA), acetic acid (AA), propionic acid (PA), and butyric acid (BA) were determined using high performance liquid chromatography (HPLC; 1260 Infinity II LC, Agilent Technologies, Santa Clara, CA, USA)as per the methods of Kudo et al. (1987) and Song et al.(2012); ammonia nitrogen (NH3-N) was analyzed by the steam distillation method of Bremner and Keeney (1965).Moreover, the anthocyanin content in silage was extracted using 1% hydrochloric acid (HCl) dissolved in 95% methanol solution at 50°C for 24 h, and then the supernatant was collected and transferred into a 50-mL volumetric flask for the determination of anthocyanin composition by HPLC according to Hosoda et al. (2009) and Yang et al. (2009).The remaining samples were dried at 65°C in a vacuum oven for 72 h, then ground and passed through a 1-mm sieve. Dry matter (DM), crude protein (CP), and ash were measured according to the feed proximate analysis of the Association of Offcialanalytical Chemists (AOAC 1990).Neutral detergentfiber (NDF) was detected with sodium sulfite and a heat stable amylase, whereas acid detergentfiber (ADF) was measured by sequentialanalysis of the residual NDF by the methods of AOAC (1990) and Van Soest et al. (1991). Each sample was run in triplicate.Organic matter (OM) and hemicellulose were calculated using the following formulas, respectively: OM=100−Ash;Hemicellulose=NDF−ADF. Gross energy (GE) was analyzed using a Parr 6200 calorimeter (Moline, Illinois, USA). Water soluble carbohydrates (WSC) were assayed by a microplate reader (SpectracountTM, Packard Canberra, Meriden, CT,USA) after reaction with anthrone reagent (Sigma-Aldrich,Pcode: 101694154) as described by McDonald and Henderson (1964). Alternatively, dry matter intake (DMI),digestible dry matter (DDM), relative feed value (RFV), and net energy for lactation (NEL) were predicted according to the following equations adapted from Lithourgidis et al. (2006): DMI=120/%NDF, DM basis; DDM=88.9−(0.779×%ADF, DM basis); RFV=%DDM×%DMI×0.775; and NEL=(1.044−0.0119×%ADF)×2.205
2.3. DPPH scavenging activity
Antioxidant activity for the 2,2-diphenyl-1-picrylhydrazyl(DPPH) scavenging activity of the corn stover silage extract was determined spectrophotometrically according to Thaipong et al. (2006) and Zhang et al. (2018) using a stable free radical DPPH (Sigma-Aldrich, Pcode: 101845869) with a minor modification. Briefly, an aliquot of 2.00 mlof the appropriate dilution (1/5, 1/4, 1/3, 1/2, 1) of two corn stover silage extracts at 21 d was added to 2.00 mlof 0.1 mmol L-1DPPH solution and then shaken vigorously. The OD value was detected at 517 nm using a microplate reader after incubation for 30 min in a 30°C water bath. The containers were wrapped in aluminum foil to ensure that the incubation process was carried out in the dark. Each sample was run in triplicate. The percentage of DPPH (%DPPHSC) was calculated by the following formula:
%DPPHSC=(Ac−As)×100/Ac
Where, Ac is the absorbance of the controland As is the absorbance of the sample. The half maximal inhibitory concentration (IC50) value was calculated by GraphPad Prism 5 software, which denotes the concentration of a substance required for 50% inhibition in vitro.
2.4. In vitro rumen fermentation
Three healthy multiparous Saanen dairy goats, body weight(42.50±0.50) kg (mean±standard deviation) were used as ruminal fluid donors. The experimentalanimals were housed in clean individual pens with free access to water and were fed diets with a concentrate/roughage ratio of 50:50 (10.13% CP and 66.33% total digestible nutrient)according to the National Research Council (NRC 1981).Ration was offered in equalamounts twice daily at 08:00 and 17:00. Ruminal fluid was obtained from goats before morning feeding via the mouth using a vacuum pump into an Erlenmeyer flask. The ruminal fluid was immediately passed through four layers of cheesecloth and mixed in equal volume, and then transported to the laboratory. The culture fluid was prepared by mixing the ruminal fluid and a phosphate-bicarbonate buffer with carbon dioxide (CO2) gas in a 39°C water bath as per Menke and Steingass (1988). A totalof 100-mL glass gas-tight syringes (Kabuskiki Kaisha,Japan) were used as fermentation vessels; they werefilled with 30-mlof mixture solution with 0.50 g of substrate in each syringe for 3, 6, 9, 12, 24, 48, 72, and 96 h incubation to estimate gas production (GP). The fermentation was stopped by submerging the syringe into ice-cold water, and then the pH of the rumen fluid was immediately measured using the portable pH meter. In the meantime, 20-mloffermentation liquid and 5 mlof HCl (6 mol L-1) were kept in a container after being mixing to gether, then stored in a refrigerator at -20°C until the samples were analyzed for NH3-N and individual volatile fatty acid (VFA) content.The total VFA (TVFA) value was calculated from the following equation: TVFA=AA+PA+BA. Each sample had three replications and two control replications. Moreover,organic matter digestibility (OMD), metabolizable energy(ME), and effective degradability (ED) were calculated using the following formulas by Menke et al. (1979), Menke and Steingass (1988), and Eliman and Ørskov (1984),respectively:
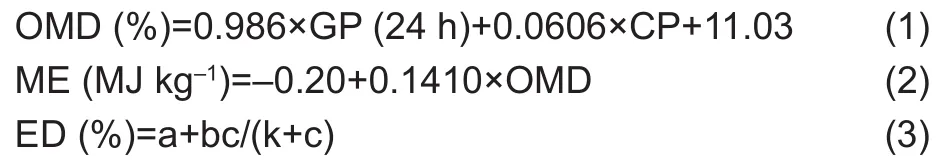
Where, k is ruminalout flow rate and the value sets as 0.031 h.
GP was calculated according to the following equation by Ørskov and McDonald (1979).
y=a+b(1-e-ct)
Where, y denotes the volume of gas produced at time t,a describes the gas production from the immediately soluble fraction (mL), b is the gas production from the insoluble fraction (mL), c is the gas production rate constant for the insoluble fraction b (% h-1), t expresses incubation time (h),and a+b represents the potential extent of gas production(mL).
2.5. Statisticalanalysis
All statisticalanalyses were performed using the SAS System version 9.1.3 (SAS Institute Inc., Cary, NC, USA).The replicate was considered the experimentalunit in allof the statisticalanalyses. Differences in the forage yield and nutritional value were analyzed using Student’s t-test. Significant differences between parameters were assessed using a 2×8 (silage treatment×day) analysis of variance (ANOVA) test in chemical composition,anthocyanin composition, and silage fermentative parameters during the storage period (0-105 d).The statistical significances of the DPPH scavenging activity between the different dilutions (1/5-1) were detected using a 2×5 (silage treatment×dilution) ANOVA test. A 2×8(silage treatment×incubation time) ANOVA test was also used for the difference in the gas production during the incubation time (0-96 h). Student’s t-test was also applied for the differences in the rumen fermentation parameters.Because the significant interactions (P<0.05) between silage and storage day were observed for WSC, malvidin-3-O-glucoside (M3G), cyanidin (Cya), and totalanthocyanins, the effect of storage day on these 4 parameters were reported.The IC50value was calculated from linear regression analysis by GraphPad Prism 5 software. Differences were considered statistically significant at P<0.05.
3. Results
3.1. Forage yield and nutritional value
As shown in Table 1, there was no statistically significant difference (P>0.05) for forage fresh weight content of sticky corn stover (SS) and anthocyanin-rich purple corn stover(PS). Compared to SS, PS had significantly higher (P<0.05)DM yield, chemical composition yields (OM, GE, CP, NDF,and ADF), and totalanthocyanin yield. In addition, PS did not affect (P>0.05) DMI, DDM, RFV, and NELfor ruminants.
3.2. Chemical composition
There was no interaction (P>0.05) between treatment and time for the DM and CP, whereas anthocyanin-rich PSS showed higher (P<0.05) levels of DM and CP compared to that of sticky corn stover silage (SSS; Table 2). The ash,OM, GE, NDF, and hemicellulose did not differ (P>0.05)between silages and were unchanged (P>0.05) throughout the experiment. The levelof ADF increased rapidly (P<0.05)prior to 7 d of ensilage, and then remained at relatively stable(P>0.05) values. There was an interaction (P<0.0001)between treatment and time was observed for the WSC.SSS had higher (P<0.05) WSC content at 0 and 7 d of ensilage relative to the anthocyanin-rich PSS. The levels of WSC in both of silages were maintained in stable values(P>0.05) from 14 to 84 d of ensilage, whereas WSC inanthocyanin-rich PSS was greater (P<0.05) than the SSS at 105 d of ensilage.
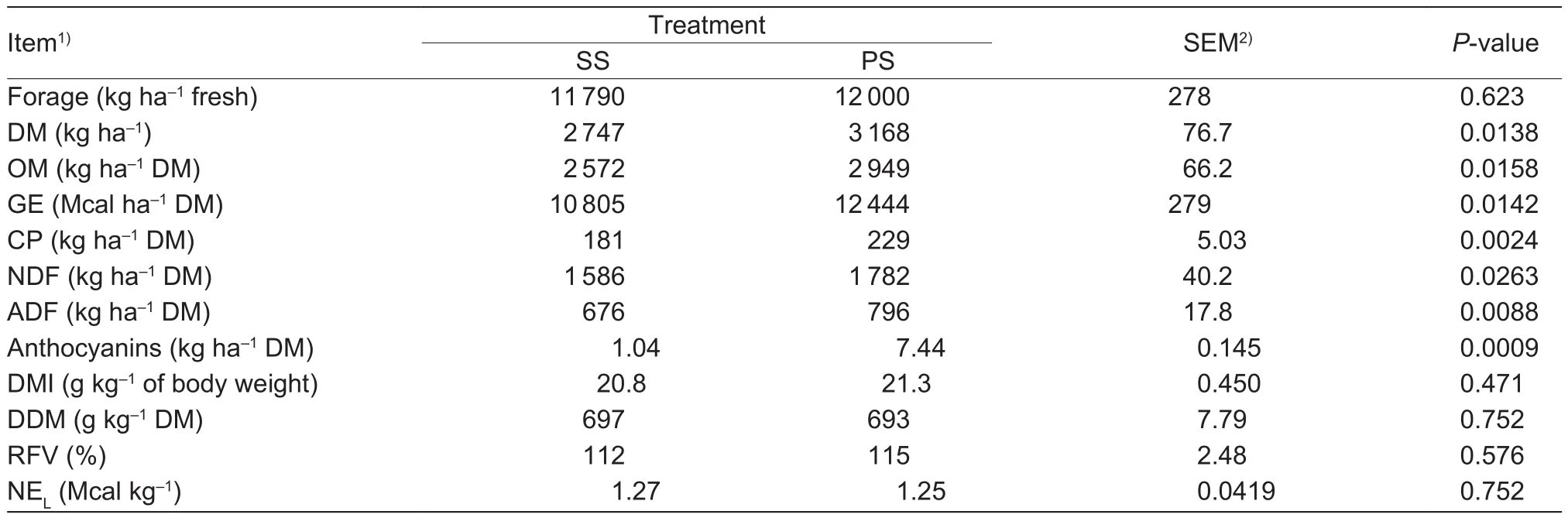
Table 1 Comparison offorage yield and nutritional value of sticky corn stover (SS) and anthocyanin-rich purple corn stover (PS)
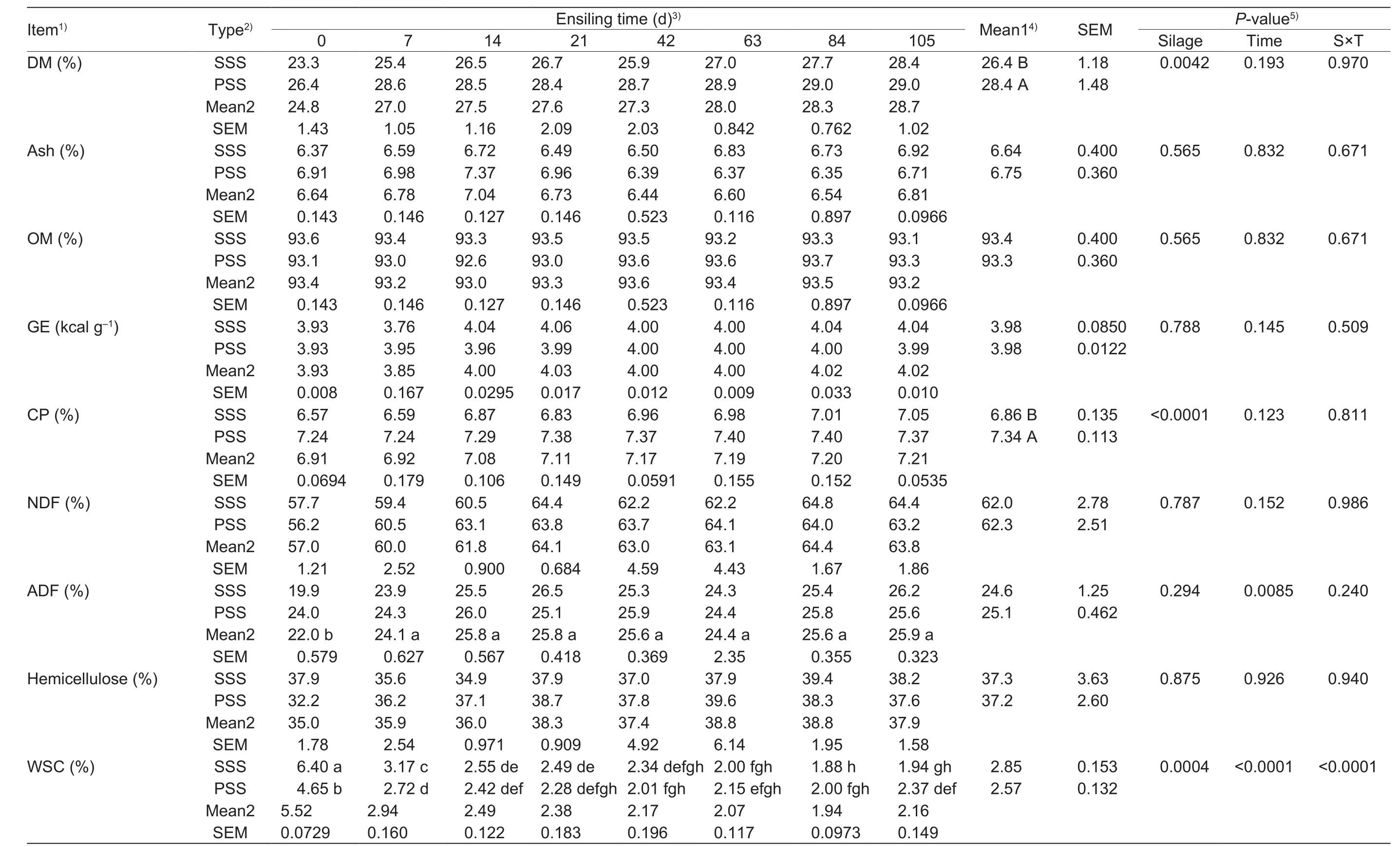
Table 2 Comparison of chemical compositionaccording to ensilingperiodof sticky corn stover silage (SSS) andanthocyanin-richpurplecornstover silage (PSS) (DM basis)
3.3. Anthocyanin composition
As shown in Table 3, cyanidin-3-glucoside (C3G) did not differ (P>0.05) between silages, and remained relatively constant (P>0.05) throughout the experiment. Delphinidin(Del) and malvidin (Mal) were unable to be detected in SSS during the entire ensilage period; in PSS, Del was not detected after 7 d of ensilage. Pelargonidin-3-glucoside(P3G) decreased rapidly (P<0.05) prior to 7 d of ensilage,and then remained at relatively stable (P>0.05) values. Of interest, the levelof P3G in the SSS was greater (P<0.05)than that of the anthocyanin-rich PSS. Differently, Peonidin(Peo) and pelargonidin (Pel) levels decreased rapidly(P<0.05) prior to 14 d of ensilage, and the means of Peo and Pel in the anthocyanin-rich PSS were greater (P<0.05)than that of the control. There were interactions (P<0.0001)between silage and storage day for the levels of M3G, Cya,and totalanthocyanins. The M3G in anthocyanin-rich PSS increased (P<0.05) prior to 21 d of ensilage compared to the SSS. M3G did not differ (P>0.05) at 42 and 63 d of ensilage in both of two treatments, whereas the sample at 84 and 105 d of ensilage also showed higher (P<0.05) M3G in anthocyanin-rich PSS than in SSS. Cya in anthocyanin-rich PSS was greater (P<0.05) than the SSS at the beginning of the fermentation (0 d), but the levelof Cya was unaffected(P>0.05) in both SSS and anthocyanin-rich PSS from 7 to 84 d of ensilage, and anthocyanin-rich PSS also displayed a higher (P<0.05) levelof Cya at 105 d of ensilage compared to the SSS. Totalanthocyanins of SSS and anthocyanin-rich PSS showed different trends because totalanthocyanins in SSS did not change (P>0.05) throughout the experiment, whereas the values in anthocyanin-rich PSS tended to decrease in response to increasing storage day.Particularly, totalanthocyanins content in anthocyanin-rich PSS was greater (P<0.05) than that of the SSS at the whole experimental period.
3.4. Silage fermentative quality
The pH value dropped rapidly (P<0.05) during the first 7 d of ensilage, and then the levels remained between 3.5 and 4.0 throughout the remainder of the experiment. Particularly,anthocyanin-rich PSS displayed a lower (P>0.05) levelof pH value relative to the control (Table 4). Although the levelof NH3-N increased (P<0.05) prior to 21 d of ensilage, NH3-N mean in PSS was significantly lower (P<0.05) than that of the control. The levels of LA and AA increased rapidly (P<0.05)during the first 7 d of ensilage, and LA in the anthocyaninrich PSS was greater (P<0.05) than that of the SSS. PA at 0 d and BA during the study period were not detected for either of the two silages.
3.5. DPPH scavenging activity
As shown in Table 5. DPPH scavenging activity of SSS and PSS extracts increased with increasing concentration in dilute solution, reaching a maximum at 73.5 and 81.1%,respectively, and the values remained relatively constant(P>0.05) after 1/3 dilution. As expected, DPPH scavenging activity mean in the anthocyanin-rich PSS extract was greater (P<0.05) than that of the control. Accordingly, PSS extract showed a lower (P<0.05) levelof IC50compared to the SSS extract (0.65 μg mL-1vs. 2.80 μg mL-1).
3.6. In vitro rumen fermentation
The levelof GP increased (P<0.05) during the first 72 h of incubation time, and then remained relatively constant(P>0.05) throughout the remainder of the experiment(Table 6). Of interest, SSS showed a higher (P<0.05) levelof GP compared to the anthocyanin-rich PSS. Moreover, SSS showed a significantly higher (P<0.05) content of the value a, and no significant differences (P>0.05) were observed in the b, c, a+b, OMD, ME, and ED compared to PSS (Table 7).As shown in Table 8, there were no significant differences(P>0.05) in the ruminal fluid pH value, NH3-N concentration,and individual VFA content of PSS and SSS. However, SSS showed a significantly higher (P<0.05) ratio of AA to PA at 12 h of incubation time compared to the anthocyanin-rich PSS.
4. Discussion
4.1. Forage yield and nutritional value
Anthocyanins showed the potential to mitigate photooxidative injury in leaves by shielding chloroplasts from excess highenergy quanta and scavenging reactive oxygen species,thereby effectively protecting plant skin from damage by ultraviolet rays (Neilland Gould 2003; Tattini et al. 2014).Meanwhile, the purple color could also help to camou flage a plant, which was likely to have a survivaladvantage over plants of other colors (Schaefer and Rolshausen 2006).As a result, PS was inclined to produce higher forage and chemical composition yields. However, anthocyanins were found to have some negative effects on the intake and production of animals, resulting from the lower palatability due to its bitter taste (Jöbstl et al. 2004). One study did show that anthocyanins could have a negative effect on digestive enzymes and the epithelial lining of the digestive tract (Davis and Milner 2009). Nonetheless, reports state
that anthocyanins have been used in ruminants because they are known to prevent bloating (Kitamura et al. 2004) and have strong antioxidant activity (Sivasankar et al. 2011). In this study, we found that PS did not affect DMI,DDM, RFV, and NELin ruminants. The possible reason was that although PSS had a higher levelof anthocyanins, resulting in negative palatability,it had relatively higher levelof chemical composition (especially CP) and stronger DPPH scavenging activity, which are known to improve animal nutrition and health.
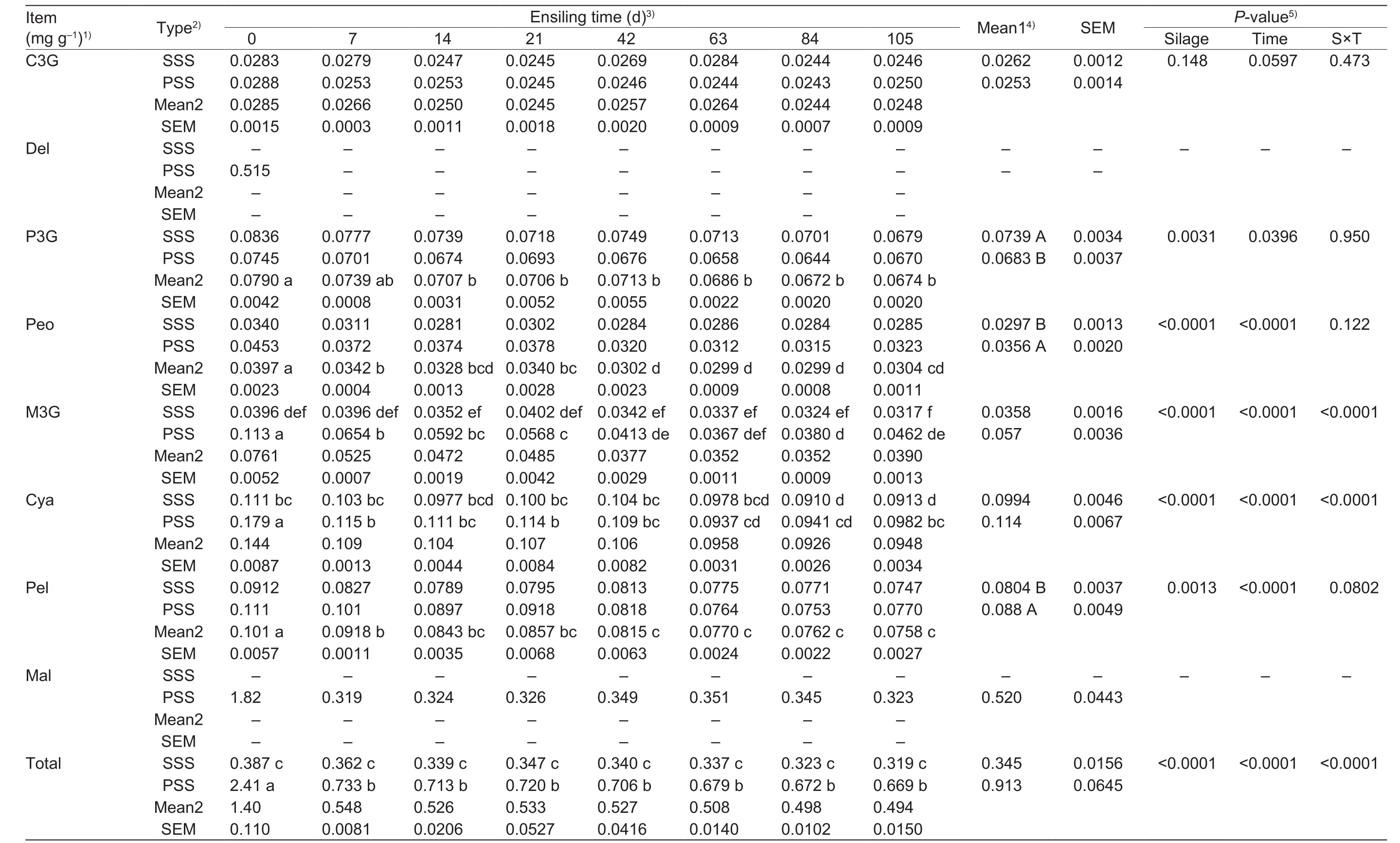
Table 3 Comparisonof anthocyanincomposition accordingtoensiling period of sticky corn stover silage (SSS) andanthocyanin-richpurplecornstover silage (PSS) (DM basis)
4.2. Silage fermentative quality and anthocyanin stability
DM and WSC content in plants are of great importance for silage(Wilkins 1982). In this study, we found that DM tended to increase during the entire ensilage period although it did not differ.Possibly because the extravasate was lost from the plant material during storage ensilage, thereby increasing DM level(Maeda et al. 2011).Conversely, a previous study showed that WSC was broken down by bacterial
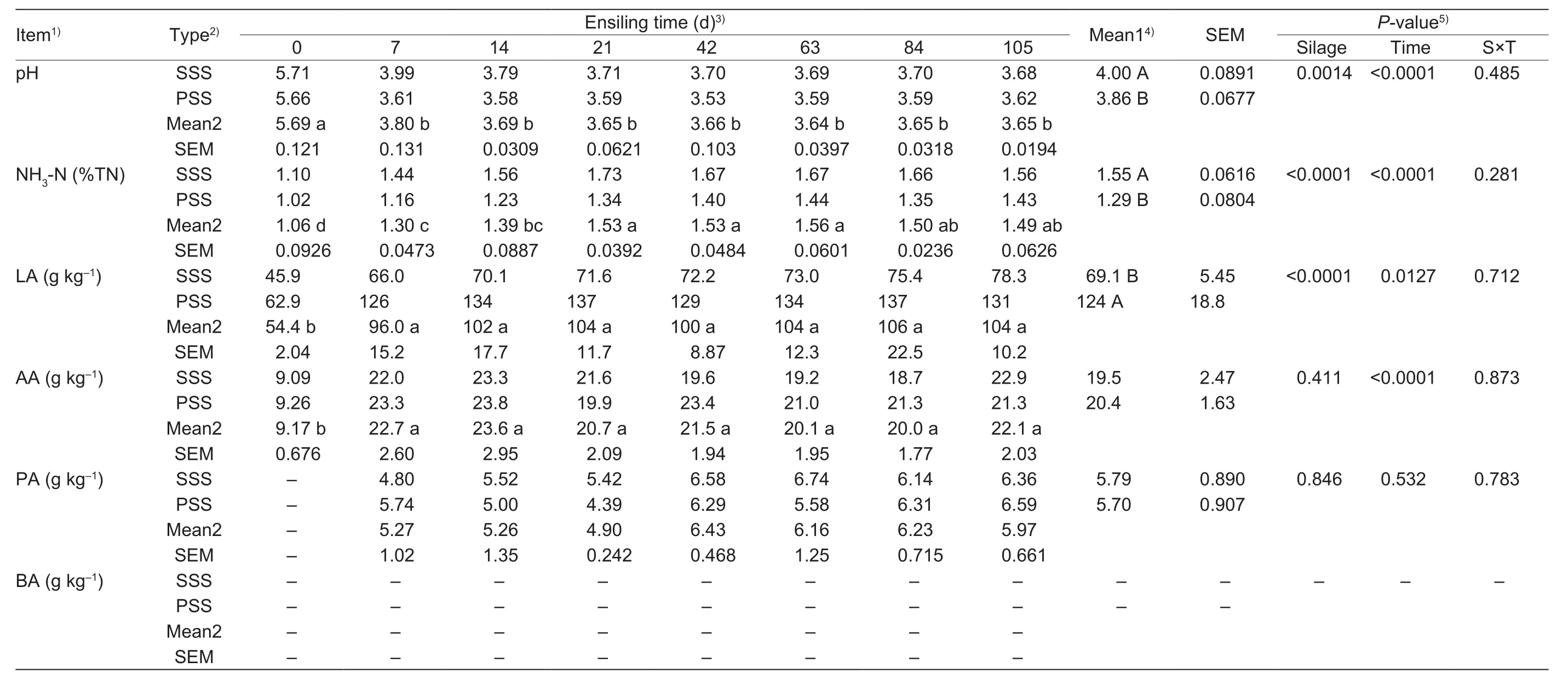
Table 4 Comparisonof silage fermentativequality accordingtoensilingperiodof sticky corn stover silage (SSS) andanthocyanin-richpurplecornstover silage (PSS) (DMbasis)
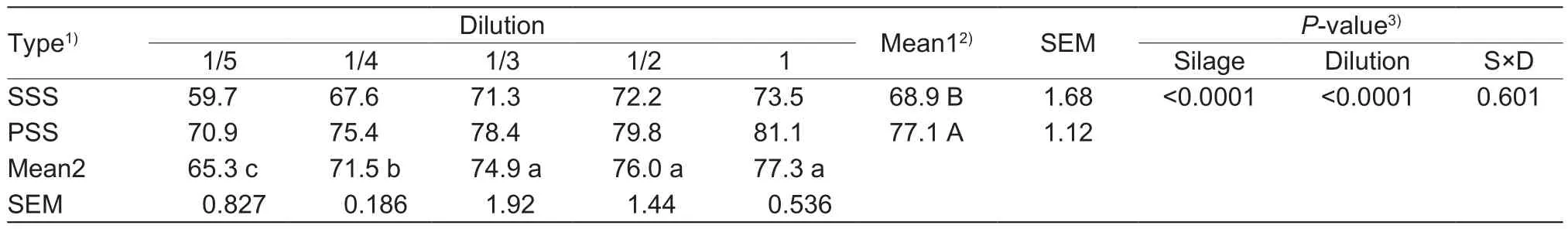
Table 5 Comparison of DPPH scavenging activity of sticky corn stover silage (SSS) and anthocyanin-rich purple corn stover silage(PSS) extracts (%)
1)Mean2, values represent the means of 6 replicates (n=6); SEM, standard error of the mean.
2)Mean1, values represent the means of 15 replicates (n=15).
3)Silage, effect of silage extract; Dilution, effect of dilution; S×D, effect of silage extract and dilution interactions.
Values represent the means of 3 replicates (n=3). Means with different lowercase letters within the same row are significantly different (P<0.05); means with different uppercase letters within the same column are significantly different (P<0.05).

Table 6 Comparison of in vitro gas production (GP) of sticky corn stover silage (SSS) and anthocyanin-rich purple corn stover silage (PSS) for 96 h
1)Mean2, values represent the means of 6 replicates (n=6); SEM, standard error of the mean.
2)Values represent the means of 3 replicates (n=3).
3)Mean1, values represent the means of 24 replicates (n=24).
4)Silage, effect of silage; Time, effect of incubation time; S×T, effect of silage and incubation time interactions.
Means with different lowercase letters within the same row are significantly different (P<0.05); means with different uppercase letters within the same column are significantly different (P<0.05).metabolism during the whole ensilage period, thus reducing its value (Sanderson 1993). The interaction between silage and storage day for WSC was the result of the high levelof WSC found in fresh SS compared to the fresh anthocyaninrich PS. Owens et al. (1999) demonstrated that sugars remaining in silage could be derived from the hydrolysis of structural carbohydrates or starch. In the current study,structural carbohydrates did not differ in two treatments,probably indicating that the starch content was the main factor that affected the levelof WSC. However, the exact reasons remain unclear and further observation is required.Fiber content was a major component of the plant cell walland was insoluble in water, making it difficult to be exploited by microorganisms during the storage period (Mertens 2003). Hence, the concentrations of NDF and hemicellulose of SSS and PSS did not differ between silages and remained fairly constant throughout the experiment. On the contrary,ensilage treatment could accelerate the growth of lactic acid bacteria (LAB), but the ensilage treatment lacked proteolytic enzyme and relevant ash/OM degrading enzyme; thus, ash and OM values also did not differ among treatments and have been quite stable during the entire ensilage period.Moreover, the levels of GE and ADF tended to increase during the ensilage period, which was reported to be related to the strength of silage fermentation, in which the loss of moisture is induced, leading to high DM, with consequent concentration of the GE and ADF (McDonald and Edwards 1976; Meineri and Peiretti 2005). Additionally, we also found the concentration of CP in PSS was significantly higher than that of SSS. The reason may be that in purple plant response to environmental stresses through anthocyanin metabolism, plant nutrients can be improved under anthocyanin protection (Chalker-Scott 1999). These results were consistent with a previous report by Hosoda et al. (2012a) who reported that anthocyanin-rich purple cornsilage showed a significantly higher CP value compared to the control.
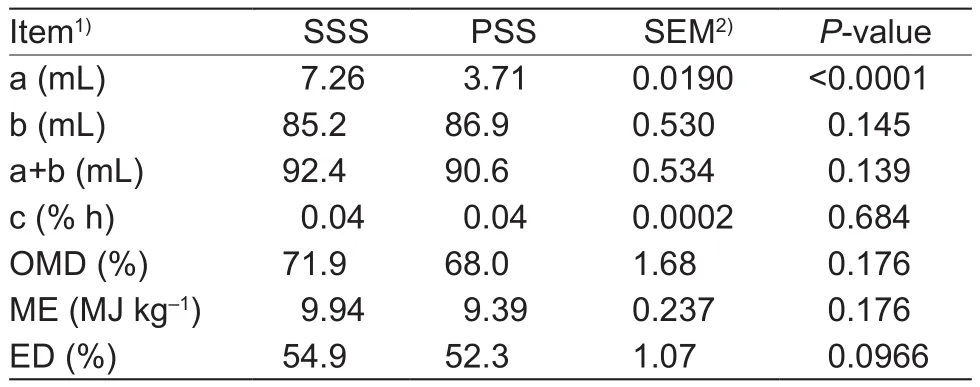
Table 7 Comparison of gas production (GP) kinetics, OMD, ME,and ED of sticky corn stover silage (SSS) and anthocyanin-rich purple corn stover silage (PSS)

Table 8 Comparison of ruminal fluid pH, NH3-N, and VFA values of sticky corn stover silage (SSS) and anthocyanin-rich purple corn stover silage (PSS)
Anthocyanins are sensitive to high pH, light, and temperature during storage period (Markakis and Jurd 1974;Francis and Markakis 1989; Laleh et al. 2006; Li et al. 2011).However, light and temperature factors could be considered to be negligent in this study because all the samples were compressed under anaerobic conditions, placed in minisilos, and kept a dark environment at 15-25°C ambient temperature. Reyes and Cisneros-Zevallos (2007) showed that the degradation rate of anthocyanins were positively correlated with the pH value under the same external conditions. Consequently, the silage had a lower pH value after 7 d of ensilage, which provided the necessary condition for the stability of anthocyanins. Specifically, anthocyanins consisted of anthocyanidin and sugar(s), which is the sugar structure used as a substrate during the ensilage period(Hosoda et al. 2009). Therefore, the decreased range of anthocyanins in PSS was greater compared to SSS, possibly indicating that more sugar in PSS took part in the silage fermentation for easier ensilage. These observations were in agreement with Song et al. (2012) who demonstrated that colored barley showed higher totalanthocyanins content and maintained a levelof 42% in storing silage. Moreover, Cya seemed to be the main anthocyanin composition in two types of silage, which is consistent with thefindings of previous studies (Pedreschi and Cisneros-Zevallos 2007; Cuevas Montilla et al. 2011). Interestingly, an interaction between treatment and time for M3G, Cya, and totalanthocyanins indicated a differential response to storage time for each of silage. One study did show that the color of the purple plant is due to anthocyanins (Aoki et al. 2002). Thus, the fresh anthocyanin-rich PS displayed high levels of M3G,Cya, and totalanthocyanins relative to the fresh SS. In addition, two samples showed different levels of DM; the trend of declining pH during the storage period was similar,whereas anthocyanin-rich PSS had a lower levelof pH compared to the control silage. Collectively, to gether these factors could cause an interaction between treatment and time for the amount of anthocyanins.
The pH value declined rapidly at the fermentation phase when the silage became anaerobic, which was usually in the range of 3.6-4.0 for the excellent silage (Guan et al.2002). This was because LAB developed, leading to it became the predominant population during anaerobic phase, resulting in large amounts of organic acid (Kang et al. 2014). Particularly, anthocyanin-rich PSS displayed a lower pH value, perhaps because the structure of purple corn anthocyanin was bonded to sugar (Aoki et al. 2002),resulting in easy fermentation during storage in anthocyaninrich PSS relative to the control. Generally, poor silage preservation had a high levelof NH3-N. This was due to breakdown of the protein in forage (proteolysis) occurring as a result of the activity of plant enzymes prior to the establishment of anaerobic conditions (Acosta et al. 1991;Hu et al. 2015). However, wetter silage also had higher concentration of NH3-N because of the potential for clostridial fermentation (Kung and Shaver 2001). Thus, SSS had a relatively high levelof NH3-N, perhaps owing to it had lower DM content in comparison to PSS. Additionally, since the sugar in anthocyanin was used as a substrate for lactic fermentation, which may have produced high LA content(Hosoda et al. 2009). AA and PA were the main energy sources for the harmful microorganisms during ensilage.Uniquely, the high levelof soluble nutrients, especially NDF and ADF in plants was usually displayed high levels of AA,PA, and BA (Kung and Shaver 2001). In this report, two types of silage had similar levels of NDF and ADF, thus there was no effect of treatments on AA and PA values. Thesefindings were in agreement with Song et al. (2012) who showed that colored barley silage had a higher levelof LA and similar levels of AA, PA, and BA compared to that of normal barley silage.
4.3. DPPH scavenging activity
Anthocyanins, as a bioactive secondary plant metabolite,have been shown to have high antioxidant activity (Akula and Ravishankar 2011). DPPH was a free radical, which was able to produce a violet solution in an organic solvent and was stable at room temperature. The value of DPPH tended to decline under antioxidant molecule circumstances(Mensor et al. 2001). Accordingly, this was a handy and rapid way of being able to assay antioxidant activity by measuring DPPH scavenging activity (Cheng et al. 2006).In this experiment, anthocyanin-rich PSS extract had a stronger levelof the DPPH scavenging activity than the control extract. One of the possible explanations was that anthocyanins could provide electrons to DPPH, so the solution was reduced to its non-free radical form to strengthen antioxidant activity (Jordão and Correia 2016).The amount of DPPH scavenging activity was in accordance with Hayashi et al. (2003). Indeed, anthocyanins are powerfulantioxidants due to their special chemical structural formula, preventing free radicals from being oxidized nearby cells (Castañeda-Ovando et al. 2009). In addition, the IC50value was negatively associated with the DPPH scavenging activity (Yang and Zhai 2010). As expected, PSS extract had lower IC50level in this study, which was consistent with a previous report by Pedreschi and Cisneros-Zevallos (2006)who demonstrated that purple corn extract had a higher antioxidant activity, primarily depending on anthocyanins involved in enzyme inactivation and scavenging of electrophiles.
4.4. In vitro rumen fermentation
Previous studies indicated that the incubation of anthocyaninrich corn or colored barley with ruminal fluid had no effect on the degradation of anthocyanin (Hosoda et al. 2009; Song et al. 2012). Their results may be a definite explanation that anthocyanins in plants are not broken down in the rumen.In the current study, anthocyanin-rich PSS had a lower levelof GP as wellas a parameter, perhaps because it had the relatively high content offiber content and relatively low levelof WSC, resulting in slower degradation rate (Brebu and Vasile 2010). It is consistent with Mangan (1988)who reported that the flavonoid family can reduce the degradability of ruminal fluid nutrients to achieve the aim of preventing bloating. A similar conclusion was also given for the lower amount of GP in anthocyanin-rich grape pulp(Spanghero et al. 2009). Therefore, anthocyanins may only have potential value in improving ruminant health rather than providing individual bodies with access to nutrients.Correddu et al. (2015) demonstrated that the rumen metabolism of lactating dairy-ewe was markedly in fluenced by dietary supplementation with anthocyanin-rich grape.Similarly, the feeding of anthocyanin-rich purple corn silage may increase superoxide dismutase (SOD) activity in the plasma, but had no effect on rumen fermentation parameters in lactating dairy cows (Hosoda et al. 2012a). In short, it was safe to assume that anthocyanins had no negative impact on rumen fermentation, but they had the potential to inhibit GP for prevention of ruminant bloating.
In this report, anthocyanins might be able to affect carbohydrate metabolism to provide more energy for ruminants by inhibiting AA production, increasing the proportion of PA. Hosoda et al. (2012a) reported that the feeding of anthocyanin-rich purple corn silage resulted in a lower AA to PA ratio in lactating dairy cows. However,Hosoda et al. (2012b) showed that the feeding of purple rice silage had higher ruminal fluid pH value and lower VFA because of poor nutrients and silage fermentative quality. As a consequence, nutrient composition could be one of the main factors affecting rumen fermentation,since anthocyanins may escape from the rumen to reach the small intestine for digestion and absorption. However,based on the present study, some chemical composition parameters in both silages were so significantly different that it was difficult to identify clearly whether the difference in rumen fermentation was responsible for the amount of anthocyanin or chemical composition difference. An interesting perspective on anthocyanins showed that they have the potential to be absorbed into milk, thereby improving consumers’ health. In this regard, additional experimental data based on in vivo studies are needed to verify the effect of antioxidant activity for dairy ruminants.
5. Conclusion
This was the first study to investigate the effects of anthocyanin-rich PSS on anthocyanin composition during the ensilage period, on its extract for DPPH scavenging activity, and on ruminal fermentation of Saanen dairy goats in vitro. The present study indicated that although the anthocyanins content in anthocyanin-rich PS declined during the ensilage period, it could be maintained in a stable condition. The anthocyanin-rich PSS showed excellent silage fermentative quality, higher levelof anthocyanins,and stronger antioxidant activity compared to the SSS.Additionally, anthocyanin-rich PSS had no negative effect on rumen fermentation parameters. Thus, anthocyaninrich PSS has the potential to become an ideal roughage for ruminants. Further studies are needed to determine the mechanism offeeding anthocyanin-rich PSS on rumen microorganisms, plasma antioxidant activities, expression of oxidative stress-related genes, and milk production in dairy ruminants.
Acknowledgements
The authors would like to thank the SUT Farm and the Center for Scientific and Technological Equipment, SUT,which provided the planting site and chemicalanalysis facilities for this study. We also thank the SUT-OROG scholarship, the Higher Education Promotion and National Research University Project of Thailand (NRU), and the Office of the Higher Education Commission (FtR 06/2559)for funding support.
杂志排行
Journal of Integrative Agriculture的其它文章
- ldentification and characterization of Pichia membranifaciens Hmp-1 isolated from spoilage blackberry wine
- Implications of step-chilling on meat color investigated using proteome analysis of the sarcoplasmic protein fraction of beef longissimus lumborum muscle
- Spatial-temporal evolution of vegetation evapotranspiration in Hebei Province, China
- Design of a spatial sampling scheme considering the spatialautocorrelation of crop acreage included in the sampling units
- Synonymous codon usage pattern in model legume Medicago truncatula
- The biotypes and host shifts of cotton-melon aphids Aphis gossypii in northern China
