MiT家族易位性肾细胞癌:15例患者影像学特征
2018-06-07陆亚文刘晓航高洪波周良平
刘 伟 陆亚文 刘晓航 高洪波 汤 伟 周良平△
(1复旦大学附属肿瘤医院放射诊断科,3病理科 上海 200032; 2上海市影像医学研究所 上海 200032; 4复旦大学上海医学院肿瘤学系 上海 200032)
2016版WHO肾脏肿瘤分类将Xp11.2/TFE3基因易位性肾癌(renal cell carcinoma with Xp11.2 translocations/TFE3 gene fusion,Xp11.2 RCC)与t(6;11) (p21;q12)基因易位性RCC[renal cell carcinoma with t(6;11) (p21;q12),t(6;11)RCC]一起归入MiT家族易位性肾细胞癌(renal cell carcinoma,RCC)[1]。Xp11.2 RCC少见,好发于儿童,占儿童RCC的20%~40%,仅占成人RCC的1%~1.6%[2]。t(6;11)RCC更为罕见,截至2016年报道仅50余例,预后较Xp11.2 RCC好[3]。虽然对Xp11.2 RCC的影像学特征已有一些总结[4-6],但仍不足以建立一个清晰的影像诊断系统,而对t(6;11)RCC描述较为详细的影像学研究仅检索到1篇[7]。本研究旨在对MiT家族易位性RCC的影像征象进行探究,以期为其术前诊断提供一定的提示价值。
资 料 和 方 法
病例来源筛选2013年1月至2017年1月于复旦大学附属肿瘤医院术前行CT和/或MRI检查并经手术病理证实的15例MiT家族易位性RCC患者,包括14例Xp11.2 RCC和1例t(6;11)RCC。其中3例行CT和MRI检查,5例行CT检查,7例行MRI检查。
影像学检查CT为西门子64排MDCT(电压120 kV、电流200 mA)。均行平扫及动态增强扫描,采用碘造影剂欧乃派克300,经肘静脉以3.0 mL/s的速率注射90 mL,注射30~35 s后行皮质期扫描,65~70s后行实质期扫描。MRI采用3.0T Signa MR扫描仪。扫描参数:层厚5~8 mm,层间距0.5~2.0 mm,扫描序列包括扰相梯度回波(spoiled gradient echo,SPRG)正相位(in-phase) (TR:150~230 ms,TE:1.4~2.4 ms)和反相位(out-phase) (TR:150~230 ms,TE:3.2~4.6 ms)T1WI序列和脂肪抑制快速自旋回波(TR:3 200~4 000 ms,TE:78~92 ms)T2WI序列。动态增强扫描采用屏气脂肪抑制的肝脏快速三维容积采集(3D gradient recalled echo,3D-LAVA)序列(TR:2.6~3.2 ms,TE:1.2~1.5 ms),造影剂为马根维显(Gd-DTPA,德国拜尔先灵公司),经静脉以2 mL/s的速率注射15 mL,注射20 s后行皮质期扫描,70 s后行实质期扫描。
影像及数据分析2位具有5~10年临床诊断经验的放射科医师来分析影像学特征,包括肿瘤的位置、大小、坏死、出血、钙化、形状、边缘、脂肪、均质性、转移、密度特征(平扫CT:CTtumour-CTrenal≥10 Hu为稍高密度,≥20 Hu为高密度,<10 Hu为等密度[4])、信号特征等。ROI的选取均在肿瘤的实性部位,避开钙化,以避免局部容积效应。在面积为40 mm2的ROI内取3次平均值,获取CT值。
病理检查2名具有5~10年经验的病理科医师进行病理诊断。在标本上常规取材、脱水、HE染色,13例行免疫组化检测,15例采用荧光原位杂交技术(fluorescencein-situhybri dization,FISH)检测TFE-3和TFE-B基因。
结 果
临床特征14例Xp11.2 RCC患者平均年龄(26±7)岁(14~42岁),临床特征见表1。1例t(6;11)RCC患者,29岁,男性,因肉眼血尿入院,触及包块,未见其他症状。
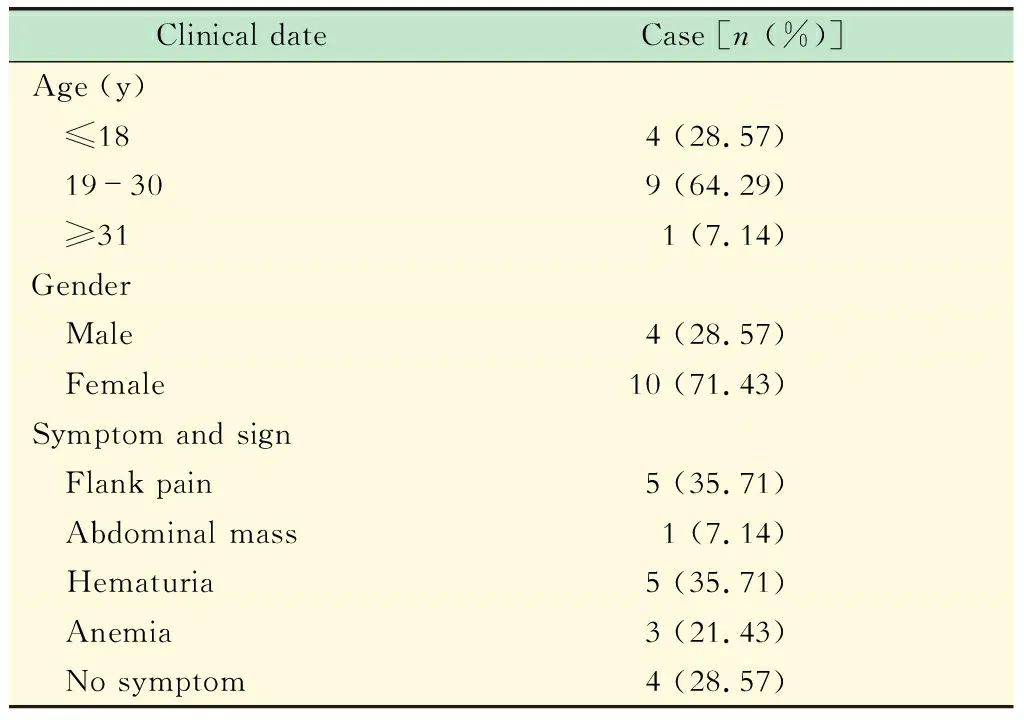
表1 14例Xp11.2 RCC的临床资料Tab 1 Clinic date of 14 cases with Xp11.2 RCC
影像学征象14例Xp11.2 RCC中,肿块主体位于肾髓质12例,最大径为 (5.6±1.5)cm(3.2~8.0 cm),形态规则12例,边缘清晰11例,周围脂肪浸润1例,腹膜后淋巴结转移3例,远处转移(肺)1例,下腔静脉有癌栓1例。8例CT征象见表2,9例MRI征象见表3。
1例t(6;11) RCC在MRI上表现为类圆形不均匀信号肿块,最大径约16 cm,边缘清晰,内见出血,主体位于肾实质内。信号混杂,主体信号在T1WI上稍高,在T2WI上稍低,呈不均匀持续性中度强化。
病理结果Xp11.2 RCC镜下细胞排列可呈巢状、腺泡状、腺管状,内可见透明细胞或嗜铬细胞乳 头状形态,伴有大量砂粒体。t(6;11)RCC镜下癌细胞呈巢状排列,由两种上皮细胞组成,其中形态较小的上皮细胞围绕着玻璃样变的基底膜样物质形成菊形团样结构,肿瘤周边常见内陷的肾小管。13例免疫组化病例检查发现特异性标记物TFE-3 (2例弱阳性、9例阳性)和TFEB(1例阳性),另外可见一些指标呈阳性表现:Vimentin(7例)、CD10(7例)、Ki67(9例)、PTEN(8例)、EGFR(10例)、VEGF(10例)等。FISH法检测t(Xp11.2)(TFE-3),14例肿瘤细胞(≥5%)内有红绿分离信号,即有TFE-3基因相关易位;FISH法检测t(6;11)(TFEB),1例肿瘤细胞(20%)内有红绿分离信号,即有TFEB基因相关易位。
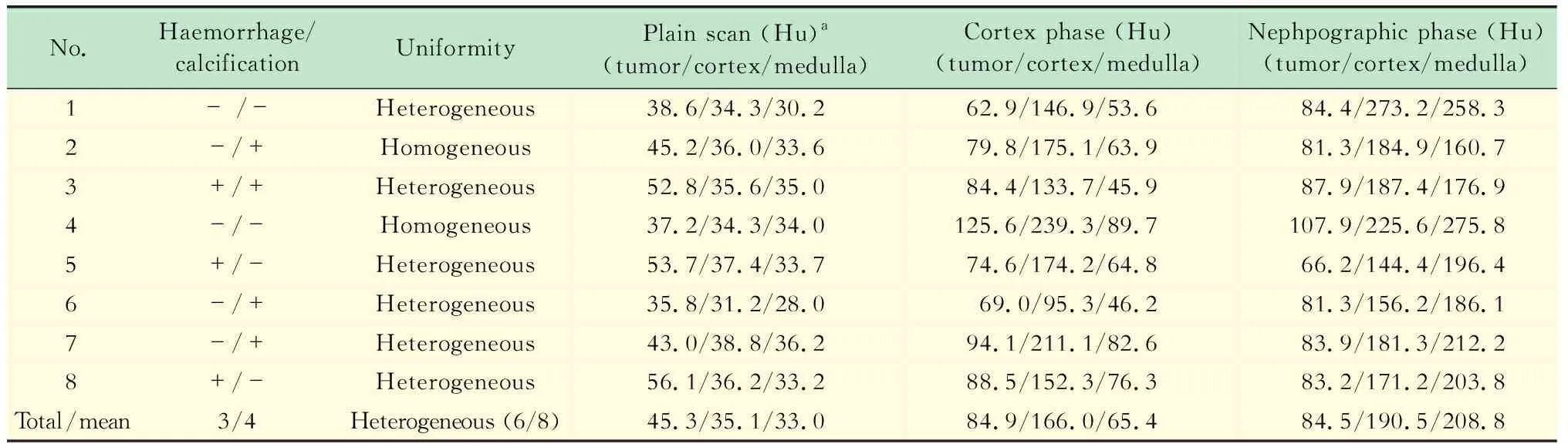
表2 8例Xp11.2 RCC的CT征象Tab 2 CT imaging findings of 8 cases with Xp11.2 RCC
aUnenhanced tumor Hu was classified as slightly high if greater than 10 HU and high greater than 20 Hu compared to normal renal cortex.
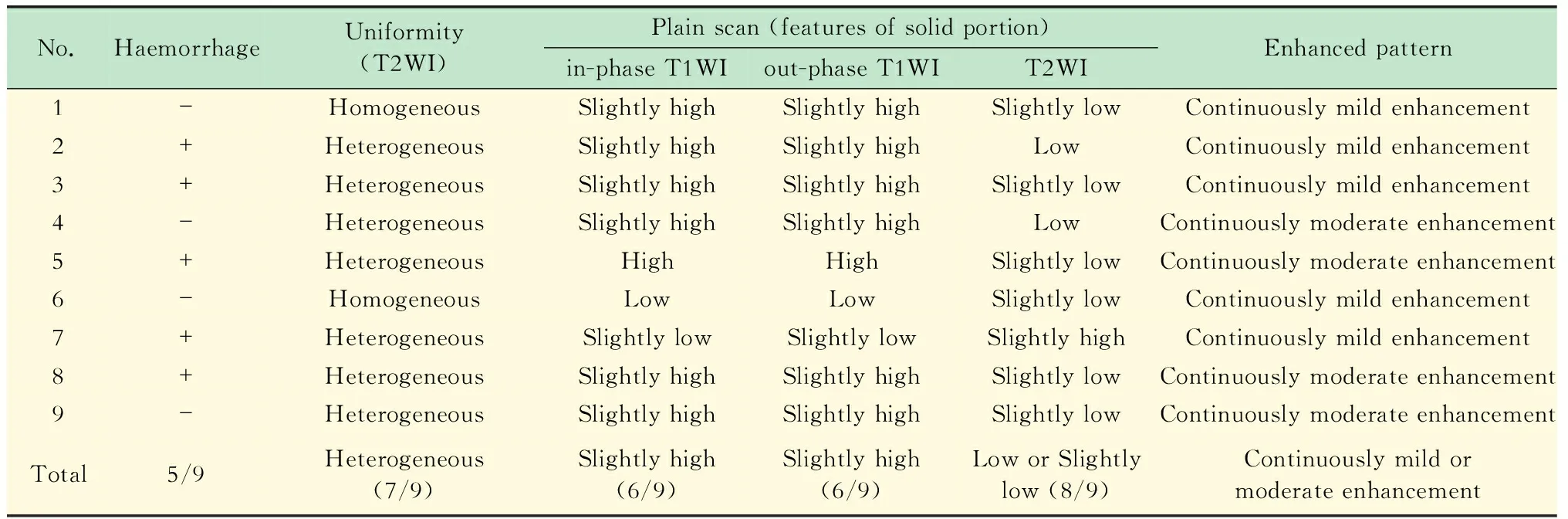
表3 9例Xp11.2 RCC的MRI表现 Tab 3 MRI features of 9 cases of Xp11.2 RCC
讨 论
目前分子遗传学检测(如FISH法)是诊断MiT易位性RCC的最佳标准[8-11],但耗时较长。Xp11.2 RCC好发于儿童及年轻人,且以女性多见(14例中13例在30岁以下,男∶女=1∶2.5),可能是因为女性的X染色体量更多,基因的易位往往发生在活跃的X染色体上[10]。临床上Xp11.2 RCC可表现为侧腹痛、腹部包块,也可无症状,因此无特异性。但血尿出现的概率相对其他RCC略高。Xp11.2 RCC患者中约1/3出现转移[12],本组研究中有27%的患者出现了转移。
Xp11.2 RCC在影像学上主要表现为较大[(5.6±1.5)cm]、界清(79%)、规则(86%)的肿块,可伴有腹膜后淋巴结转移(21%),也可伴有远处转移及下腔静脉癌栓,这与多数既往研究的结果相符[4-6,13-14]。与病理对照发现,Xp11.2 RCC有一层不完整的假包膜[15],因而肿块在CT增强实质期及MRI上表现出清晰的边缘(图3),也使其很少累及肾周和肾窦脂肪(14例中仅1例有肾窦脂肪浸润)。Chen等[4]及Zhu等[16]等报道Xp11.2 RCC位于肾髓质,Liu等[17]报道5例均位于肾皮质,而He等[5,13]及Wang 等[6]发现肿瘤位置多涉及肾皮质及实质。我们发现,虽然Xp11.2 RCC的体积较大,但突出肾脏轮廓之外的部分很小,其主体位于肾髓质。故我们认为,Xp11.2 RCC位于肾髓质,因其体积较大而向外累及肾皮质,向内与肾集合系统毗邻。但其他肾脏肿瘤也可位于肾髓质,如部分肾透明细胞癌、乳头状癌、嫌色细胞癌、髓样癌、集合管癌,因此位置的诊断价值有限。
Xp11.2 RCC在CT上主要表现为混杂密度(75%)(图1),这与之前的研究相符[4-5,13]。另外,本组研究中有半数肿瘤出现点片样钙化,He等[5,13]认为典型者呈环形分布,本组研究中2例有此表现(图1、2)。Xp11.2 RCC实性部分密度较肾皮质高,其肿瘤/皮质指数约为1.65,这可能与出血、丰富的蛋白成分、较高的细胞密度等有关[15,18]。在MRI上(图2),Xp11.2 RCC大多为混杂信号(78%),与肾皮质相比,主体信号在T1WI上稍高(67%),在T2WI上呈低或稍低(89%),且反相位上并无信号的减低。Liu等[17]的研究与本研究一致,但是Zhong等[19]、Chen等[4]及Wang等[6]研究发现Xp11.2 RCC在T1WI上为中等或稍低信号,在T2WI上呈稍低信号。这可能是因为Xp11.2 RCC内部常见出血、囊变坏死、钙化及含铁血黄素的沉积[15],因而信号混杂多变,且不同的设备、场强、扫描序列对组织信号也有影响。MRI因其对出血检测的敏感性,因而其发现肿瘤内部出血(56%)比CT(38%)敏感。Xp11.2 RCC主体位于肾髓质,易出血,假包膜不完整,且往往较大,这些可能是患者容易出现血尿的原因。
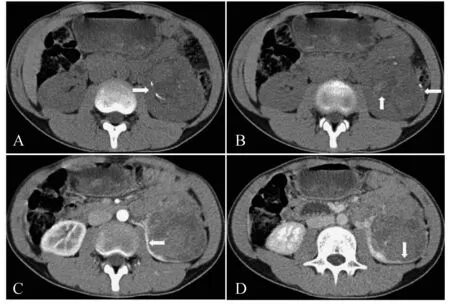
A-B:Unenhanced CT scan showed a heterogeneously slightly hyperdensive mass (45 Hu) compared with renal parenchyma (35 Hu) in the renal medulla and some calcification distributing like a circle.Additionally,patchy hyper-dense part in the neoplasm indicated hemorrhage.C-D:It was less enhancing than the cortex on all phases,while it was more enhancing than the medulla on arterial phase,and less enhancing on nephrographic phase.A clear boundary could be found on enhanced CT scan.
图126岁女性患者左肾Xp11.2RCC的CT影像
Fig1CTimagingofleftkidneyofa26-year-oldfemalepatientwithXp11.2RCC
CT增强后,Xp11.2 RCC实性部分强化程度在皮质期较肾皮质弱而较肾髓质高,在实质期较肾皮质及髓质均低,平扫、皮质期、实质期CTtumour/CTcortex分别为1.65、0.51、0.44,CTtumour/CTmedulla分别为1.69、1.30、0.40。MRI T1WI增强上,大多为不均匀持续性轻中度强化(图3)。这与之前的研究符合[4-6,14,20]。有研究表明,周围假包膜呈渐进性强化,与肿瘤内部实质部分的持续性强化不同,这种渐进性强化的表现也出现在肿瘤坏死囊变区域的周围[6]。
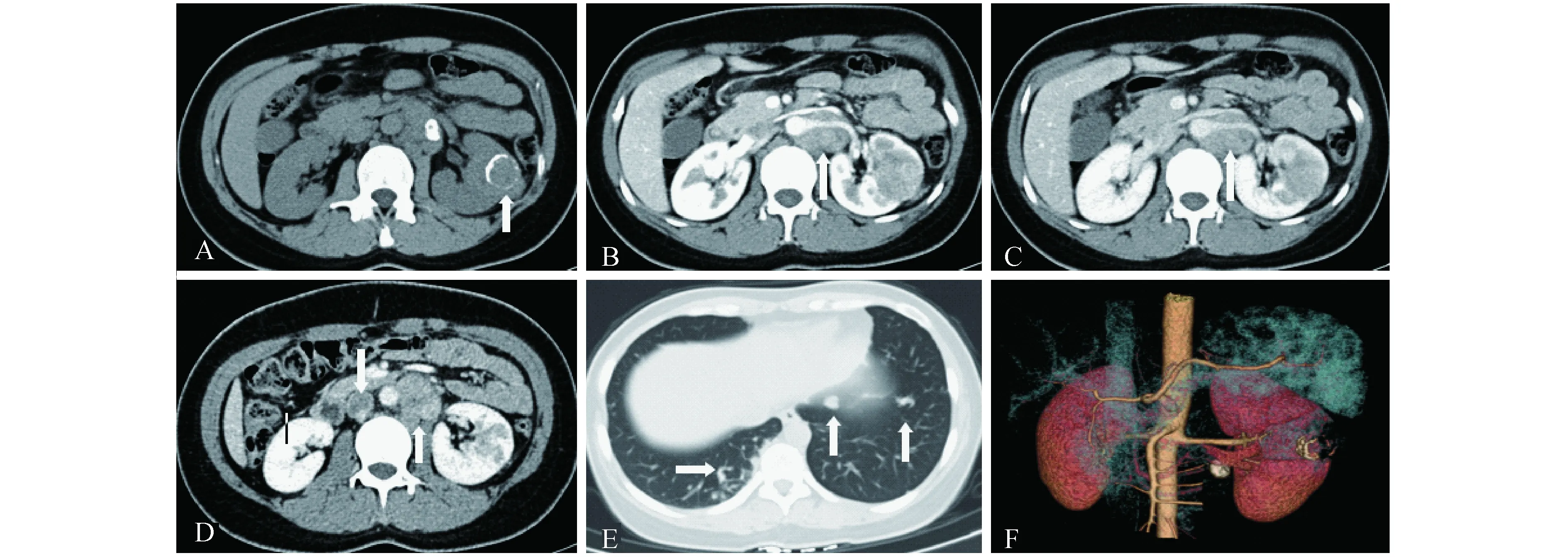
A:A circular calcification could be seen on plain CT.B-D:Metastatic Lymph nodes were found in the retroperitoneal area,and inferior vena cava tumor thrombosis was shown on the picture D.E:In the lung some nodes can be seen,which were derived from the left renal tumor confirmed by pathology.F:On this reconstructed 3-dimension image,the tumor was not obviously out of the left renal outline.
图228岁女性患者左肾Xp11.2RCC且表现为肉眼血尿的MRI影像
Fig2MRIfindingsofgrosshematuriainleftkidneyofa28-year-oldfemalepatientwithXp11.2RCC
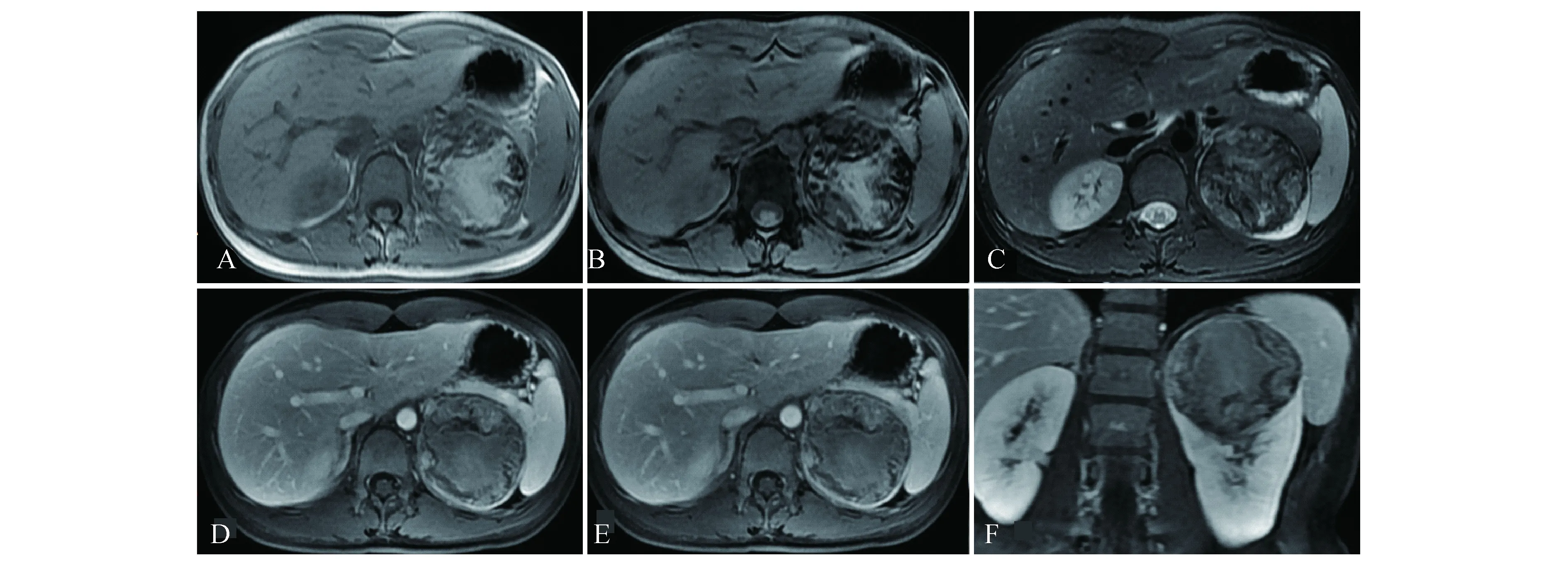
A:T1-weighted MR imaging revealed a heterogeneously left renal mass with a large part of slight hyperintensity.B:Reduction of signal intensity could not be found on the out-phase T1WI.C:T2-weighted MRI showed a mass slightly hypointense relative to the renal parenchyma.D-F:T1-weighted enhanced MRI on all phase revealed a heterogeneous mass with persistently slight enhancement,and was less enhancement relative to the renal cortex.A clear boundary was seen on every pictures because of the false capsule sign.
图313岁男性患者左肾Xp11.2RCC的MRI影像
Fig3MRIfindingsofleftrenalofa13-year-oldmalepatientwithXp11.2RCC
MiT家族易位性RCC需要与其他类型的肾脏肿瘤鉴别。RCC好发于50~60岁,而 MiT家族易位性RCC好发于儿童及年轻成人(≤30岁)。肾透明细胞癌是最常见的RCC,可有坏死囊变、出血及淋巴节转移[21-22],因而需与Xp11.2 RCC鉴别。但肾透明细胞癌是富血供肿瘤,其强化廓清较快,外生表现较为常见,且实性成分较所占比例较大,钙化少见[13],因而较易鉴别。肾乳头状细胞癌通常表现为边界清楚的、形状较规则的乏血供肿瘤,其密度与肾皮质相比呈等或稍高密度[23]。与肾乳头状癌相比,Xp11.2 RCC往往较大,囊变坏死改变更加明显,点片样钙化、较高密度的实性部分及转移的出现也起到一定的提示作用[5,14]。然而2类肾乳头状癌往往较大,钙化出现的概率增加,与Xp11.2 RCC难以鉴别,但Xp11.2 RCC有更清晰的边缘,更明显的囊变坏死区[5],在T2WI上表现为更加混杂的信号[6]。肾嫌色细胞癌好发于肾皮质,往往呈现较大、界清、均质实性的肿块,出血及钙化少见,皮质期强化明显,实质期强化减退,典型者瘤内可见延迟强化的轮辐射状纤维瘢痕[24],因此与Xp11.2 RCC较易鉴别。肾集合管癌多位于中心区域,大多有肾窦脂肪浸润表现,而Xp11.2 RCC很少侵犯肾窦脂肪(1/14例),且多呈浸润性生长,边缘模糊,因此较易鉴别[16]。乏脂肪血管平滑肌脂肪瘤(angiomyolipoma,AML)血供相对较多,皮质期强化与肾皮质接近,实质期减退明显,钙化罕见[25-26],有时在正反相位上能够发现微脂肪灶,因而两者较易鉴别。
本研究中t(6;11) RCC(图4)为类圆形不均质的巨大肿块,最大径约16 cm,边界清晰,这与Zhao等[7]的研究结果一致。但增强扫描呈持续性中度强化,而Zhao等[7]认为呈渐进性强化。肿瘤呈混杂信号,主体信号在T1WI上较肾皮质稍高,在T2WI上稍低,肿块内有出血信号,反相位上未见信号减低,故与Xp11.2 RCC无法区分,但两者最大径差异较大。我们查阅文献获取62例Xp11.2 RCC[6,15,17,27-28]及38例t(6;11) RCC[3,7,29-32]的肿瘤最大径,采用SPSS 20进行独立样本t检验,提示两者差异有统计学意义(P=0.013),这可能是因为t(6;11)RCC的恶性度较低而更易形成巨块。

A-C:Plain T1WI (A and B,in-phase and out-phase) and Axial T2WI (C) showed a large,well-defined,regular mass with slightly high mixed signal intensity on T1WI and slightly low mixed signal intensity on T2WI relative to renal cortex signal.We could see many necrotic focus in the tumor.D-E:The heterogeneous mass on T1-weighted enhanced MRI showed moderate enhancement on all phases.
图429岁男性患者右肾t(6;11)RCC且表现有肉眼血尿的MRI影像
Fig4MRIfindingsofgrosshematurisainrightkidneyofa29-year-oldmalepatientwitht(6;11)RCC
本研究存在一些不足:(1)样本量不够大,尤其是t(6;11) RCC;(2)文献中所筛选出的肿瘤最大径评估标准可能不一;(3)影像资料齐全(CT和MRI)的病例较少。年轻RCC患者若病灶主体部分位于肾髓质,界清,相对较大,呈明显不均质表现,与其他类型RCC相比,更易发生坏死囊变,增强呈持续轻中度强化,其实性部分强化程度在动脉期较肾皮质低,较肾髓质高,在实质期较肾皮质及肾髓质均低,则应考虑MiT家族易位性RCC可能,若有环形分布的点片样钙化,则可能性增加。目前尚不能完全从影像上区分Xp11.2 RCC与t(6;11)RCC,肿瘤大小有一定参考价值。
参 考 文 献
[1] MOCH H,CUBILLA AL,HUMPHREY PA,etal.The 2016 WHO classification of tumours of the urinary system and male genital organs-part A:renal,penile,and testicular tumours[J].EurUrol,2016,70(1):93-105.
[2] KMETEC A,JERUC J.Xp 11.2 translocation renal carcinoma in young adults;recently classified distinct subtype[J].RadiolOncol,2014,48(2):197-202.
[3] PECKOVA K,VANECEK T,MARTINEK P,etal.Aggressive and nonaggressive translocation t(6;11) renal cell carcinoma:comparative study of 6 cases and review of the literature[J].AnnDiagnPathol,2014,18(6):351-357.
[4] CHEN X,ZHU Q,LI B,etal.Renal cell carcinoma associated with Xp11.2 translocation/TFE gene fusion:imaging findings in 21 patients[J].EurRadiol,2017,27(2):543-552.
[5] HE J,ZHOU K,ZHU B,etal.Dynamic contrast-enhanced CT characterization of Xp11.2 translocation/TFE3 gene fusions versus papillary renal cell carcinomas[J].BiomedResInt,2015,2015:298679.
[6] WANG W,DING J,LI Y,etal.Magnetic resonance imaging and computed tomography characteristics of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion[J].PLoSOne,2014,9(6):e99990.
[7] ZHAO Y,YAO J,CHEN N,etal.Renal cell carcinomas with t(6;11) (p21;q12):presentation of two cases with computed tomography findings[J].JpnJRadiol,2015,33(6):380-383.
[8] QU Y,GU C,WANG H,etal.Diagnosis of adults Xp11.2 translocation renal cell carcinoma by immunohistochemistry and FISH assays:clinicopathological data from ethnic Chinese population[J].SciRep,2016,6:21677.
[9] XIA Q,SHI S,SHEN Q,etal.Renal cell carcinoma with t(6;11)(p21.2;q13)/MALAT1-TFEB fusion:a clinical and pathological analysis[J].ZhonghuaBingLiXueZaZhi,2015,44(12):895-899.
[10] CHENG X,GAN W,ZHANG G,etal.Clinical characteristics of XP11.2 translocation/TFE3 gene fusion renal cell carcinoma:a systematic review and meta-analysis of observational studies[J].BMCUrol,2016,16(1):40.
[11] KLATTE T,STREUBEL B,WRBA F,etal.Renal cell carcinoma associated with transcription factor E3 expression and Xp11.2 translocation:incidence,characteristics,and prognosis[J].AmJClinPathol,2012,137(5):761-768.
[12] SUDOUR-BONNANGE H,LEROY X,CHAUVET MP,etal.Cutaneous metastases during an aggressive course of Xp11.2 translocation renal cell carcinoma in a teenager[J].PediatrBloodCancer,2014,61(9):1698-1700.
[13] HE J,GAN W,LIU S,etal.Dynamic computed tomographic features of adult renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions:comparison with clear cell renal cell carcinoma[J].JComputAssistTomogr,2015,39(5):730-736.
[14] WOO S,KIM SY,LEE MS,etal.MDCT findings of renal cell carcinoma associated with Xp11.2 translocation and TFE3 gene fusion and papillary renal cell carcinoma[J].AJRAmJRoentgenol,2015,204(3):542-549.
[15] KOO HJ,CHOI HJ,KIM MH,etal.Radiologic-pathologic correlation of renal cell carcinoma associated with Xp11.2 translocation[J].ActaRadiol,2013,54(7):827-834.
[16] ZHU QQ,WANG ZQ,ZHU WR,etal.The multislice CT findings of renal carcinoma associated with XP11.2 translocation/TFE gene fusion and collecting duct carcinoma[J].ActaRadiol,2013,54(3):355-362.
[17] LIU K,XIE P,PENG W,etal.Renal carcinomas associated with Xp11.2 translocations/TFE3 gene fusions:findings on MRI and computed tomography imaging[J].JMagnResonImaging,2014,40(2):440-447.
[18] SILVERMAN SG,MORTELE KJ,TUNCALI K,etal.Hyperattenuating renal masses:etiologies,pathogenesis,and imaging evaluation[J].Radiographics,2007,27(4):1131-1143.
[19] ZHONG Y,WANG HY,CHEN X,etal.MRI findings of renal cell carcinoma associated with Xp11.2 translocations/TFE3 gene fusions[J].ZhonghuaYiXueZaZhi,2016,96(33):2635-2639.
[20] CHEN X,ZHU Q,LI B,etal.Renal cell carcinoma associated with Xp11.2 translocation/TFE gene fusion:imaging findings in 21 patients[J].EurRadiol,2017,27(2):543-552.
[21] SAUK SC,HSU MS,MARGOLIS DJ,etal.Clear cell renal cell carcinoma:multiphasic multidetector CT imaging features help predict genetic karyotypes[J].Radiology,2011,261(3):854-862.
[22] YOUNG JR,MARGOLIS D,SAUK S,etal.Clear cell renal cell carcinoma:discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT[J].Radiology,2013,267(2):444-453.
[23] LEVERIDGE MJ,BOSTROM PJ,KOULOURIS G,etal.Imaging renal cell carcinoma with ultrasonography,CT and MRI[J].NatRevUrol,2010,7(6):311-325.
[24] SASAGURI K,IRIE H,KAMOCHI N,etal.Magnetic resonance imaging of large chromophobe renal cell carcinomas[J].JpnJRadiol,2010,28(6):453-459.
[25] SUNG CK,KIM SH,WOO S,etal.Angiomyolipoma with minimal fat:differentiation of morphological and enhancement features from renal cell carcinoma at CT imaging[J].ActaRadiol,2016,57(9):1114-1122.
[26] CORNELIS F,TRICAUD E,LASSERRE AS,etal.Routinely performed multiparametric magnetic resonance imaging helps to differentiate common subtypes of renal tumours[J].EurRadiol,2014,24(5):1068-1080.
[27] KMETEC A,JERUC J.Xp11.2 translocation renal carcinoma in young adults;recently classified distinct subtype[J].RadiolOncol,2014,48(2):197-202.
[28] HE J,HUAN Y,QIAO Q,etal.Renal carcinomas associated with Xp11.2 translocations:are CT findings suggestive of the diagnosis?[J].ClinRadiol,2014,69(1):45-51.
[29] ARNEJA SK,GUJAR N.Renal cell carcinoma with t(6:11) (p21;q12).A case report highlighting distinctive immunohistologic features of this rare tumor[J].IntJSurgCaseRep,2015,7C:16-19.
[30] SMITH NE,ILLEI PB,ALLAF M,etal.t(6;11) renal cell carcinoma (RCC):expanded immunohistochemical profile emphasizing novel RCC markers and report of 10 new genetically confirmed cases[J].AmJSurgPathol,2014,38(5):604-614.
[31] CHASTE D,VIAN E,VERHOEST G,etal.Translocation renal cell carcinoma t(6;11)(p21;q12) and sickle cell anemia:first report and review of the literature[J].KoreanJUrol,2014,55(2):145-147.
[32] INAMURA K,FUJIWARA M,TOGASHI Y,etal.Diverse fusion patterns and heterogeneous clinicopathologic features of renal cell carcinoma with t(6;11) translocation[J].AmJSurgPathol,2012,36(1):35-42.
