五氧化二碘辅助亲电碘化1-甲基萘制备高残炭沥青
2018-06-28杨海潇韩贺祥王际童乔文明凌立成
杨海潇, 韩贺祥, 王际童, 乔文明,2, 凌立成,2
(1. 华东理工大学,化学工程联合国家重点实验室, 上海200237;2. 华东理工大学,特种功能高分子材料及相关技术教育部重点实验室, 上海200237)
1 Introduction
Pitches, usually fabricated from coal tar, petroleum residues, polyvinyl chloride (PVC)or polycyclic aromatic hydrocarbon (PAH) monomers, exhibit such properties as high molecular weight, wide molecular weight distribution and high carbon yield[1]. Many high performance carbon products such as carbon fibers[2,3], carbon foams[4], advanced carbon/carbon composites[5]and anode materials for lithium ion batteries[6]can be developed from carbonaceous pitches. Among the aforementioned original materials for pitches, pure PAHs have features of highly aromatic, free from heteroatoms and inorganic ash, which make it ideal raw materials for high-quality pitches. However, the coupling reaction of PAHs is not an easy task because of its low reactivity. Considerable insight into this kind of reaction has been gained in past decades. Greinke et al[7]. converted monomeric aromatics into oligomeric compounds via thermal polymerization, but this process has some drawbacks, for example, extreme conditions (high temperature above 470 ℃ and high pressure up to 9.3 MPa) and a relatively low yield of approx. 30%. Mochida et al[8-10]. employed diverse catalysts to condense model aromatics such as naphthalene, methylnaphthalene and anthracene. It was reported that isotropic carbons could be synthesized through dehydrogenative polymerization in the presence of alkali metals[8], while graphitizable carbons could be prepared through non-dehydrogenative condensation under catalysis of aluminum chloride[9]or HF-BF3[10]. However, alkali metals or AlCl3interact intimately with pitches which could not be removed completely, and fatally trace residues can cause sharp volume expansion in subsequent carbonization. Spinnable mesophase pitches were effectively prepared from arenes by the aid of HF-BF3on an industrial scale. Despite the catalysts could be easily recovered through distillation, the super-acid catalyst still presented extremely strict requirements for reactors.
Recently, new routes have been created to polymerize methylnaphthalene by thermal bromination or visible light irradiation assisted bromination methods. Kim et al[11]. reported that isotropic pitches with high softening points and high solubilities were prepared through thermal-bromination of 2-methylnaphthalene (2-MNP) followed by dehydrobromination at 320 ℃. Ge et al[12]. obtained graphitizable methylene-bridged pitches from 1-methylnaphthalene (1-MNP) by adjusting bromination conditions, and found that visible light irradiation induced methyl bromination was the key point. Since iodine shares many similarities to bromine due to its location in the same group of periodic table, it is assumed that iodine with a low volatility and high stability may be an alternative to bromine because of its advantages, such as milder reaction conditions, lower bond enthalpies of C-I and less dependence on external conditions[13]. To date, many technologies have been proposed for synthesis of aromatic iodides[14-19].Among these methods, I2O5assisted electrophilic iodination has turned out to be an effective, environment-friendly and regioselective choice because it can eliminate heavy metal salt wastes and improve iodine atom utilization[20,21]. It was reported that acid and iodine were firstly adsorbed and activated onto the surface of I2O5respectively, thenπ-complex was formed between arene and the activated I2species under the action of delocalized electron cloud, and finally aromatic iodide was produced through transformation ofπ-complex intoσ-complex and cleavage of C-H bond. It should be noted that dose of H2SO4catalyst was essential to boost iodination. To the best of our knowledge, iodination of 1-MNP using diiodine pentoxide, iodine and sulfuric acid has not been reported before, and nor for thermal-dehydroiodination investigation of iodinated methylnaphthalenes. Hence, it is necessary to study I2O5assisted iodination of 1-MNP as well as thermal-dehydroiodination behavior of iodinated species.
Herein, a new approach to prepare high carbon yield polymerized pitches from 1-MNP through oxidative iodination/dehydroiodination is developed. 1-MNP is firstly iodinated in the presence of I2O5, I2and sulfuric acid. Then, iodinated methylnaphthalenes are thermally-treated at an elevating temperature of 130-180 ℃ to cleave C-I bond, thus polymerizing 1-MNP monomers. The influence of solvent, reaction temperature and molar ratio of raw materials in iodination is investigated. Moreover, the effect of dehydroiodination temperature and time on physical properties of obtained pitches is investigated as well. It is the first try to polymerize methylnaphthalene through I2O5assisted electrophilic iodination to develop pitches with high carbon yields.
2 Experimental
2.1 Preparation of polymeric pitches
1-MNP (97%, Acros Organics), I2O5(AR, Aladdin Industrial Corporation), sulfuric acid (AR, Shanghai Lingfeng Chemical Reagent Co., Ltd.), I2(AR, Shanghai Macklin Biochemical Co., Ltd.) and H2SO4(AR, Shanghai Lingfeng Chemical Reagent Co., Ltd.) were used without further purification. AR grade acetic acid, acetone, methanol and n-heptane were used as reaction solvents for the iodination for comparison.
The synthesis was started from the iodination of 1-MNP in the presence of I2O5,I2and concentrated H2SO4. The reaction was performed in a 500 mL three-necked round-bottom Pyrex glass flask (TPF) equipped with a water condenser. Typically, 71.1 g 1-MNP was dissolved in a solvent to a concentration of 1 mol/L by vigorous stirring under a N2gas flow of 100 mL/min. After uniform mixing, stoichiometric I2O5and I2were added into the flask and stirred for 5min. Then, 545 μL sulfuric acid was added dropwise and kept at 60 ℃ for 24 h. The product was obtained by rotary evaporation and the solvent was recovered.
Since the optimal iodination condition was determined, dehydroiodination was carried out at 130, 140, 160 and 180 ℃ for a total duration ranging from 3 to 9 h under nitrogen flow. The obtained pitch was extracted with methanol to remove unreacted monomers and light fractions. The resulting pitches were denoted as ID-T-t, whereTandtwere the dehydroiodination temperature (℃) and time (h), respectively.
2.2 Characterization
The molecular structure of iodination products and their contents were characterized by a GC/MS instrument (Shimadzu GCMS-QP2010 plus) equipped with a DB-5 mass capillary column. Samples of 1 μL were injected into the injector with a split ratio of 1∶30. Oven temperature was kept at 100 ℃ for 5 min, then increased to 260 ℃ at a rate of 7 ℃/min and held for 3 min. The inlet temperature was kept at 300 ℃ with helium as a carrier gas. Quantitative analysis of each essential component was obtained from peak area normalization measurement.
The carbon and hydrogen contents of the prepared pitches were determined using an elemental analyzer (elementar vario EL III, Germany). The residual iodine content was obtained by difference.
The pitch was fractionated by sequential Soxhlet extraction using toluene, pyridine and quinolone to ascertain its group composition. The softening points of the extracted pitches were measured by thermomechanical analysis (DMA2980, America). The sample was initially melted in an aluminum crucible and then placed in the chamber with a probe on the surface of the sample exerting a constant force of 0.001 N. The pitch was heated from ambient to 300 ℃ at a heating rate of 5 ℃/min in an inert atmosphere. The softening point was determined by the intersection of tangents before and after the insertion.
Pyridine soluble fractions (PS) of the pitches were analyzed with H-NMR (Bruker Avance 400 spectrometer). The sample was dissolved in CDCl3using tetramethylsilane as an internal standard.
The thermogravimetric analysis (TA Instrument Q600 Analyzer) was carried out in a nitrogen flow rate of 100 mL min-1. The samples were heated to 850 ℃ at a rate of 10 ℃ min-1. Fourier transform infrared (FTIR) spectra of samples were collected by a Nicolet 6700 FTIR spectrometer. The samples were firstly fine ground and then diluted with KBr. The FTIR spectra were recorded by accumulating 128 scans at a spectra resolution of 4 cm-1.
The molecular weight of the extracted polymeric pitch was measured with a 4 800 Plus LDI TOF/MS analyzer (AB Sciex) which was equipped with 355 nm nitrogen laser and used in linear, positive-ion mode. Pyridine soluble fractions of the pitches were dissolved in pyridine.
3 Results and discussion
3.1 Electrophilic iodination of 1-MNP


Fig. 1 Mechanism scheme for I2O5 assisted electrophilic iodination of 1-MNP.
Fig. 2 illustrates GC-MS spectra of products iodinated at 60 ℃ in acetic acid with the molar ratio of 1-MNP, I2and I2O5of 1∶0.4∶0.4. As can be seen, the spectrum exhibits three major peaks A, B and C with the retention time of 7.4, 16.0 and 22.6 min, respectively. Mass spectra suggest that molecular ion peaks of A, B and C are located at m/z 141, m/z 268 and m/z 394, indicating that A, B and C belong to 1-MNP, 1-iodo-4-MNP and diiodo-substituted derivatives. To be noted, para position of methyl group is to be iodinated at first due to denser electron cloud and the steric effect, while diiodo-substituted arenes have five isomers due to the high reactivity of electrophilic iodine species.

Fig. 2 (a) GC-MS spectrum of iodination products, (b) mass spectrum of peak A, (c) mass spectrum of peak B and (d) mass spectrum of peak C.
Since Stavber et al[14]. reported that the solvent played a crucial role in the iodination of arenes, we first investigated the effect of different solvents, reaction temperature and the molar ratio of reactants on the conversion of 1-MNP into the corresponding iodo-substituted derivatives. Fig. 3 illustrates the distribution of iodinated products obtained with different solvents. The detailed information concerning synthesis conditions is summarized in Table 1. Four types of solvent including acetone, acetic acid, n-heptane and methanol are used for comparison. It can be seen that the property of solvent influences the distribution of iodinated products significantly. Here, acetic acid (entry 3) is the most suitable solvent compared with methanol, n-heptane and acetone in terms of iodina tion efficiency and selectivity. As for the reason, acetic acid probably stabilizes the transition state of theπ-complex due to unclear interactions between I2and the oxygen in acetic acid molecule and boosts desorption of 1-iodo-4-MNP from surface of I2O5. In addition, the steric hindrance of CH3COO-facilitates para substitution, thus achieving an extraordinarily high selectivity. In other cases, methanol promotes substitution on alkyne groups, and hydroxyl in alcohol initiates side reactions easily, decreasing selectivity substantially. Acetone and n-heptane could not stabilize the intermediate electrophilic iodine species.

Fig. 3 GC-MS spectra of iodination products obtained from different solvents.

Table 1 The product distribution of iodination under various conditions.
Note:s: solvent;T: reaction temperature, ℃; molar ratio of 1-MNP, I2and I2O5; A, B and C demonstrate the fractions mentioned in Fig. 1(a);Smb: mono-iodination selectivity among products,Smb=100B/ (A+B+C).
Fig. 4 shows the influence of reaction temperature on the product distribution. At a relatively low temperature of 30 ℃, only 6.6% 1-MNP is iodinated (entry 5). With the increase of temperature to 60 ℃, almost all 1-MNP could be iodinated with a mono-iodination selectivity (Smb) of 96.1% (entry 3). Further increase of temperature to 80 ℃ results in a higher content of diiodo-methylnaphthalenes with aSmbof 91.5% (entry 6). Brazdil[21]reported that rate constant of iodination reactions using iodine, diiodine pentoxide and sulfuric acid steadily increased as a function of temperature. So an increase of iodination temperature results in a higher rate constant and more 1-MNP could be iodinated in unit time. However, if activated I2species are generated too fast, mono-iodinated products could be re-iodinated to form di-iodinated aromatics before desorbing from the surface of I2O5, and the selectivity is greatly reduced as a result.

Fig. 4 GC-MS spectra of iodination products obtained under different temperatures.
Fig. 5 presents the influence of reactant stoichiometric ratio on the product distribution. The addition amount of I2O5strongly affects the kind and content of iodination products.Diiodo-methylnaphthalene exhibits a sharp rise from 0% (entry 7) to 32.1% (entry 9) with increasing the amount of I2O5from 0.15 to 0.4 molar equivalents. The optimumSmbof 96.4% is achieved when 0.18 mol I2O5and 0.75 mol I2are reacted with 1 mol 1-MNP at 60 ℃ in acetic acid. I2O5supplies sites for iodination and could not be consumed in the whole process. So an increase in amount of I2O5implies an addition of reaction sites, thus more iodinated methylnaphthalenes could be generated in unit time. However, too many I2O5particles dispersed in solutions enhance possibilities of re-iodination, and diiodinated arenes are produced. So iodination at 60 ℃ in acetic acid with the molar ratio of 1-MNP, I2and I2O5of 1∶0.75∶0.18 is proposed as the optimized conditions.

Fig. 5 GC-MS spectra of iodination products obtained from different molar ratios of reactants.
3.2 Dehydroiodination/polycondensation of iodinated aromatics
After the optimized iodination conditions had been determined, thermal-treatment was carried out at an elevating temperature to study the influence of temperature and time on dehydroiodination products. Table 2 summarizes the various dehydroiodination conditions in detail. With the increase of reaction temperature and time, the polymerization degree is gradually enhanced, which is reflected by viscosity. Dehydroiodination under 130 ℃ for 3 h results in liquid products. With the reaction time increased to 9 h, the product becomes soft solids. When reaction temperature is further increased to 140 ℃, the product becomes solids after 6 h.

Table 2 Dehydroiodination products synthesized under various conditions.
Note: L-liquids; SS-soft solids; S-solids.
As we all know, pitches are complex mixtures composed of aliphatic hydrocarbons and aromatic hydrocarbons with at least three condensed rings. And it’s hardly to find a solvent to dissolve completely all the components, which brings about difficulties to elucidate its structure. An effective way is to capture structure information of light components, which could be analyzed with many techniques. Here, GC-MS is applied to elucidate dehydroiodination process by analyzing methanol-soluble fractions of pitches. Fig. 6 illustrates the composition of methanol-soluble fractions of dehydroiodination products synthesized at 130 ℃ for 3, 6 and 9 h. The integration results based on the area of the whole spectra are available in Table 3. As can be seen from the spectra, an obvious drop of fraction B can be observed for ID130-9, which is attributed to extensive dehydroiodination. This is confirmed by some weak peaks with the retention time above 26.8 min, which corresponds to dimer of methylnaphthalene units (see Fig. S1). No obvious difference can be found for ID130-3 and ID130-6, suggesting dehydroiodination at 130 ℃ is conducted at a relatively slow rate. So a further increase of dehydroiodination temperature is needed.
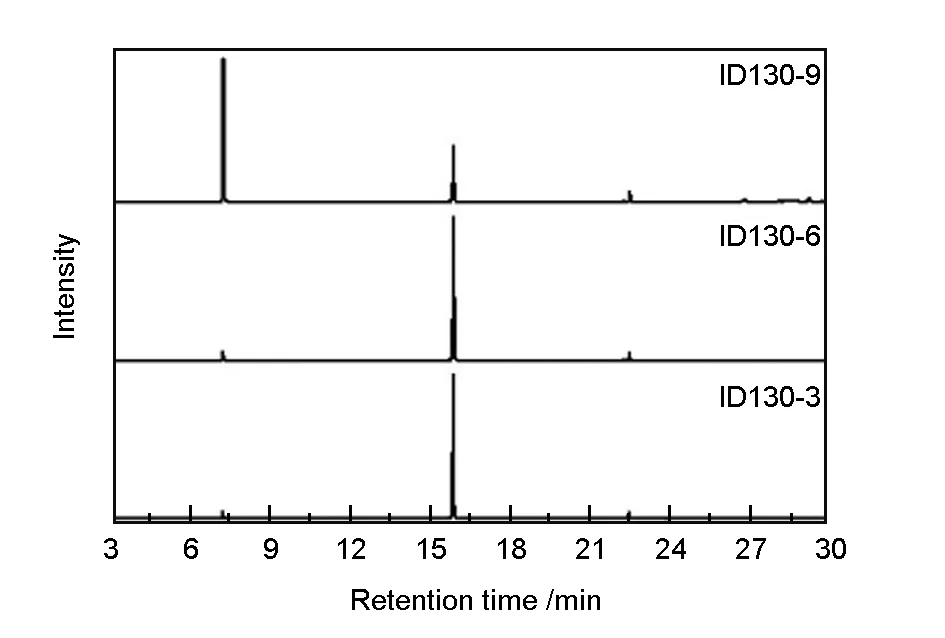
Fig. 6 GC-MS spectra of products dehydroiodinated at 130 ℃ for 3, 6 and 9 h.

SamplesArea percentage /%ABCID130-34.191.84.1ID130-65.290.44.4ID130-960.630.39.1
Fig. 7 shows composition of methanol-soluble fractions for dehydroiodination products prepared at 140 ℃ for 3, 6 and 9 h. A list of the identified PAHs is given in Table 4.

Fig. 7 GC-MS spectra of products dehydroiodinated at 140 ℃ for 3, 6 and 9 h.
Compared with those of ID130, methanol-soluble fractions of ID140 show more complicated compositions, such as tetralin, naphthalene, 2-methylnaphthalene and multiple methylated naphthalenes. Component distribution of ID140-3 is similar to ID130-9, the difference is that ID140-3 contains less 1-iodo-4-MNP, indicating the rate of dehydroiodination reaction is enhanced substantially at 140 ℃. However, ring-hydrogenation products (tetralin, 5-methyltetralin) and methyl migration products (2-MNP, dimethylnaphthalene and trimethylnaphthalene) are generated with an increase of reaction time. In addition, a long reaction time favors methyl migration reactions, which can be evidenced by the intensity of the peak 5 and 6 in Fig. 7. The formation of methyl migration products and ring-hydrogenation products is probably due to the existence of diverse iodonium valency states. Maccoll[24]reported that the released HI with a strong reducibility could react with aromatic iodides, generating iodine. For this system, aromatics could be re-iodinated in the presence of remaining I2O5and re-generated iodine, and atomic hydrogens are produced following step 6 in Fig. 1. Atomic hydrogens collide with methyl groups and activate methyl into free-state CH3·, then CH3· migrates in the solution until running into naphthalene rings, thus forming dimethyl-naphthalenes or trimethyl-naphthalenes. Otherwise, atomic hydrogens attack naphthalene rings directly and ring-hydrogenation products are formed[25]. The longer the reaction time, the more the free-state atomic hydrogens would be formed, consequently their collision with methyl group is facilitated and the content of methyl migration products is increased. So ID140-9 shows stronger responses for 2-MNP, dimethyl-naphthalenes and trimethyl-naphthalenes than ID-140-6.

Table 4 Identification of the components in products dehydroiodinated at 140 ℃.
In summary, dehydroiodination process involves complicated hydrogen transfer reactions and methyl migration reactions, which is ascribed to free-state atomic hydrogens. Dehydroiodination efficiency is substantially enhanced when the reaction temperature is increased to 140 ℃ and products exhibit solid state only after 6 h.
3.3 Characterization of polymeric pitches
Some physical properties of the extracted pitches including softening point, carbon yield, H/C ratio and solubility are summarized in Table 5.

Table 5 General physical properties of the synthesized pitches.
a: Softening point measured by TMA.b: Carbon yield at 850 ℃.c: TS: toluene soluble fraction; TI-PS: toluene insoluble-pyridine soluble fraction; PI-QS: pyridine insoluble-quinoline soluble fraction; QI: quinolone insoluble fraction.
As shown in Fig. S2, the softening points, ranging from 148 to 184 ℃, are increased with the dehydroiodination temperature and time. When iodinated arenes are thermal-treated at 180 ℃ for 3 h, the resultant pitch is non-meltable solids, which is attributed to drastic and repetitive iodination/dehydroiodination accompanied by coking. The sum of C and H is increased with reaction temperature and time, distributed from 74.71% of ID140-6 to 86.08% of ID180-3, which suggests a deeper dehydroiodination at a higher temperature for a longer time. In addition, compared with other pitches, H/C ratio of ID180-3 experiences an obvious decrease to 0.51, indicating a higher content of intramolecular C—C bond among aromatic rings.
The solubility of obtained pitches is characterized by sequential Soxhlet extraction. As shown in Table 5, TS content of pitches is decreased with dehydroiodination temperature and time from 11.2 to 6.5 wt.%, indicating the polymerization degree is steadily increased with an increment of dehydroiodination temperature and time. Besides, QI content of pitches varies greatly with reaction conditions. ID140-6 is all quinolone-soluble. However, QI jumps to 32.3 wt.% when increased to 180 ℃, which demonstrates the vital role of the introduced iodine on the formation of C—C bonds and the growth of molecules. Due to a relatively low bond enthalpy of C—I, free radicals could easily be formed through cleavage of C—I bond and thus oligomers are formed. However, as mentioned earlier, there is a significant chance for iodinated aromatics to be re-iodinated, and this would finally lead to multiple aromatic units linking to one nucleus. Therefore, the resultant pitches are mainly composed of highly condensed molecules, which can be evidenced by carbon yields of pitches in the range from 53.9 to 65.9 wt.% (see Fig. S3).
The hydrogen distributions of as-prepared pitches were determined by1H-NMR spectra in terms of pyridine soluble fractions, as shown in Fig. 8. The integration results based on peak area normalization measurements are summarized in Table 6.
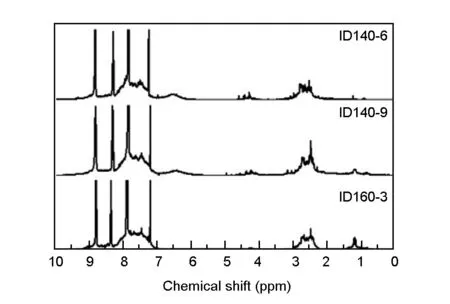
Fig. 8 1H-NMR spectra for pyridine-soluble fractions of extracted pitches.

Pitches1H-NMRHarHαHCH2HβHγfaID140-674.318.33.63.20.60.92ID140-970.820.72.35.11.20.91ID160-374.717.11.25.41.60.93
Har: 9-6 ppm, aromatic hydrogen; Hα: 5.2-2.0 ppm, alpha aliphatic hydrogen; Hβ: 2.0-1.0 ppm, beta aliphatic hydrogen; Hγ: 1.0-0.5 ppm, gamma aliphatic hydrogen;fa: aromaticity factor.
It is found that the pitches have a relatively high aromaticity of ca. 0.91-0.93[26], indicating that the pitches mainly consist of condensed polycyclic structures. Besides, all pitches have signals of Hβand Hγdue to the existence of hydrogenation structures. It’s strange that the aromatic hydrogen of ID140-9 is lower than ID140-6. As previously noted, a longer reaction time promotes methyl migration initiated by free-state hydrogens. The most reasonable explanation of this phenomenon is that components with multi alkyl substituents in ID140-9 account for a larger proportion and contribute to a higher aliphatic hydrogen content. Moreover, the spectra also reveal bonding sites of the oligomers. Hαof polymeric pitches exhibits a broad peak rather than a sharp one with its chemical shift ranging from 2.3 to 3.0 ppm. Ge et al[12]reported that the oligomers with exclusively 1,4’- or 1,5’- connections in alpha-position of naphthalene rings show major signals at ca. 2.7 ppm, and the peak corresponding to Hαshifts to ca. 2.5 ppm whenβ-position connection structures are introduced. So the polymeric pitches have characteristics of diverse connection patterns including both alpha-position connection and beta-position connection, thus forming complicated cross-linking structures.


Fig. 9 FT-IR spectra of extracted pitches.
The molecular weight distributions of the pitches in terms of pyridine-soluble components are measured by laser desorption/ionization time-of-flight mass spectroscopy (LDI-TOF/MS) as illustrated in Fig. 10 (a). All pitches consist of sharp peaks that correspond to various oligomers and methyl migration derivatives. Molecular weight distributions of the polymeric pitches are in the range of 250-800 with its abundance in dimer and trimer. Take ID160-3 as an example, the sample exhibits intense peaks at M’-14, M’, M’+14, M’+28 and M’+42, which suggests that the extensive methyl migration reaction occur. Besides, peak M’-2, M’+4, M’+12 can also be observed. Components with M’-2 is generated through dehydrogenation to form perylene while M’+4 results from hydrogenation to form naphthenic structures[28]. Some possible molecular structures are proposed in Fig. 10 (b). It is worth noting that the development of molecular chain is restricted to tetramer. Due to repeated iodination/dehydroiodination mode, oligomers containing excessive cross-linking structures are formed and viscosities are sharply increased, hindering molecular assembly and further chain growth.

Fig. 10 (a) LDI-TOF/MS spectra for pyridine-soluble components of as-prepared pitches and (b) predicted molecular structures of representative components.
4 Conclusions
Novel highly condensed polymeric pitches have been successfully prepared through I2O5assisted electrophilic iodination of 1-MNP followed by dehydroiodination/polycondensation and the removal of light fractions by methanol extraction. The key to the preparation depends on iodination of 1-MNP, which could produce 1-iodo-4-methylnaphthalene with a high yield and selectivity by the aid of I2O5and sulfuric acid. Dehydroiodination investigation reveals that hydrogen transfer and methyl migration reactions are available, which might be caused by free-state atomic hydrogen. And iodinated aromatics could be polymerized into solids under 140 ℃ for 6 h. As-prepared pitches show excellent physical properties, such as high aromaticity (0.91-0.93), high carbon yield (53.9-65.9 wt.%) and high purity. The structural elucidation suggests that dehydroiodination could effectively condense 1-MNP molecules into oligomers with an extraordinarily high degree of polymerization. All results indicate that the iodination/dehydroiodination process is a simple, mild and clean approach to prepare high quality pitches.
[1] Edwards W F, Jin L, Thies M C. MALDI-TOF mass spectrometry: obtaining reliable mass spectra for insoluble carbonaceous pitches[J]. Carbon, 2003, 41(14): 2761-2768.
[2] Mochida I, Yoon S H, Takano N, et al. Microstructure of mesophase pitch-based carbon fiber and its control[J]. Carbon, 1996, 34(8): 941-956.
[3] Maeda T, Zeng S M, Tokumitsu K, et al. Preparation of isotropic pitch precursors for general purpose carbon fibers (GPCF) by air blowing-I. Preparation of spinnable isotropic pitch precursor from coal tar by air blowing[J]. Carbon, 1993, 31(3): 407-412.
[4] Chen C, Kennel E B, Stiller A H, et al. Carbon foam derived from various precursors[J]. Carbon, 2006, 44(8): 1535-1543.
[5] White J L, Sheaffer P M. Pitch-based processing of carbon-carbon composites[J]. Carbon, 1989, 27(5): 697-707.
[6] Huang S, Guo H, Li X, et al. Carbonization and graphitization of pitch applied for anode materials of high power lithium ion batteries[J]. Journal of Solid State Electrochemistry, 2013, 17(5): 1401-1408.
[7] Greinke R A, Lewis I C. Carbonization of naphthalene and dimethylnaphthalene[J]. Carbon, 1984, 22(3): 305-314.
[8] Mochida I, Nakamura E, Maeda K, et al. Carbonization of aromatic hydro carbons-III: carbonization catalyzed by alkali metals[J]. Carbon, 1975, 13(6): 489-493.
[9] Mochida I, Kudo K, Fukuda N, et al. Carbonization of pitches-IV: Carbonization of polycyclic aromatic hydrocarbons under the presence of aluminum chloride catalyst[J]. Carbon, 1975, 13(2): 135-139.
[10] Mochida I, Shimizu K, Korai Y, et al. Structure and carbonization properties of pitches produced catalytically from aromatic hydrocarbons with HF/BF3[J]. Carbon, 1988, 26(6): 843-852.
[11] Kim B J, Kil H, Watanabe N, et al. Preparation of novel isotropic pitch with high softening point and solvent solubility for pitch-based electrospun carbon nanofiber[J]. Current Organic Chemistry, 2013, 17(13): 1463-1468
[12] Ge C, Yang H, Miyawaki J, et al. Synthesis and characterization of high-softening-point methylene-bridged pitches by visible light irradiation assisted free-radical bromination[J]. Carbon, 2015, 95: 780-788.
[13] Blanksby S J, Ellison G B. Bond dissociation energies of organic molecules[J]. Accounts of chemical research, 2003, 36(4): 255-263.
[14] Stavber S, Jereb M, Zupan M. Electrophilic iodination of organic compounds using elemental iodine or iodides[J]. Synthesis, 2008, 2008(10): 1487-1513.
[15] Wing-Wah S. Iodination of methoxyamphetamines with iodine and silver sulfate[J]. Tetrahedron letters, 1993, 34(39): 6223-6224.
[16] Orito K, Hatakeyama T, Takeo M, et al. Iodination of alkyl aryl ethers by mercury (II) oxide-iodine reagent in dichloromethane[J]. Synthesis, 1995(10): 1273-1277.
[17] Sathiyapriya R, Karunakaran R J. A convenient procedure for the iodination of arenes[J]. Journal of Chemical Research, 2006(9): 575-576.
[18] Lulinski P, Skulski L. Oxidative iodination of arenes with manganese (IV) oxide or potassium permanganate as the oxidants[J]. Bulletin of the Chemical Society of Japan, 1999, 72(1): 115-120.
[19] Olah G A, Wang Q, Sandford G, et al. Iodination of deactivated aromatics with N-iodosuccinimide in trifluoromethanesulfonic acid (NIC-CF3SO3H) via in situ generated superelectrophilic iodine (I) trifluoromethanesulfonate[J]. Journal of organic chemistry, 1993, 58(11): 3194-3195.
[20] Brazdil L C, Cutler C J. Selective production of diiodobenzene and iodobenzene from benzene[J]. The Journal of Organic Chemistry, 1996, 61(26): 9621-9622.
[21] Brazdil L C, Fitch J L, Cutler C J, et al. Kinetics of aromatic iodination reactions using iodine, diiodine pentoxide and sulfuric acid in acetic acid[J]. Journal of the Chemical Society, Perkin Transactions 2, 1998 (4): 933-936.
[22] Ogata Y, Aoki K. Iodination of aromatic compounds with a mixture of iodine and peracetic acid. III. Autocatalysis and relative rates[J]. Journal of the American Chemical Society, 1968, 90(22): 6187-6191.
[23] Ogata Y, Nakajima K. Iodination of aromatic compounds with a mixture of peroxyacetic acid and iodine[J]. Tetrahedron, 1964, 20(1): 43-47.
[24] Maccoll A. Heterolysis and the pyrolysis of alkyl halides in the gas phase[J]. Chemical Reviews, 1969, 69(1): 33-60.
[25] Szwarc M, Ghosh B N, Sehon A H. The C-Br bond dissociation energy in benzyl bromide and allyl bromide[J]. The Journal of Chemical Physics, 1950, 18(9): 1142-1149.
[26] Masuda K, Okuma O, Nishizawa T, et al. High-temperature nmr analysis of aromatic units in asphaltenes and preasphaltenes derived from Victorian brown coal[J]. Fuel, 1996, 75(3): 295-299.
[27] Korai Y, Nakamura M, Mochida I, et al. Mesophase pitches prepared from methylnaphthalene by the aid of HFBF3[J]. Carbon, 1991, 29(4-5): 561-567.
[28] Ida T, Akada K, Okura T, et al. Carbonization of methylene-bridged aromatic oligomers[J]. Carbon, 1992, 30(2): 165-171.
