水热环境下棉纤维水解炭化机理
2018-06-28崔丽萍侯文生阎智锋戴晋明
崔丽萍, 史 晟, 侯文生, 阎智锋, 戴晋明
(1. 太原理工大学 煤化工研究所, 山西 太原030024;2. 太原理工大学 轻纺工程学院, 山西 太原030024)
1 Introduction
Presently, the processing of waste textiles has become one of the biggest concerns for experts and the public in general. Given the wide range of textiles, the recycling of cotton fibers, which amount to about 85% of the total natural fibers produced, has attracted the attention of many researchers. At present, physical methods, such as loosening of fabrics to obtain recycled fibers, are used, which reduce the value of the textiles after weaving or gluing. However, repeated loosening and weaving of recycled textiles cannot be carried out many times. Hence, finally, they have to be discarded and the problem of repeated reuse of cotton fibers is far from being solved. Cotton is mainly composed of cellulose, which is a polysaccharide, a natural polymer with the highest carbon content (as high as 44%). It is a linear polymer, which consists ofβ-D-glucopyranosyl residues linked to each other byβ-1,4-glycosidic bonds[1]. The glycosidic bond is sensitive to a high concentration of H+, which makes cotton fibers susceptible to hydrolysis, forming polysaccharide in concentrated acids. The structure also has a large number of hydrogen-bonded linkages besides the stable glycosidic linkages[2,3]. Due to the length of cotton cellulose-macromolecule, which is 5 000 nm or more[4], almost all the hydroxyl groups are involved in hydrogen bonding, which increases the number of intermolecular hydrogen bonds to a large extent. The stability of the cellulose-macromolecule is precisely due to the glycosidic bonds and the presence of hydrogen bonds[5,6]. Hence, chemical treatment is relatively more difficult.
In recent years, technologies involving hydrothermal carbonization of biomass materials have attracted more and more attention[7,8]. Many researchers world-wide have focused on producing coke from carbohydrates using subcritical water (temperature of 180-350 ℃ and pressure of 2-20 MPa)[9-11]. Mi[9]synthesized carbon micro-spheres with the aqueous glucose solution as starting materials in a stainless steel autoclave at 500 ℃ for 12 h. The carbon micro-spheres have a regular and perfect shape, high yields and narrow-range distributions, and diameters ranging from 1 to 2 μm. Sevilla[10]studied hydrothermal carbonization of simple sugars. Zhang et al[11]. used hydrothermal synthesis method utilizing glucose as carbon source to obtain homogeneous nano-sized carbon spheres. Studies on hydrothermal treatment of monosaccharides or oligosaccharides showed that temperature was the main factor that influences carbonization of carbohydrates. However, there are only a few researches on the hydrothermal behavior of cellulose-macromolecule having a high degree of polymerization. According to the structure of cellulose, pH of the hydrothermal system is one of the main factors, which also has an impact on carbonization, structure, and properties. Therefore, this paper mainly focuses on the discussion on the effects of initial pH of hydrothermal reaction on the carbonization of cotton, thus paving a new way for obtaining the high-value-added use of waste cotton.
2 Experimental
2.1 Chemicals and instruments
Waste cotton fabrics were procured from Shanxi Greenland Textile Co. Ltd. The high-temperature autoclave (1 L capacity with a maximum operating pressure and temperature of 42 MPa and 500 ℃, respectively, and equipped with a feeding valve) was purchased from Run Chang Dalian Petrochemical Equipment Co., Ltd.
2.2 Preparation
NaOH and HCl solutions (0.05 mol/L), prepared in distilled water, were mixed to obtain aqueous solutions with a pH value from 10 to 2. The pH values of the solutions were measured by a pH meter. Then waste cotton debris (12 g, washed and decolorized) was collected, divided into several parts and added to 600 mL of the aqueous solution. This mixture was then transferred into an autoclave to 60% of its capacity and sealed. The autoclave was then heated rapidly to 280 ℃, allowed to react for 8 h and then cooled to room temperature. Thereafter, the reaction products were taken out and centrifuged to separate the solid and liquid products. The solid was later washed repeatedly with alcohol and distilled water, and placed in a drying oven at 120 ℃ for 4 h.
The hydrothermal carbon yield and calorific value (h) were calculated as follows:
Yield of hydrothermal carbon (mass fraction) = (quantity of hydrothermal carbon/ quantity of raw dried material) × 100%,
(1)
h(MJ/Kg)=0.3383ωC+1.422(ωH-ωO/8)
(2)
WhereωC,ωHandωOare quality score of C, H, O, respectively.
2.3 characterization
Field emission scanning electron microscopy (JSM-6700F, Japan-Electronics Company) was used to study the morphologies and structures of products. The crystal structures of samples were characterized by X-ray diffraction using a Y-2000 (Dandong Tong Da Technology Co., China). C and H elemental contents were determined using a Vario Micro cube elemental analyzer (ELEMENTAR, Germany ). Laser Raman scattering spectra of the samples were recorded using an InVia Raman spectrometer (Renishaw Company,UK). Liquid products were characterized by Flexar LC-Chromera high performance liquid chromatography using a PerkinElmer instrument, US. Refraction-differential detector RID, SH1011 and chromatographic columns from Shodex, Japan was used. The mobile phase was 5 × 10-3mol/L H2SO4solution, the column temperature was 50 ℃, the flow rate was 0.5 mL/min, and the sample volume was 10 μL.
3 Results and discussion
3.1 Effect of pH value on the physical properties of hydrothermal products

Fig. 1 SEM images of cotton fiber products, carbonized at different pH values:
Fig. 1 shows the SEM images of products of cotton fibers, carbonized at different pH values. From the images, it was clear that the structure of the material obtained from cotton after hydrothermal treatment was globular. It was also evident that pH value of carbonization had a remarkable impact on the morphologies of the hydrothermal products. Under weakly basic conditions, the hydrothermal products appeared as irregular particles and the range of particle sizes was large with a strong fusion of particles. When the initial pH of carbonization was neutral, although some spherical particles appeared in the picture, the overall appearance of the product was not changed. When the pH decreased further, the shape of the products became spherical and the number of particles decreased. When the pH was 3.5, the product particles were mainly spherical and the particle sizes were between 0.8-3 μm. When the pH values of carbonization was <2.5, the product appears as jelly-like balls, bonded with each other.
The morphologies and crystal structures of cotton fibers underwent significant changes after hydrothermal treatment. Fig. 2 shows the XRD diffraction patterns of cotton and its carbonized products. When the pH was 8, the crystal structure of cotton fiber could not be observed. With the decrease in pH value till 2.5, the crystal structure of carbonized products did not show any significant changes. Broad diffraction peak appears for the products at 2θ= 22.7° (Crystal face index 002), which could be attributed to the diffraction peak of graphitic carbon. The larged002suggested that the structure be highly disordered and the degree of graphitization be very low[12].

Fig. 2 XRD patterns of cotton fibers and cotton fiber products carbonized at different pH values:
Fig. 3 shows the Raman spectra of carbon microspheres. The peak at 1 590 cm-1(G-band) corresponded to theE2gvibrational mode of graphite. It was due to the vibrations of sp2carbon atoms of graphite in a two-dimensional hexagonal lattice[13]. The peak at 1 355 cm-1(D-band) could be attributed to the dangling bonds, which induced disorders in the two dimensional hexagonal lattice. This peak indicated defects in the hexagonal lattices of graphite sheets. Values of relative intensity ratios (IG/ID) inGandDbands is a reflection of the degree of graphi tization of carbonaceous materials. TheIG/IDvalues for pH = 8, pH = 6, pH = 3.5, and pH = 2.5, were 1.291, 1.316, 1.480, and 1.489, respectively. Degree of graphitization in the carbonized products increased with decreasing pH value, but this change was small. These results were consistent with the X-ray diffraction, according to which the graphitization degree of hydrothermal products was far below than those of the other graphitic materials.
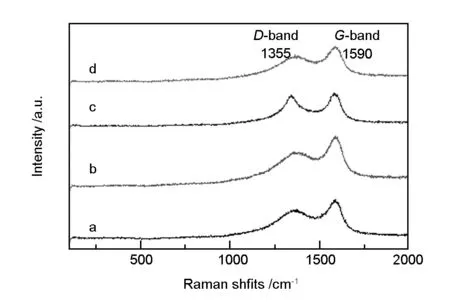
Fig. 3 Raman spectra of carbon microspheres:
3.2 Effect of pH value on chemical properties of hydrothermal products
Table 1 lists the yield of hydrothermal products of cotton fibers and the results of elemental compositions at different pH values. In Table 1,wis the quality score, whereasγrepresents the atomic ratios of the constituent elements. Cotton fibers had different degrees of carbonization under alkaline, neutral, and acidic conditions. At pH = 10, carbon content was 68.02%, which was higher than that of cotton (43.11%). However, a reduction of pH valueimproved the degree of carbonization of cotton fibers. Carbon content of the product obtained in neutral water was 73.90%, and when the pH value was 3, it was 75.34%. The carbon content increased much slowly with a further reduction of pH value, whereas at the same time,γ(C/O) andγ(C/H) increased gradually. This was mainly due to the fact that dehydration and decarboxylation caused the hydrothermal carbonization of cotton. H and O in cotton formed H2O and CO2[14,15].
A high calorific value is also another feature of carbide. High carbon and low oxygen contents lead to a high calorific value[16]. Thus lowering the pH value of water not only increased the carbon content of the products, but also increased the calorific value. When pH was 3.5, the calorific value was 27.84 MJ/kg. However, under the same conditions of holding time and temperature, the effect of pH on the yield was not obvious. Overall, due to the low levels of carbonization yields under alkaline conditions, the yields of the products were significantly higher than those of their counterparts under acidic conditions. With a decrease in pH value, the yield was slightly lowered and remained around 20%.
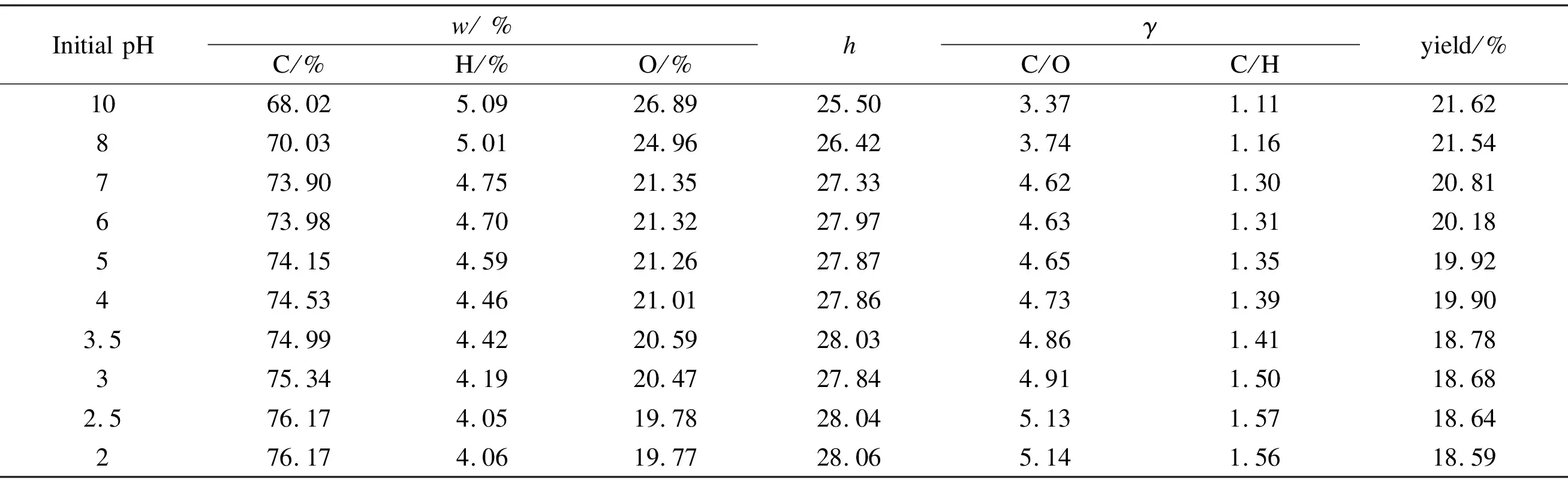
Table 1 Productivity and elemental analysis of hydro-char from cotton at different pH values.
3.3 Influence of pH value on the liquid products in hydrothermal treatment
At first, the pH variations of the reaction system before and after hydrothermal treatment of cotton fibers were studied (Table 2). It was found that large amounts of organic acids were generated during the hydrolysis of cellulose[17,18]. These acids neutralized the alkaline compounds in the system. Hence, when the initial pH value was more than or equal to 7, the pH was lowered after the reaction. Moreover, with increasing alkalinity of the reaction system, acidic intermediate products were generated[19]. When the initial pH was 2- 4, due to the higher H+concentration in the reaction solution, pH was not significantly reduced after the reaction.

Table 2 Initial and final pH values of cotton fibers in hydrothermal carbonization.
In addition, the liquid components in the hydrothermal process were analyzed by high performance liquid chromatography, and the results showed that the main liquid product during hydrothermal reaction was glucose, and that pH value had a great influence on the glucose yield[20]. Fig. 4 shows the curves for the formation of glucose at different pH values. It was evident that the rate of glucose formation reached 25.3% at pH 2.5 for 6 min. The rate increased to 40.5% when the time was increased to 20 min. However, if the reaction time was increased further, the rate of glucose formation declined rapidly. This was due to the complete hydrolysis of cotton to glucose under these conditions, and glucose could be easily broken down to form small molecular compounds in subcritical water[21]. When pH was 8, glucose yield in the hydrothermal system was very low, and the highest yield that was obtained for 10 min was only 2.1%. It is noteworthy that the glucose yield when the pH was 3.5 was lower than that when the pH was 2.5. This was due to the breakdown of glucose into many compounds with different functional groups, such as carboxylic acids, aldehydes, and furan. The dehydration and condensation of these compounds generated compounds of aromatic family, which then became spherical carbonaceous materials. When the H+concentration in reaction system was high, the hydrolysis of cotton could not utilize all these H+. The surplus H+hindered the cracking of glucose, which affected the morphology and reduced the carbon content of the products[22].
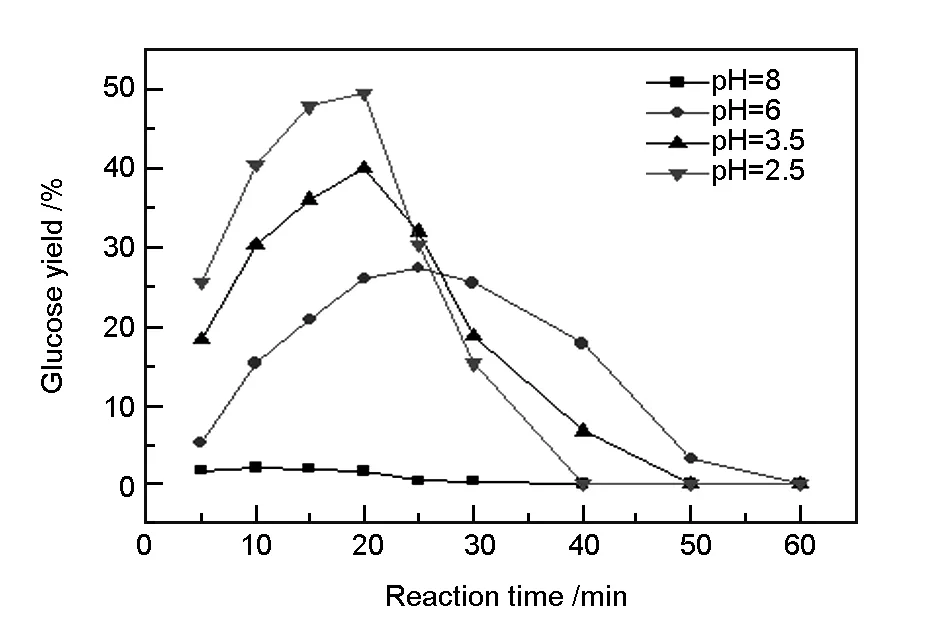
Fig. 4 Curves for glucose yields from hydrolysis of waste cotton fibers at different pH values.
3.4 Mechanism of influence of pH value on hydrothermal carbonization of cotton
The analytical results discussed above showed that cotton fibers could be carbonized to form spherical carbonaceous material in subcritical water. The concentration of H+in the reaction system had a great impact on the morphology of the products. However, XRD and Raman spectral analysis showed that the crystal structure of cellulose could be damaged in subcritical water, causing gradually dehydration of cotton. In this process, the effect of H+was relatively small. However, a high concentration of H+could improve the formation rate of the intermediate products, glucose and carbide. Based on the above analysis, the study suggested that factors that determine the carbonization and product morphology be different. According to the literature, glucose is an important intermediate product that determines whether cellulose can be carbonized[23]. In the hydrothermal processing, subcritical water could provide enough energy to destroy cellulose molecules, but this was complicated and cellulose could not be hydrolyzed to a stable state. So, although cotton could react under neutral or alkaline hydrothermal conditions, its morphology was not good enough. However, in the presence of H+in the reaction system, theβ-1,4 glycosidic linkage of cellulose was attacked, which formed —OH at one end of glycosidic linkage and a carbocation (R—CH+) at the other end. It could neutralize the effect of H2O, resulting in the combination of —OH of H2O and R—CH+to form R—CH—OH, with the simultaneous release of H+[24]. The released H+continued to attack the glycosidic linkages of the cellulose macromolecules. The above process kept on and cellulose was fully hydrolyzed to monosaccharides or oligosaccharides (as shown in Fig. 5). The dehydration reaction that followed formed the carbon spheres. Although the ionic product constant of subcritical water was 1 000 times more than that of the ambient water[8], in the hydrolysis of cotton fibers, the effect of H+to quickly break down the glycosidic linkages of the fiber molecules was not large enough. Under alkaline condition, a large number of H+ions in the reaction system were consumed, causing insufficient hydrolysis of the cotton fibers. A good morphological structure of carbon products could not be formed, but it could not prevent the damage to the crystal structure of the cotton fibers.

Fig. 5 Scheme for hydrolysis of cotton fibers.
4 Conclusion
In subcritical water, the crystal structures of cotton fibers were damaged and hydrolyzed to form monosaccharides. Then the amorphous carbonaceous materials were formed, which had spherical morphology and a specific structure through the dehydration reaction. Changes in pH value would not affect the process of crystal structure destruction of cotton fibers. The dehydration of fibers could be carried out under alkaline, neutral, and acidic conditions, but the dehydration degree varied greatly. In the reaction system, H+had a major influence on the morphology, due to their ability to hydrolyze cotton fibers to form monosaccharides. Spherical carbonaceous materials with uniform sizes could be obtained at pH = 3.5. Their carbon content reached 74.99%, with a calorific value up to 27.84 MJ/kg. A high concentration of H+was beneficial for the improvement of the degree of carbonization and calorific value of the carbonaceous material.
[1] Peter Zugenmaier. Conformation and packing of various crystalline cellulose fibers[J]. Progress in Polymer Science, 2001, 26(9): 1341-1417.
[2] Shaka S, Ueno T. Chemical conversion of various celluloses to glucose and its derivatives in supercritical water[J]. Cellulose, 1999, 6(3): 177-191.
[3] Xiao Z, Ge Q, Xing C. Self-reducing bifunctional Ni-W/SBA-15 catalyst for cellulose hydrogenolysis to low carbon polyols[J]. Journal of Energy Chemistry, 2016, 20(3): 434-444.
[4] Klemm D, Heublein B, Fink H., et al. Cellulose: fascinating biopolymer and sustainable raw material[J]. ChemInform, 2005, 44(36): 3358-3393.
[5] Gardner K.H., Blackwell J. The structure of native cellulose[J]. Biopolymers, 1974, 13(10): 1975-2001.
[6] 胡玉洁. 天然高分子材料改性与应用[M]. 化学工业出版社, 2003.
(Hu Yujie. Natural Polymer Materials Modification and Application [M]. Chemical Industry Press, 2003.)
[7] Khajenoori M, Haghighi A, Hormozi F. Proposed models for subcritical water extraction of essential oils[J]. Chinese Journal of Chemical Engineering, 2009, 17(3): 359-365.
[8] Fu Y, Song R J, Yao N, et al. Separations of some alcohols, phenols, and caboxylic acids by coupling of subcritical water chromatography and flame ionization detection with postcolumn splitting[J]. Chinese Journal of Analytical Chemistry, 2007, 35(9): 1335-1338.
[9] Mi Y, Hu W, Dan Y, et al. Synthesis of carbon micro-spheres by a glucose hydrothermal method[J]. Materials Letters, 2008, 62(8-9): 1194-1196.
[10] Sevilla M, Fuertes A. B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides[J]. Chemistry-A European Journal, 2009, 15(16): 4195-4203.
[11] Zhang N, Cui X Y, Zhao X J, et al. Preparation of nanometer carbon spheres by hydrothermal synthesis method[J]. Key Engineering Materials, 2012, 512-515: 257-260.
[12] Xu Yixiang, Anita Scales, Jordan Krystle. Starch nanocomposite films incorporating grape pomace extract and cellulose nanocrysta[J]. Journal of Applied Polymer Science, 2017, 134(6): 38-44.
[13] Tuinstra F, Koenig J L. Raman spectrum of graphite[J]. The Journal of Chemical Physics, 1970, 53(3): 1126-1130.
[14] Gao Ying, Yuan Qiaoxia, Chen Hanping. Forming property of water hyacinth hydro-char physicochemical structure during hydrothermal carbonation[J]. J. huazhong univ. of SCI. & tech. (natural science edition), 2015, 43(6): 116-121.
[15] Liu Juan, Chi Yong, Shu Di. Effects of process parameters on hydrothermal carbonization of cellulose[J]. CIESC Journa, 2015, 66(12): 4980-4987.
[16] Liu Z, Balasubramanian R. Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTP): A comparative evaluation[J]. Applied Energy, 2014, 114(2): 857-864.
[17] Yin S D, Mehrotra A K, Tan Z C. Alkaline hydrothermal conversion of cellulose to bio-oil: Influence of alkalinity on reaction pathway change[J]. Bioresource Technology, 2011, 102(11): 6605-6610.
[18] Klingler D, Vogel H. Influence of process parameters on the hydrothermal decomposition and oxidation of glucose in sub- and supercritical water[J]. Journal of Supercritical Fluids, 2010, 55(1): 259-270.
[19] Kishida H, Jin F, Yan X. Formation of lactic acid from glycolaldehyde by alkaline hydrothermal reaction[J]. Journal of Materials Science, 2007, 341(24): 2619-2623.
[20] Nore Struyf, Jitka Laurent, Bianca Lefevere. Establishing the relative importance of damaged starch and fructan as sources of fermentable sugars in wheat flour and whole meal bread dough fermentations[J]. Food Chemistry, 2017, 218, 89-98.
[21] Ryu Jihye, Suh Young-Woong, Suh Dong Jin. Hydrothermal preparation of carbon microspheres from mono-saccharides and phenolic compounds[J]. Carbon, 2010, 48(7): 1990-1998.
[22] Sevilla M, Fuertes A B. The production of carbon materials by hydrothermal carbonization of cellulose[J]. Carbon, 2009, 47(9): 2281-2289.
[23] Shi Sheng, Hou Wensheng, GaoJuan. Microstructure rvolution of votton giber obtained from hydrothermal treatment[J]. Fine Chemicals, 2016, 33(4): 365-371.
[24] Kupiainen L, Ahola J, Tanskanen J. Distinct effect of formic and sulfuric acids on cellulose hydrolysis at high temperature[J]. Ind.eng.chem.res. ,2012, 51(8): 3295-3300.
