生物质莲杆废弃物制备高比表面积多孔炭及其CO2吸附性能
2018-06-28吴星星张乘云田忠卫蔡进军
吴星星, 张乘云, 田忠卫, 蔡进军
(1. 湘潭大学 化工学院, 湖南 湘潭411105;2. 清华大学 固体废物处理与环境安全教育部重点实验室, 北京100084)
1 Introduction
The CO2emitted from excessive burning of fossil fuels is regarded as one of the main greenhouse gas for global warming[1-3].It is urgent to design new route for the reduction of CO2emission. Carbon capture method is a promising option owing to its low energy requirement and easy operation where the adsorption using sorbents is an efficient way without production of pollution[4-6].Porous carbons have aroused the most attention for CO2capture among the various adsorbents including zeolite, polymers, silica, and metal-organic frameworks[7], because carbons have all pre-requisite attributes for the capture such as tailored pores, large surface areas, hydrophobicity, and viable adsorptive sites on the carbon surface. Therefore, it is not difficult to understand the situation that although porous carbons have possible slightly inferior uptakes during the CO2capture as compared with other adsorbents, they have still seized the greatest interest in the area of CO2capture.
Carbons are often synthesized from precursors ranging from coal to biomass through direct pyrolysis or hydrothermal carbonization followed by activation treatment[1,2,8].The past five decades have witnessed tremendous progress in preparation of porous carbons and their application in CO2capture, indicating that surface area and pore size distribution of carbons are two of the most critical points for CO2capture[9,10].It is thought that not only activation affects microstructure of carbons, but the precursors used. For a large-scale sustainable production, biomass should be used as the precursor because biomass is renewable, environment friendly, easily accessible in the nature, and low cost. Nowadays, more and more scientists have focused on biomass-derived porous carbons for CO2capture[11-14]. Significantly, various agriculture wastes have been used to obtain carbons, including corn, leaves, longan shells, shrimp, cotton, mango, cocoa, sawdust, straw, asphalt, grape fruit, aloe, bagasse, and rice husk, which have been fully summarized in several reviewsreported by others[15-18].Using agriculture wastes as precursors will reduce waste pollution and acquire commercial value[11]. For example, Han et al[12]. reported carbons from longan shells with KOH activation showed a CO2uptake of 4.3 mmol/g at 25 ℃ and 1 bar. Jaroniec et al[19].reported coconut- based carbons from CO2activation had an uptake of 3.9 mmol/g at 25 ℃. Recently, Jiang et al[20]. reported carbons from KOH-activated pine cone had an uptake of 7.63 mmol/g at 0 ℃ while carbon with the highest surface area did not show the highest value, implying that surface area is not the single factor for uptake, and pores less than 0.7 nm have an important role in the CO2capture. Compared with the direct pyrolysis method, hydrothermal treatment has great advantages because it can operate at mild conditions and handle biomass without drying pre-treatment. For example, Wu et al[11]. reported carbon spheres with a surface area of 3 350 m2/g from hydrothermally carbonized starch and CO2activation, which exhibited a CO2uptake of only 2.5 mmol/g at 25 ℃ and 1 bar, but a high uptake of 21.2 mmol/g at 20 bar, among the largest values ever reported up to date. Comparatively, Liu et al[21]. reported N-doped carbons with a surface area of 1 405 m2/g from waste Coca Cola had a CO2uptake of up to 5.22 mmol/g at 25 ℃ and 1 bar, the largest value ever reported for carbons. Therefore, it is not difficult to imagine that the CO2uptake is closely related to the precursor and final microstructure of carbons. How to select a proper biomass precursor for preparation of carbons is one of critical points for the success of CO2capture.
Lotus is a perennial aquatic plant and the living environment of lotus makes them rich in pores in their stem structure to suffice their respiration[22-24]. There are abundant lotus resources in south China. As well known, most of lotus will be directly wasted due to less value except that some proportion of lotus root or leaves were used as food or tea. Each autumn, most of lotus stem (LS) were abandoned in the pond and decayed with little or no use, resulting in solid waste and environmental pollution. It has enormous interest to transform lotus-related waste into other high-value-added matters rather than decay naturally. Guo et al[25]. and Li et al[26]. have separately reported that carbons from lotus root shells by direct pyrolysis or hydrothermal carbonization followed by KOH activation have an excellent capacitive performance. Luo et al[27]. reported carbons from the pyrolysis of lotus leaves or stems, and the LS-derived carbon has a surface area of 1 610 m2/g, which is about 55% higher than the one from leaves and exhibits a specific capacitance of 174 F/g at 5 mV/s in 6 M KOH electrolytes. However, there is no report about the carbon from LS waste using the hydrothermal carbonization method in published literatures, to the best of our knowledge. Considering the fact that fresh LS usually has a high content of O-species and fiber combined with lavish pores in the structure[26],it is reasonable to believe that LS should be a promising precursor for carbons. Considering the advantages of hydrothermal pyrolysis like no drying pretreatment of precursors and high yields of ultimate carbons[1,11,21,25], in this work, LS-derived carbons with abundant O-species were prepared from the hydrothermal pyrolysis followed by KOH activation using 1 wt% sulfuric acid instead of pure water as medium to assist hydrothermal pyrolysis. As-obtained carbon has a surface area of 2 893 m2/g with CO2uptakes of 6.17 and 3.85 mmol/g at 0 and 25 ℃, respectively. The importance of micropores in structure of carbons for the CO2capture is highlighted. The low-cost production together with high CO2uptakes imply that LS waste is a good precursor for porous carbon to achieve waste recycling, and LS-based carbon is a good candidate for the next generation CO2absorbents.
2 Experimental
2.1 Preparation of LS-derived carbons
Activated carbons were derived by hydrothermal pyrolysis followed by KOH activation using lotus stem (LS) as precursor. Fresh LS was collected from a local plantation in south China, washed with distilled water, cut into pieces, and dried in an oven at 80 ℃ for 24 h. In a typical run, 5 g dried LS was dispersed into 85 mL of 1 wt.% sulfuric acid solution[28], and then transferred into a 100 mL Teflon-lined autoclave to carry out the hydrothermal pyrolysis at 180 ℃ for 24 h. The product was collected by filtration, washed with distilled water and dried at 110 ℃ for 24 h. The dried solid was pyrolyzed at 450 ℃ with a heating rate of 4 ℃/min under N2flow for 2 h to get char. After that, the char was mixed with KOH in a char/KOH mass ratio of 1∶0, 1∶2 or 1∶4, and then activated at 800 ℃ for 1 h with a heating rate of 2 ℃/min under N2flow of 80 mL/min. The resulting carbons were treated with 2 M HCl at 60 ℃ for 3 h followed by leaching with distilled water until neutral pH was reached, and dried at 110 ℃ for 24 h. The carbons were denoted as AC-x, where x (x =0, 2 or 4) stands for the char/KOH mass ratio. The ultimate yields of carbons based on dried LS are about 27-38 wt% varying with activation conditions.
2.2 Characterization
Thermal analysis of the hydrothermal product was performed on a thermogravimetric device (TGA/DSC 1/1600HT) up to 800 ℃ at a heating rate of 10 ℃/min under N2or air flow. The structure and morphology of carbons were studied by the X-ray diffraction (XRD, Bruker D8 ADVANCE), Raman spectra (Renishaw-inVia, UK), scanning electron microscopy (SEM, JSM6610LV) and high-resolution transmission electron microscopy (HRTEM, Tecnai G2 F20). Surface chemistry was analyzed by photoelectron spectroscopy (XPS, ESCALab 250Xi). Pore structure was studied by N2adsorption performed on a volumetric sorption analyzer (Micromeritics, TriStar II 3020) after evacuation for 6 h at 200 ℃. Brunauer-Emmett-Teller (BET) method was used to calculate specific surface area. Total pore volume was determined from adsorbed N2amount at thep/p0value of 0.99. Pore size distributions (PSDs) were calculated from adsorption branch of isotherms based on density functional theory (DFT) model by assuming a slit-pore geometry. To evaluate capture performance, the CO2adsorption measurements were performed on a sorption analyzer at 25 or 0 ℃ and 1 bar, after the evacuation at 200 ℃ for 6 h.
3 Results and discussion


Fig. 1 Thermogravimetric analysis for hydrothermal solid under nitrogen and air atmosphere.
The XRD patterns of carbons in Fig.2 show two characteristic peaks at around 24° and 44°, corre sponding to the (002) and (100) planes from turbos tratic carbons, respectively[30].The almost smooth diffraction curves suggest that all the carbons comprise of mainly amorphous carbons. In addition, the intensity of (002) plane for AC-0 shown in inset of Fig.2 is much lower than the other two activated samples, indicating that the KOH activation moderately inhibit the graphitization of carbon to some extent. The broad weak diffractions located at around 2θ=24° and 44° suggest the presence of some domains of graphene-like sheets in the carbons[2, 14]. However, the weak intensity in the two typical diffraction patterns indicate that the carbons are composed of a significant content of amorphous carbon and their graphitization degreesare very low. On the other hand, the typical SEM image for hydrothermal solid from LS precursor at 180 ℃ shown in Fig.3a displays an apparent fiber-like structure. It can be observed from Fig.3 that the typical morphology of carbons changes little after KOH activation and the smooth surface with a typical fiber-structure remains, implying that the fiber-structure is not broken by the KOH activation. All the carbons have irregularly shaped holes in their structures, while the carbon surface seems to be etched at high KOH/char ratios. Therefore the two samples of AC-2 and AC-4 have relatively more eroded cavities with larger surface areas. A close inspection of AC-2 sample through HRTEM in Fig.3e,f show randomly distributed pores, which is a typical characteristics of activated carbon with a developed microporous structure. Moreover, the enlarged TEM image of AC-2 in Fig.3f shows some disordered layered-graphite structures at edge positions, indicating a less ordered graphitic structure. All the morphological and microstructure results are in good agreement with the XRD results in Fig.2 that the present LS-based carbons are largely amorphous.
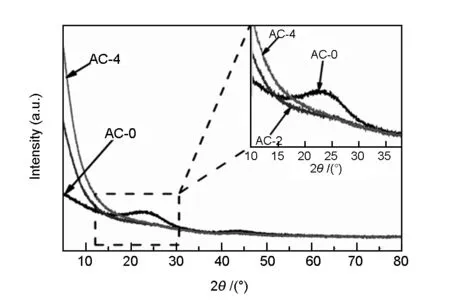
Fig. 2 XRD patterns for the LS-derived carbons.
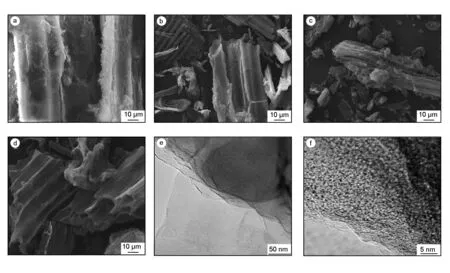
Fig. 3 The typical SEM images: (a) hydrothermal product from LS precursor, (b) AC-0, (c) AC-2, and (d) AC-4, and (e, f) typical TEM images for AC-2.
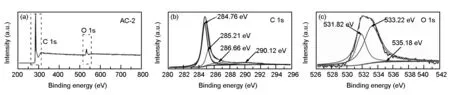
Fig. 4 XPS spectra of the AC-2: (a) XPS survey spectrum, (b) C 1s spectrum, and (c) O 1s spectrum.

Sample[a]SBET(m2/g)[b]Vtotal(cm3/g)[c]Vmicro(cm3/g)[d]Dp(nm)XPS composition (at.%)COCO2 uptake (mmol/g)25 ℃0 ℃AC-0422 (399)0.160.151.5291.638.372.333.02AC-22091 (1644)0.870.651.6790.499.513.856.17AC-42893 (1105)1.590.702.2088.8011.202.844.61
Note:[a]BET surface areas calculated from N2adsorption data atp/p0=0.02-0.10, and values in parenthesis are microporous surface areas;[b]Total pore volumes estimated from the adsorption data atp/p0=0.99;[c]Mesopore volumes calculated by BJH method using adsorption volume of pores from 1.7 to 300 nm;[d]Average pore size determined from Dubinin-Astakhov (DA) method.
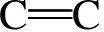
N2sorption isotherms for LS-based carbons are shown in Fig. 5a. The pore size distributions (PSDs) obtained from adsorption branch using density functional theory (DFT) model are shown in Fig. 5b and the detailed results are listed in Table 1. As can be seen from Fig.5a, the adsorbed N2amount for AC-0 without activation is the least in series of carbons, and adsorption amount levels off at relative pressures (p/p0) beyond 0.1, which is a typical isotherm characteristics for microporous materials[33]. After KOH activation, N2uptakes for AC-2 and AC-4 at the very low pressure ofp/p0<0.05 are much high, but the adsorbed N2amounts gradually increase atp/p0up to 0.1 for AC-2 and 0.5 for AC-4, then knee off thereafter and reach horizontal plateau with a further increase ofp/p0, indicating the presence of some small-sized mesopores in the carbons[11]. The larger ‘knee’ turning point for AC-4 indicates that the pore sizes for AC-4 are much wider than AC-2 and AC-0. It should be noted that N2adsorption isotherms for all the three carbons do not show an obvious hysteresis loop over the whole pressure range, implying the presence of almost micropores in carbons and absence of large-sized mesopores or macropores. As shown in Fig.5b, KOH activation has a remarkable influence on the PSDs for the carbons, in which AC-0 has almost pure micropores while AC-2 and AC-4 have some small-sized mesopores less than 3.0 and 5.0 nm, respectively. Changes in the pore sizes are probable due to a partial collapse of pores for existing micropores and mesopores, and also the generation of new micropores on the surface of carbons[34]. Using KOH as reagents to activate carbons is a traditional technique for creating micropores involving a reaction between KOH and carbon[29,30]:
6KOH+2C↔2K+3H2+2K2CO3
(1)
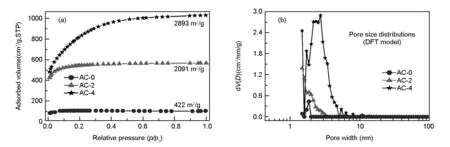
Fig. 5 (a) N2 adsorption isotherms, and (b) pore size distributions for LS-derived carbons calculated from DFT model.
Reaction between potassium and carbon during etching is mainly responsible for creating micropores, and the decomposition of K2CO3at high temperatures to form small molecules (e.g. H2O, CO2and CO, etc) also contributes to the generation of micropores. Generally, the smoother is N2isotherm, the more micropores in the carbons, and vice versa[2]N2isotherms for AC-0 are evidently much smoother than AC-4 and AC-2 (Fig.5a), and AC-0 with the lowest surface areas of only 422 m2/g shows the highest microporosity (Table 1). It can be observed from Table 1 that an increase of KOH/char ratio enlarges the surface area and pore volume of carbons. For instance, the calculated BET surface areas and total pore volumes for AC-0 are only 422 m2/g and 0.16 cm3/g, respectively, while the corresponding values for AC-4 are 2 893 m2/g and 1.59 cm3/g, and the values for AC-2 are 2 091 m2/g and 0.87 cm3/g. The high ratios of KOH/cha increase surface area and pore volume, yet widen the PSDs for carbons (Fig.5b). Therefore, AC-0 has the highest microporosity of 93.7% while AC-4 has a microporosity of only 44% (Table 1). This could be caused by the fact that KOH activation widens small-sized micropores to form small-sized mesopores by a breakage between adjacent micropores.
The CO2adsorption isotherms on the carbons at ambient pressure and 0 and 25 ℃ are illustrated in Fig.6 and the detailed uptakes are summarized in Table 1. It can be observed from Fig.6 that the trend for CO2adsorption at 0 and 25 ℃ are quite similar. The CO2uptakes sharply decrease with temperature, demonstrating a physisorption nature. It is generally believed that the carbon with a larger surface area will also have a larger uptake[1]. However, the CO2uptakes on AC-4 are much lower than those on AC-2 at 0 or 25 ℃ even though AC-4 has the largest surface area and pore volume. Very interestingly, although AC-0 has a surface area of only 422 m2/g which is much lower than the the values for AC-2 (2 091 m2/g) and AC-4 (2 893 m2/g), it can be observed from Fig.6 that the CO2uptake for AC-0 is higher than those for AC-2 and AC-4 at the low pressures. For instance, the CO2uptake at 25 ℃ for AC-0 is higher than AC-2 and AC-4 as pressures are lower than 216 and 654 mbar, respectively. The CO2uptake for AC-0 at 0 ℃ is higher than those for AC-2 and AC-4 as pressures are lower than 135 and 426 mbar, respectively. The variation of turning points for pressure imply that CO2adsorption in the carbons is physisorption, and the adsorbed CO2molecules reach the equilibrium more easily in pores at lower temperatures, because the effective micropores for CO2adsorption in the carbons decrease with temperature[35].However, the CO2uptakes at 1 bar for AC-0 are of 2.33 and 3.02 mmol/g at 25 and 0 ℃, respectively, which are the lowest data in series of carbons due to smallest surface area of 422 m2/g and pore volume of 0.16 cm3/g. The CO2uptakes on the AC-4 with the largest surface areas of 2 893 m2/g are 2.84 and 4.61 mmol/g at 25 and 0 ℃ and 1bar, respectively. Significantly, the CO2uptake at 1 bar and 25 ℃ for AC-2 is 3.85 mmol/g, comparable to those previously reported for some high-surface-areas N-doped carbons from polymers, biomass wastes and metal-organic frameworks (MOFs) designed for CO2capture[2,19,36-41].It should be admitted that the CO2uptake is still lower than some N-doped microporous carbons from other kinds of biomass wastes[21,33,35]. For instance, Sevilla et al[33].reported that the CO2uptake on a sawdust-based carbon with a surface area of 1 260 m2/g is 4.8 mmol/g at 25 ℃ and 1 bar. Deng et al[35]. reported a carbon from pine nut shells has a CO2uptake of 5.0 mmol/g at 25 ℃ and 1 bar. There are some researches that indicate that N-doped group on the surface of carbons increase CO2uptakes. Liu et al[21]. reported a N-doped carbon with a surface area of 1 405 m2/g from waste Coca Cola has a CO2uptake of 5.22 mmol/g at 25 ℃ and 1 bar, the largest uptake ever reported for carbons up to date, which is resulted from the fact that 55.8% pores in the carbon are less than 0.8 nm. All the results imply that the selection of precursor and activation condition is very important for improving CO2capture performance. Detailed comparative results of the CO2uptake at 25 ℃ and 1 bar for AC-2 and other carbons derived from other precursors are listed in Table 2.
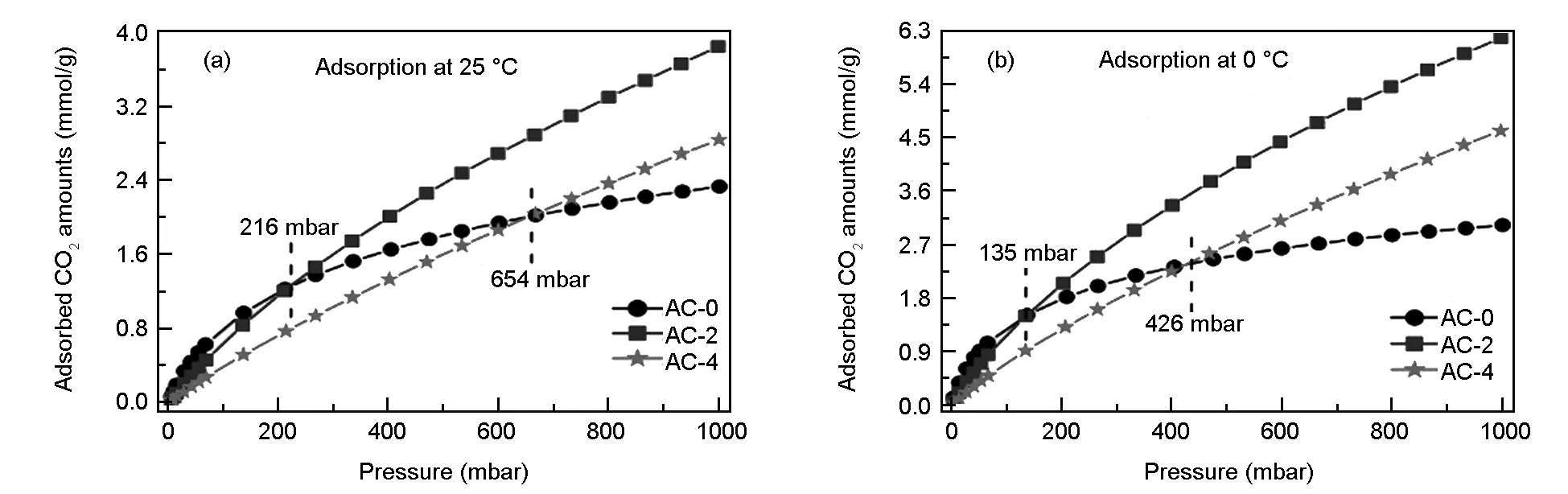
Fig. 6 The CO2 adsorption isotherms at (a) 25 and (b) 0 ℃ for LS-derived carbons.

Table 2 The comparison of CO2 uptakes at 25 ℃ and 1 bar for carbons derived from various precursors.
Combined with the PSD curves shown in Fig.5b, the superior CO2uptake at 1 bar for AC-2 is mainly due to the presence of moderate surface areas and high microporosity, and the inferior CO2uptake for AC-4 is due to some small-sized mesopores in carbon frameworks caused by the severe activation, which result in different interactions between adsorbed CO2molecules and pores[2],reflecting the importance of some small-sized micropores for CO2capture. In addition, the CO2uptake per unit surface area is proportional to the contents of O-species on the carbons (Table 1), implying that there is also a partial enhancement of the uptake by introduction of O-species[32].
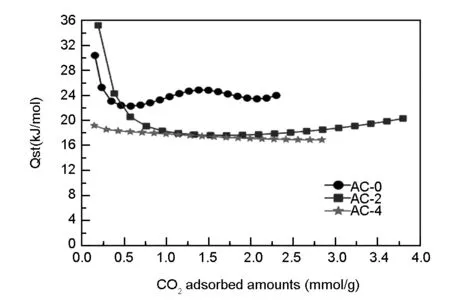
Fig. 7 Isosteric heat of CO2 adsorption as a function of adsorbed amounts on LS-derived carbons.
Apart from the uptake for carbons, isosteric heat (Qst) of CO2adsorption is also an important indicator for regenerability of carbons as solid adsorbents. It is generally believed that a largerQstvalue indicates relative stronger interactions between the adsorbed CO2molecules and pores[2]. TheQstof CO2adsorption on LS-derived carbons in relation to the adsorbed amounts is calculated from the Clausius-Clapeyron formula using adsorption data at 25 and 0 ℃, and the results are illustrated in Fig.7. As can be clearly observed from Fig.7 that theQstof CO2adsorption on the AC-0 with a surface area of 422 m2/g is in the range of 22-32 kJ/mol, comparable to those previously reported values on high-surface-area carbons (e.g.25-20 kJ/mol)[11,33], and much larger than the values on ordered mesoporous carbons (e.g.18.2 kJ/mol)[45], due to its high microporosity and small-sized micropores. In contrast, AC-4 with the largest surface area has the lowest stableQstof around 17 kJ/mol, which is probable due to the fact that AC-4 has the majority of pores in the 2-5 nm range. Interestingly, theQstdata on AC-2 first decrease and then increase slightly, indicating interaction between adsorbed CO2molecules is more pronounced in pores under high surface coverages, resulting from the high surface area and large quantity of pores in the 2-3 nm range generated by KOH activation (Fig.2b). Significantly, initialQstof CO2adsorption on AC-2 is highly up to 36 kJ/mol, indicating that the AC-2 strongly interacts with CO2molecules at low surface coverages. It should be noted thatQstdata for CO2adsorption are much lower than the typical values for chemisorption (>60 kJ/mol)[11],indicating that the interaction between the adsorbed CO2molecules and pores is mainly physical interactions involving weak Van Der Waals and electrostatic interaction. All trends inQstdata for CO2adsorption imply that micropores in the carbons are occupied first and achieve a saturation level after certain amounts of CO2uptakes. Considering the high uptake of 3.85 mmol/g at 25 ℃ and moderateQstvalues, these LS-derived carbons are excellent adsorbents for CO2capture.
4 Conclusion
O-rich carbons with high surface areas were obtained by hydrothermal pyrolysis followed by KOH activation using LS waste as the precursor. This type of carbon has the highest surface area of up to 2 893 m2/g and pore volumes up to 1.59 cm3/g. KOH activation enlarges surface area and pore volumes in addition to broaden pore size. The carbons have a good CO2capture performance at ambient pressures, with uptakes to 3.85 and 6.17 mmol/g at 25 and 0 ℃, respectively. Significantly, the carbons with narrower pore sizes have greater affinity with CO2than the ones with a high surface area in the CO2capture at ambient pressure owing to the relative strong interactions between pore walls of carbons and CO2molecules. The results indicate that LS waste-derived carbons are promising materials for CO2capture.
[1] Yang G, Ye J, Yan J, et al. Preparation and CO2adsorption properties of porous carbon from camphor leaves by hydrothermal carbonization and sequential potassium hydroxide activation[J]. RSC Advances, 2017, 7: 4152-4160.
[2] Cai J, Qi J, Yang C, et al. Poly(vinylidene chloride)-based carbon with ultrahigh micro- porosity and outstanding performance for CH4and H2storage and CO2capture[J]. ACS Applied Materials & Interfaces, 2014, 6: 3703-3711.
[3] Tang Z, Han Z, Yang G, et al. Preparation of nanoporous carbons with hierarchical pore structure for CO2capture[J]. New Carbon Materials, 2013, 28: 55-60.
[4] D'Alessandro D M, Smit B, Long J R. Carbon dioxide capture: prospects for new materials[J]. Angewandte Chemie-International Edition, 2010, 49: 6058-6082.
[5] MacDowell N, Florin N, Buchard A, et al. An overview of CO2capture technologies[J]. Energy & Environmental Science, 2010, 3: 1645-1669.
[6] Lu A H, Hao G P. Porous materials for carbon dioxide capture[J]. Annual Reports Section "A" (Inorganic Chemistry), 2013, 109: 484-503.
[7] Fu R W, Li Z H, Liang Y R, et al. Hierarchical porous carbons: design, preparation, and performance in energy storage[J]. New Carbon Materials, 2011, 26: 171-179.
[8] Yang M, Guo L, Hu G, et al. Adsorption of CO2by petroleum coke nitrogen-doped porous carbons synthesized by combining ammoxidation with KOH activation[J]. Industrial & Engineering Chemistry Research, 2016, 55: 757-765.
[9] Sevilla M, Parra J B, Fuertes A B. Assessment of the role of micropore size and N-doping in CO2capture by porous carbons[J]. ACS Applied Materials & Interfaces, 2013, 5: 6360-6368.
[10] Zhang Z, Zhou J, Xing W, et al. Critical role of small micropores in high CO2uptake[J]. Physical Chemistry Chemical Physics, 2013, 15: 2523-2529.
[11] Li Y, Li D, Rao Y, et al. Superior CO2, CH4, and H2uptakes over ultrahigh-surface-area carbon spheres prepared from sustainable biomass- derived char by CO2activation[J]. Carbon, 2016, 105: 454-462.
[12] Wei H, Chen H, Fu N, et al. Excellent electro- chemical properties and large CO2capture of nitrogen-doped activated porous carbon synthesised from waste longan shells[J]. Electrochimical Acta, 2017, 231: 403-411.
[13] Singh G, Kim I Y, Lakhi K S, et al. Single step synthesis of activated bio-carbons with a high surface area and their excellent CO2adsorption capacity[J]. Carbon, 2017, 116: 448-455.
[14] Zhu X L, Wang P Y, Peng C, et al. Activated carbon produced from paulownia sawdust for high-performance CO2sorbents[J]. Chinese Chemical Letters, 2014, 25: 929-932.
[15] Yahya M A, Al-Qodah Z, Ngah C W Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review[J]. Renewable and Sustainable Energy Reviews, 2015, 46: 218-235.
[16] Tekin K, Karagöz S, Bektas S. A review of hydrothermal biomass processing[J]. Renewable and Sustainable Energy Reviews, 2014, 40: 673-687.
[17] Wang J, Nie P, Ding B, et al. Biomass derived carbon for energy storage devices[J]. Journal of Materials Chemistry A, 2017, 5: 2411-2428.
[18] Gao Z, Zhang Y, Song N, et al. Biomass-derived renewable carbon materials for electrochemical energy storage[J]. Materials Research Letters, 2017, 5: 69-88.
[19] Ello A S, de Souza L K C, Trokourey A, et al. Coconut shell-based microporous carbons for CO2capture[J]. Microporous and Mesoporous Materials, 2013, 180: 280-283.
[20] Li K, Tian S, Jiang J, et al. Pine cone shell-based activated carbon used for CO2adsorption[J]. Journal of Materials Chemistry A, 2016, 4: 5223-5234.
[21] Boyjoo Y, Cheng Y, Zhong H, et al. From waste Coca Cola® to activated carbons with impressive capabilities for CO2adsorption and supercapacitors[J]. Carbon, 2017, 116: 490-499.
[22] Tian Z, Qiu Y, Zhou J, et al. The direct carbonization of algae biomass to hierarchical porous carbons and CO2adsorption properties[J]. Materials Letters, 2016, 180: 162-165.
[23] Wu X, Tian Z, Hu L, et al. Macroalgae-derived nitrogen-doped hierarchical porous carbons with high performance for H2storage and supercapacitors[J]. RSC Advances, 2017, 7: 32795-32805.
[24] Tian Z, Xiang M, Zhou J, et al. Nitrogen and oxygen-doped hierarchical porous carbons from algae biomass: direct carbonization and excellent electrochemical properties[J]. Electrochimical Acta, 2016, 211: 225-233.
[25] Wang X, Wang M, Zhang X, et al. Low-cost, green synthesis of highly porous carbons derived from lotus root shell as superior performance electrode materials in supercapacitor[J]. Journal of Energy Chemistry, 2016, 25: 26-34.
[26] Liu B, Zhou X, Chen H, et al. Promising porous carbons derived from lotus seedpods with outstanding supercapacitance performance[J]. Electrochimical Acta, 2016, 208: 55-63.
[27] Zhang Y, Liu S, Zheng X, et al. Biomass organs control the porosity of their pyrolyzed carbon[J]. Advanced Functional Materials, 2017, 27: 1604687.
[28] Sun W, Lipka S M, Swartz C, et al. Hemp- derived activated carbons for supercapacitors[J]. Carbon, 2016, 103: 181-192.
[29] Wang K, Zhao N, Lei S, et al. Promising biomass-based activated carbons derived from willow catkins for high performance supercapacitors[J]. Electrochimical Acta, 2015, 166:1-11.
[30] Wang R, Wang P, Yan X, et al. Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2capture performance[J]. ACS Applied Materials & Interfaces, 2012, 4: 5800-5806.
[31] Yu W, Wang H, Liu S, et al. N, O-codoped hierarchical porous carbons derived from algae for high-capacity supercapacitors and battery anodes[J]. Journal of Materials Chemistry A, 2016, 4: 5973-5983.
[32] Xing W, Liu C, Zhou Z, et al. Oxygen- containing functional group-facilitated CO2capture by carbide-derived carbons[J]. Nanoscale Research Letters, 2014, 9: 189.
[33] Sevilla M, Fuertes A B. Sustainable porous carbons with a superior performance for CO2capture[J]. Energy & Environmental Science, 2011, 4: 1765-1771.
[34] Sevilla M, Fuertes A B, Mokaya R. Preparation and hydrogen storage capacity of highly porous activated carbon materials derived from polythio- phene[J]. International Journal of Hydrogen Energy, 2011, 36: 15658-15663.
[35] Deng S, Wei H, Chen T, et al. Superior CO2adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures[J]. Chemical Engineering Journal, 2014, 253: 46-54.
[36] Liu L, Xu S D, Wang F Y, et al. Nitrogen-doped carbon materials with cubic ordered mesostructure: low-temperature autoclaving synthesis for electrochemical supercapacitor and CO2capture[J]. RSC Advances, 2017, 7: 12524-12533.
[37] Wang L, Yang R T, Significantly increased CO2adsorption performance of nanostructured templated carbon by tuning surface area and nitrogen doping[J]. The Journal of Physical Chemistry C, 2011, 116: 1099-1106.
[38] Wang J, Senkovska I, Oschatz M, et al. Highly porous nitrogen-doped polyimine-based carbons with adjustable microstructures for CO2capture[J]. Journal of Materials Chemistry A, 2013, 1: 10951-10961.
[39] Hao G P, Li W C, Qian D, et al. Structurally designed synthesis of mechanically stable poly(benzoxazine-co-resol)-based porous carbon monoliths and their application as high- performance CO2capture sorbents[J]. Journal of the American Chemical Society, 2011, 133: 11378-11388.
[40] Pan Y, Xue M, Chen M, et al. ZIF-derived in situ nitrogen decorated porous carbons for CO2capture[J]. Inorganic Chemistry Frontiers, 2016, 3: 112-1118.
[41] Luo H, Zhu C C, Tan Z C, et al. Preparation of N-doped activated carbons with high CO2capture performance from microalgae (Chloro-coccum sp.)[J]. RSC Advances, 2016, 6: 38724-38730.
[42] Bezerra D P, Oliveira R S, Vieira R S, et al. Adsorption of CO2on nitrogen-enriched activated carbon and zeolite 13X[J]. Adsorption, 2011, 17: 235-246.
[43] Zhou J, Li W, Zhang Z, et al. Carbon dioxide adsorption performance of N-doped zeolite Y templated carbons[J]. RSC Advances, 2012, 2: 161-167.
[44] Mahurin S M, Górka J, Nelson K M, et al. Enhanced CO2/N2selectivity in amidoxime- modified porous carbon[J]. Carbon, 2014, 67: 457-464.
[45] Yuan B, Wu X, Chen Y, et al. Adsorption of CO2, CH4, and N2on ordered mesoporous carbon: approach for greenhouse gases capture and biogas upgrading[J]. Environmental Science & Technology, 2013, 47: 5474-5480.
