长链非编码RNA UCA1靶向miR-582-5p对膀胱癌UM-UC-3细胞生存和运动能力的调节作用及机制研究①
2018-05-25陈怀安苗文隆
王 哲 张 敬 陈怀安 张 潮 刘 硕 苗文隆
(河北北方学院附属第一医院泌尿外科,张家口 075000)
膀胱癌是临床常见恶性肿瘤之一,因其具有高转移率及复发率的特点而备受关注[1,2]。但膀胱癌发病机制尚不明确,导致目前仍无治疗药物能明显影响其预后[3]。因此,探究膀胱癌发病机制是寻找治疗膀胱癌新药及新方法的关键。已有研究表明长链非编码RNA UCA1在膀胱癌中呈高表达状态,并且能够促进膀胱癌细胞的侵袭迁移,其机制与调控其下游microRNAs(miRNAs)的表达有关[4-6]。miR-582-5p 是一类抑癌基因,上调miR-582-5p表达可明显抑制肝癌、结肠癌及前列腺癌等癌细胞增殖[7-9]。 也有研究表明,miR-582-5p低表达也与膀胱癌的发展密切相关[10]。并且生物信息预测表明UCA1可能与miR-582-5p之间存在靶向关系。但UCA1能否通过靶向调控miR-582-5p的表达影响膀胱癌的增殖和迁移能力还未见报道。因此本文将以膀胱癌UM-UC-3为对象,探讨UCA1对膀胱癌细胞生存及运动能力的作用及作用机制。
1 材料与方法
1.1材料
1.1.1试剂及细胞 MEM细胞培养液、胎牛血清、胰酶和转染试剂盒均购自美国Invitrogen公司;Matrigel购自美国BD公司;TRIzol试剂盒、反转录试剂盒和荧光定量试剂盒购自美国ThermoFisher公司;Ki67、cleaved caspase-3和VEGF一抗购自美国Millipore公司;HRP标记的山羊抗小鼠二抗购自美国Santa Cruz公司;人膀胱癌细胞UM-UC-3购自美国ATCC,货号为CRL-1749TM;UCA1 shRNA(sh-UCA1)由上海生工生物公司设计合成,miR-582-5p inhibitor购自美国Invitrogen公司。
1.1.2仪器 ELX808酶标仪购自美国Bio-Tek公司。BD LSRFortessaTM流式细胞仪购自美国Biosciences公司。PCR仪、电泳仪及半干转膜仪均购自美国伯乐公司。Gel View 6000化学发光凝胶成像系统购自广州云星仪器有限公司。
1.2方法
1.2.1细胞培养 人膀胱癌细胞用含10% 胎牛血清的MEM培养液于37℃ 5% CO2的培养箱中培养,隔天换液。细胞融合率达到85%时进行传代培养。
1.2.2细胞转染 将细胞接种于6孔板中。培养24 h后根据转染试剂盒说明书用Lipofectamine 2000分别或同时转染sh-UCA1和miR-582-5p inhibitor,48 h后进行相应检测。
1.2.3CCK8检测细胞活性 将UM-UC-3细胞传代培养于96孔板中,培养24 h后将细胞随机分为sh-Ctrl组、sh-UCA1组、sh-UCA1+miR-582 mock组和sh-UCA1+miR-582 inhib组,对细胞进行相应转染后,根据试剂盒说明书每孔加入10 μl CCK8试剂并于培养箱中继续孵育2 h,用酶标仪检测各组吸光度,计算细胞生长速度(增殖倍数=细胞吸光度/0 h 细胞吸光度)。
1.2.4流式细胞术检测细胞凋亡 细胞转染后48 h,用胰酶收集细胞,将细胞以1 000 r/min转速离心4 min,用缓冲液清洗3次后重悬沉淀,使细胞密度为3×106个/ml。加入FITC-Annexin V和PI溶液,避光孵育15 min后用流式细胞仪检测细胞凋亡情况。
1.2.5划痕实验 实验前用Maker笔于培养板背面划平行的5条直线,灭菌后备用。将细胞传代培养于12孔板中,用sh-UCA1和miR-582-5p inhibitor转染后,用10 μl枪头垂直于培养板背面直线划痕,用预冷的PBS洗涤3次后,加入无血清培养液进行培养,于0 h、24 h进行拍照记录,并计算划痕愈合率[划痕闭合率 =(0 h划痕宽度-24 h划痕宽度)/0 h划痕宽度]×100%。
1.2.6侵袭实验 将膀胱癌细胞以无血清培养液培养24 h后,用0.25%胰蛋白酶消化细胞并传代接种于用Matrigel预处理的Transwell小室中,细胞密度为3×105个/ml。小室上层加入无血清培养液培养细胞,下层则加入含血清的正常培养液。48 h后用无菌棉签擦去小室上层细胞,下层细胞HE染色后计数统计。
1.2.7荧光素酶报告实验 首先通过生物信息预测miR-582-5p在UCA1上的结合位点并用PCR对UCA1上miR-582-5p的结合位点进行扩增。随后将扩增片段插入到pMIR-REPORT载体,构建UCA1野生型质粒。用基因突变技术将部分核苷酸突变,构建UCA1变型质粒。用UCA1野生型质粒、UCA1变型质粒和miR-582 mimic转染细胞后,用荧光素酶检测试剂盒进行检测,最后检测各组荧光素酶活性。
1.2.8qRT-PCR 用TRIzol试剂盒提取各组细胞总RNA。根据逆转录试剂盒说明书合成cDNA,用PCR仪对cDNA进行扩增后用荧光定量试剂盒对进行定量分析。以β-actin为内参。反应条件为:95℃ 反应10 s、5 s,总共40个循环,最后60℃反应30 s,70℃反应30 s。实验所用的引物序列如下:UCA1 上游引物:5′-CTCTCCATTGGGTTCACCATTC-3′,下游引物:5′-GCGGCAGGTCTTAAGAGATGAG-3′;miR-582-5p上游引物:5′-GCACACATTGAAGAGG-ACAGAC-3′,下游引物:5′-TATTGAAGGGGGTT-CTGGTG-3′,β-actin 上游引物:5′-GGCACCACCATGTACCCTG-3′,下游引物:5′-CACGGAGTACTTGC-GCT CAG-3′。
1.2.9Western blot 用RIPA裂解液提取各组细胞总蛋白。用BCA试剂盒检测总蛋白浓度,10% SDS-PAGE分离蛋白后用半干转膜仪转移蛋白质至PVDF膜。用5%脱脂牛奶室温封闭蛋白2 h,随后加入一抗(Ki67,1∶1 000;cleaved caspase-3,1∶1 000;VEGF,1∶1 000)于4℃封闭过夜,第2天加入对应二抗室温封闭1 h,最后滴加ECL曝光显影。
2 结果
2.1沉默UCA1对膀胱癌细胞miR-582-5p表达的影响 实验结果表明,sh-UCA1转染细胞后,sh-UCA1组UM-UC-3细胞UCA1表达水平较sh-Ctrl组比较明显降低,表明转染成功(P<0.05,图1);与sh-Ctrl组比较,sh-UCA1组和sh-UCA1+miR-mock组miR-582-5p表达水平明显升高(P<0.05,图1);与sh-UCA1+miR-mock组比较,sh-UCA1+miR-inhib组细胞miR-582-5p表达水平明显降低(P<0.05,图1)。
2.2UCA1与miR-582-5p的靶向关系 生物信息预测结果表明,UCA1上存在miR-582-5p的结合位点。因此,本研究采用荧光素酶报告实验进一步确定UCA1和miR-582-5p的靶向关系。实验结果表明,miR-582 mimic可显著降低UCA1野生型质粒荧光素酶的活性(P<0.05,图2);结合位点突变后,miR-582 mimic对UCA1质粒荧光素酶活性的调控关系消失,表明UCA1上存在miR-582-5p的结合位点。
2.3沉默UCA1对膀胱癌细胞生存能力的影响 实验结果表明,与sh-Ctrl组比较,细胞转染后5 d,sh-UCA1组和sh-UCA1+miR-mock组膀胱癌细胞生长速度明显降低(P<0.05,图3);sh-UCA1+miR-inhib组细胞生长速度与sh-UCA1+miR-mock组比较显著升高,差异有统计学意义(P<0.05,图3)。
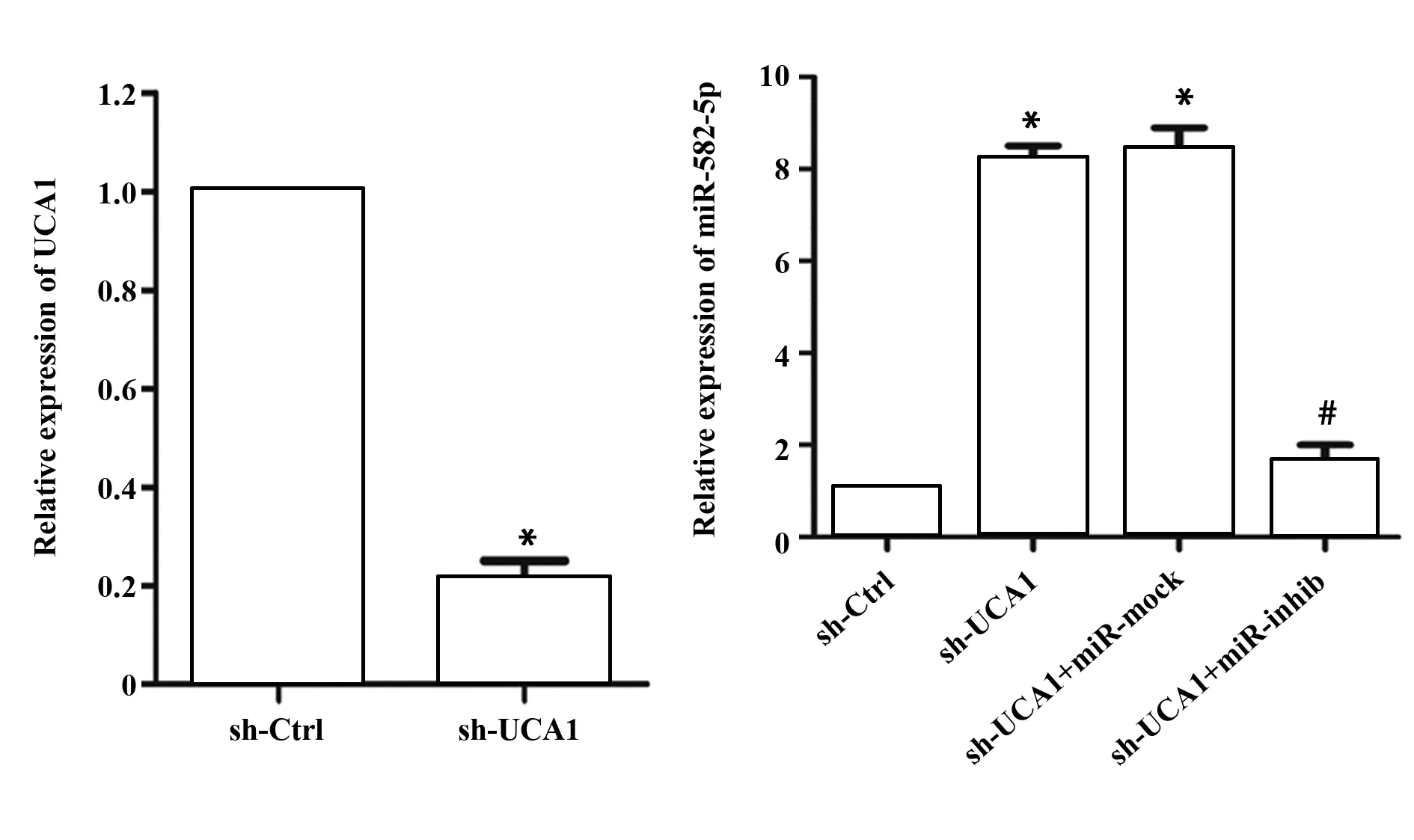
图1 沉默UCA1对膀胱癌细胞miR-582-5p表达的影响Fig.1 Effect of silencing UCA1 on expression of miR-582-5p in bladder cancer cellsNote:Cells were transferred with sh-UCA1 and miR-582-5p inhibitor.mRNA levels of UCA1 and miR-582-5p were measured by RT-PCR.n=6,β-actin was used as loading control.*.P<0.05 versus sh-Ctrl group;#.P<0.05 versus sh-UCA1+miR-mock group.
同时,sh-UCA1组和sh-UCA1+miR-mock组细胞增殖标记蛋白Ki67表达水平与sh-Ctrl组比较明显降低(P<0.05,图4);sh-UCA1+miR-inhib组细胞Ki67的表达明显升高,与sh-UCA1+miR-mock组比较差异有统计学意义(P<0.05,图4);此外,sh-UCA1还能显著诱导膀胱癌细胞凋亡,促进凋亡标记蛋白cleaved caspase-3表达(P<0.05,图4、5); miR-582-5p inhibitor能明显减弱sh-UCA1对膀胱癌细胞凋亡和cleaved caspase-3表达的促进作用(P<0.05,图4、5)。
2.4沉默UCA1对膀胱癌细胞运动能力的影响为了进一步探究UCA1通过靶向miR-582-5p对UM-UC-3细胞运动能力的影响,我们用Transwell实验和划痕实验检测sh-UCA1和miR-582-5p inhibitor转染后细胞的侵袭及迁移能力。Transwell实验结果表明,与sh-Ctrl组比较,sh-UCA1组和sh-UCA1+miR-mock组细胞侵袭数明显减少(P<0.05,图6);与sh-UCA1+miR-mock组比较,sh-UCA1+miR-inhib组细胞侵袭数目明显增多(P<0.05,图6);同时,细胞转染48 h后,sh-UCA1组和sh-UCA1+miR-mock组划痕闭合程度明显低于sh-Ctrl组(P<0.05, 图7),sh-UCA1+miR-inhib组划痕闭合程度较sh-UCA1+miR-mock组明显升高,差异有统计学意义(P<0.05,图7)。此外,sh-UCA1还能显著抑制VEGF的表达(P<0.05,图4);下调miR-582-5p表达则能明显减弱sh-UCA1对VEGF表达的抑制作用(P<0.05,图4)。
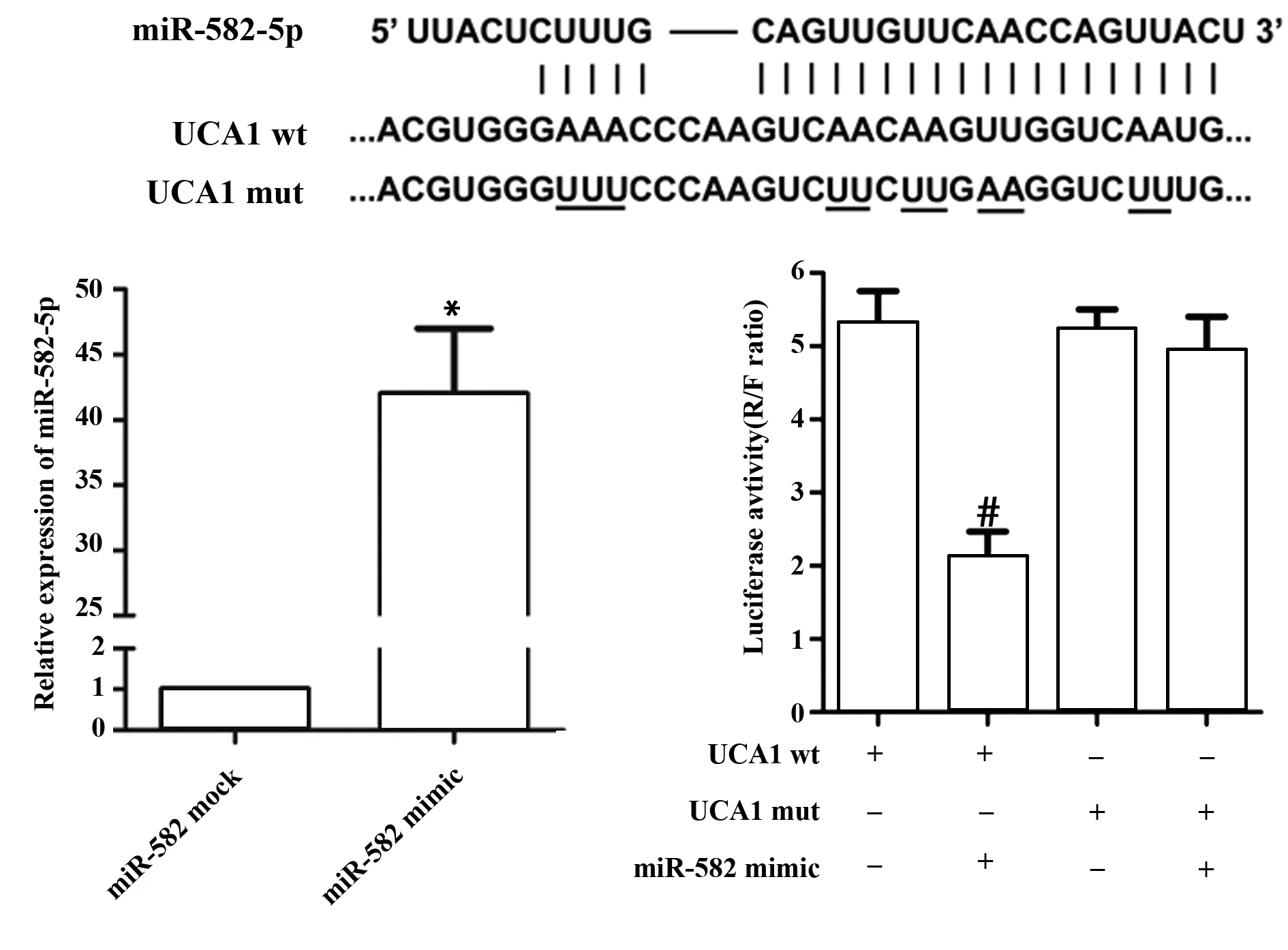
图2 UCA1与miR-582-5p的靶向关系Fig.2 Relationship of UCA1 and miR-582-5pNote:Luciferase reporter assay was performed for targeted-relationship of UCA1 and miR-582-5p.*.P<0.05 versus miR-582 mock group;#.P<0.05 versus UCA1 wt group.

图3 沉默UCA1对膀胱癌细胞生长的影响Fig.3 Effect of silencing UCA1 on growth of bladder cancer cellsNote:Cell viability was measured by CCK8 assay after cells were transferred with sh-UCA1 and miR-582-5p inhibitor.*.P<0.05 versus sh-Ctrl group;#.P<0.05 versus sh-UCA1+miR-mock group.
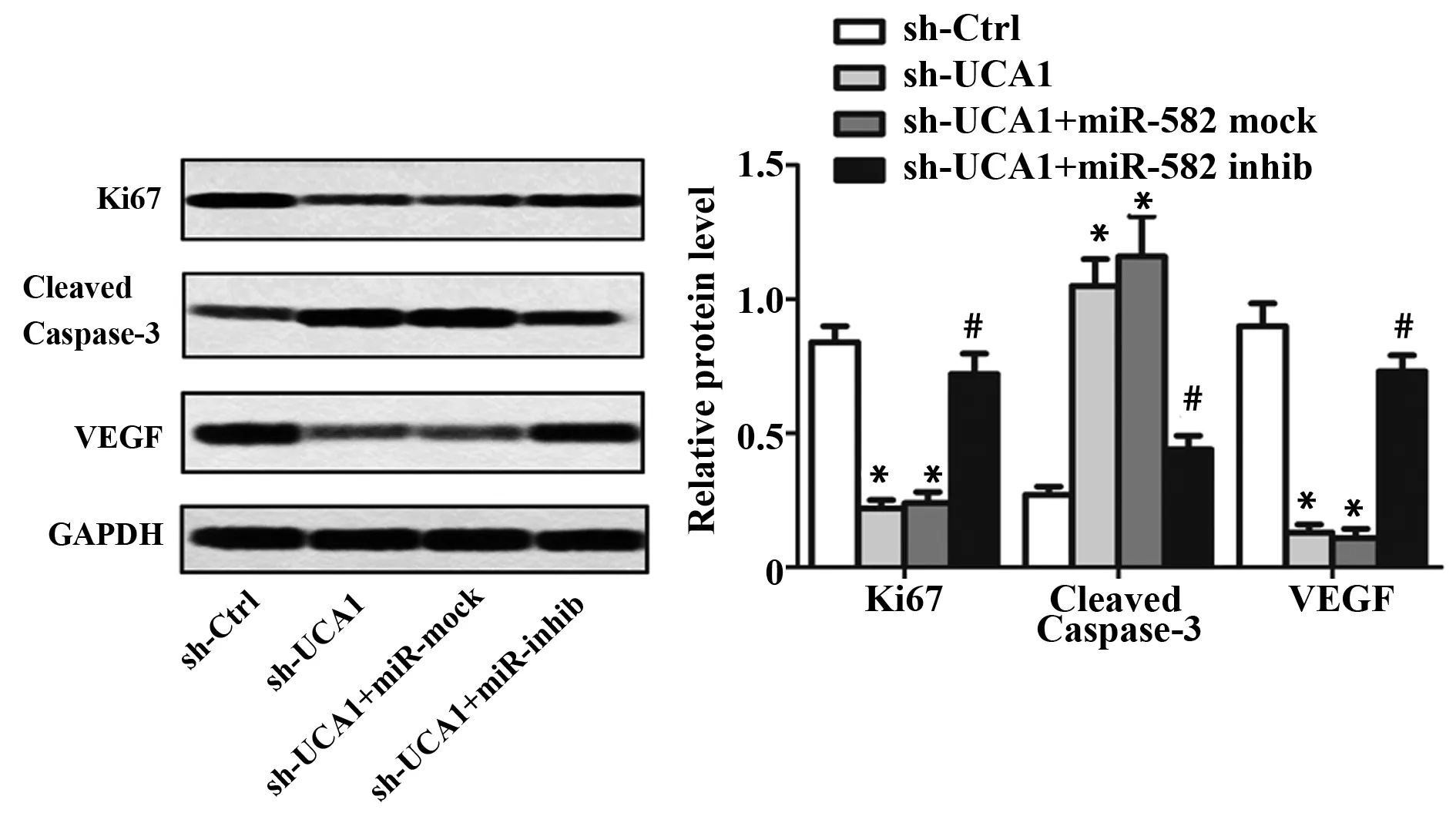
图4 沉默UCA1对膀胱癌细胞增殖、凋亡及侵袭迁移相关蛋白表达的影响Fig.4 Effects of silencing UCA1 on expression levels of proliferation-,apoptosis-and migration-related proteinsNote:Western blot was performed for protein levels of Ki67,cleaved caspase-3 and VEGF.GAPDH was used as loading control.*.P<0.05 versus sh-Ctrl group;#.P<0.05 versus sh-UCA1+miR-mock group.
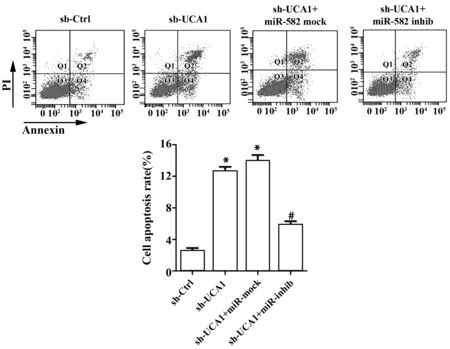
图5 沉默UCA1对膀胱癌细胞凋亡的影响Fig.5 Effect of UCA1 silencing on apoptosis of bladder caner cellsNote:Apoptosis was determined by flow cytometry.*.P<0.05 versus sh-Ctrl group;#.P<0.05 versus sh-UCA1+miR-mock group.
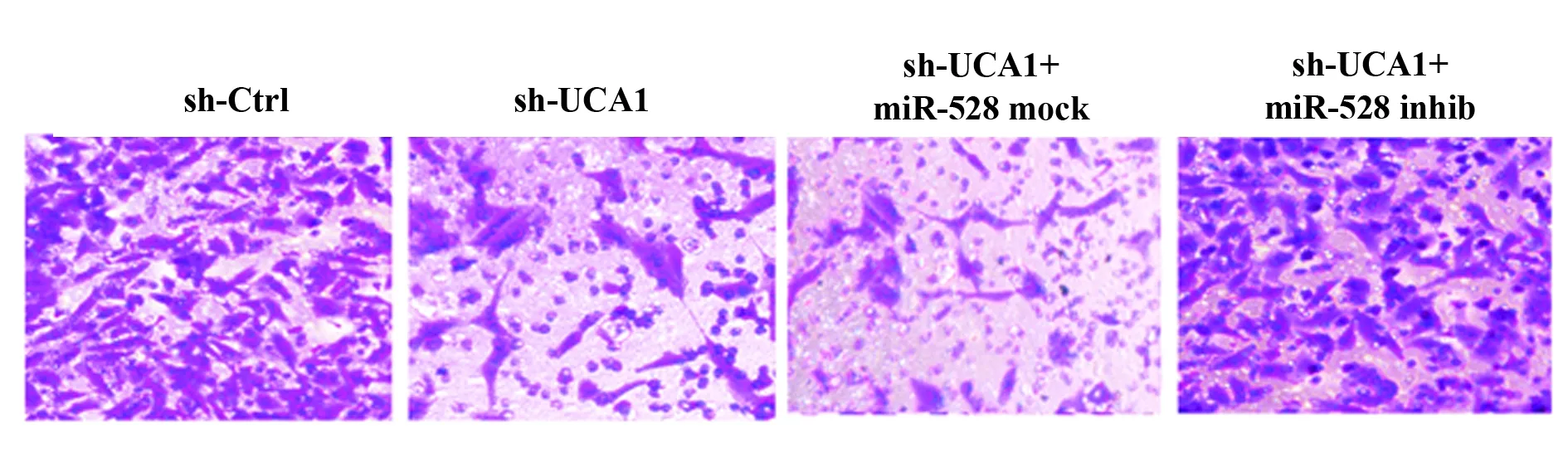
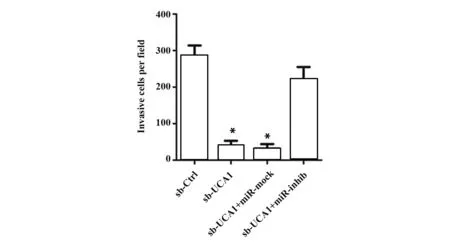
图1 沉默UCA1对膀胱癌细胞侵袭能力的影响。

图7 沉默UCA1对膀胱癌细胞迁移能力的影响Fig.7 Effect of silencing UCA1 on migration ability of bladder cancer cellsNote:Wound healing assay was performed for migration ability.*.P<0.05 versus sh-Ctrl group;#.P<0.05 versus sh-UCA1+miR-mock group.
3 讨论
大量研究表明,长链非编码RNA在癌症的发展和转移过程中发挥重要作用[11,12]。长链非编码RNA UCA1是一类癌症诱导基因,其在多类癌症中均呈高表达状态,如乳腺癌、结肠癌、胃癌和肝癌等[6,12-15]。其在多类膀胱癌细胞系中表达显著升高,促进膀胱癌细胞增殖和迁移[16,17]。本研究发现UCA1在膀胱癌细胞UM-UC-3中呈高表达状态,且与癌症的发生发展有关。
研究表明,非编码RNA发挥生物学功能与抑制或上调下游靶标miRNA的表达密切相关[18-20]。UCA1可通过调控多种miRNA的表达影响癌症的发展。但UCA1是否能通过靶向调控抑癌基因miR-582-5p的表达影响膀胱癌的恶性进展未见报道。本研究发现miR-582-5p在膀胱癌细胞中表达明显降低,这也与之前的报道一致[10]。沉默UCA1能显著上调膀胱癌细胞miR-582-5p的表达。用miR-582-5p inhibitor抑制miR-582-5p表达后,sh-UCA1对miR-582-5p表达的促进作用明显减弱,提示UCA1和miR-582-5p之间可能存在靶向调控关系。生物信息预测结果表明,UCA1上存在miR-582-5p的结合位点,荧光素酶报告实验发现,miR-582-5p过表达可明显减弱UCA1野生质粒的荧光素酶活性,进一步表明miR-582-5p可与UCA1结合,UCA1与miR-582-5p之间存在靶向调控关系。
癌细胞无限增殖和细胞凋亡抑制是癌症发生发展的重要机制。已有研究表明UCA1能明显促进膀胱癌细胞增殖、诱导癌细胞凋亡[21]。本文研究发现,下调UCA1表达能显著抑制膀胱癌细胞增殖,还可降低细胞增殖标记蛋白Ki67的蛋白表达水平,而通过抑制miR-582-5p表达可部分恢复UCA1沉默引起的膀胱癌细胞的增殖受抑,说明UCA1促进膀胱癌细胞增殖与其抑制miR-582-5p表达有关。此外,沉默UCA1还能诱导膀胱细胞凋亡,促进cleaved caspase-3表达,miR-582-5p inhibitor可明显减弱sh-UCA1对膀胱癌细胞凋亡的作用,提示UCA1调控膀胱癌细胞凋亡与其靶向调控miR-582-5p表达有关。
癌细胞转移是导致癌症恶化和癌症患者死亡的主要原因[22,23]。抑制癌细胞转移能明显延长癌症患者的生存时间,降低癌症术后复发率[24,25]。UCA1过表达能促进癌细胞转移[26,27],可被作为恶性肿瘤转移和不良预后的指标[28]。本文研究发现,沉默UCA1表达能明显降低膀胱癌细胞的侵袭及迁移能力,并能抑制VEGF的表达。VEGF是血管新生重要调控分子,其可通过促进细胞外基质降解促进细胞的迁移,促进VEGF表达可促进膀胱癌细胞迁移[29]。同时,抑制miR-582-5p表达能明显减弱sh-UCA1对膀胱癌细胞侵袭、迁移的抑制作用,提示UCA1促进膀胱癌细胞转移与其下调 miR-582-5p的表达有关。
综上所述,沉默UCA1能明显抑制膀胱癌细胞增殖、侵袭和迁移,同时还能诱导膀胱癌细胞凋亡,减缓膀胱癌的恶性进展。抑制miR-582-5p表达能明显减弱sh-UCA1的抑癌作用,表明UCA1可通过靶向miR-582-5p增强膀胱癌细胞的生存及运动能力。本研究进一步阐明了UCA1促进膀胱癌发展的机制,可能为膀胱癌的治疗提供了又一新的靶标。
参考文献:
[1] Chen Q,Chong T,Yin J,etal.Molecular events are associated with resistance to vinblastine in bladder cancer[J].Cell Mol Biol(Noisy-le-grand),2015,61(2):33-38.
[2] Barocas DA,Clark PE.Bladder cancer[J].Curr Opin Oncol,2008,20(3):307-314.
[3] Valastyan S,Weinberg RA.Tumor metastasis:molecular insights and evolving paradigms[J].Cell,2011,147(2):275-292.
[4] Wang F,Li X,Xie X,etal.UCA1,a non-protein-coding RNA up-regulated in bladder carcinoma and embryo,influencing cell growth and promoting invasion[J].Febs Lett,2008,582(13):1919-1927.
[5] Li HJ,Li X,Pang H,etal.Long non-coding RNA UCA1 promotes glutamine metabolism by targeting miR-16 in human bladder cancer[J].Jpn J Clin Oncol,2015,45(11):1055-1063.
[6] Wang XS,Zhang Z,Wang HC,etal.Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma[J].Clin Cancer Res,2006,12(16):4851-4858.
[7] Yi Z,Wei H,Yan R,etal.miR-582-5p inhibits proliferation of hepatocellular carcinoma by targeting CDK1 and AKT3[J].Tumor Biol,2015,36(11):8309-8316.
[8] Zhang X,Zhang Y,Yang J,etal.Upregulation of miR-582-5p inhibits cell proliferation,cell cycle progression and invasion by targeting Rab27a in human colorectal carcinoma[J].Cancer Gene Ther,2015,22(10):475-480.
[9] Maeno A,Terada N,Uegaki M,etal.Up-regulation of miR-582-5p regulates cellular proliferation of prostate cancer cells under androgen-deprived conditions[J].Prostate,2014,74(16):1604-1612.
[10] Uchino K,Takeshita F,Takahashi R,etal.Therapeutic effects of microRNA-582-5p and -3p on the inhibition of bladder cancer progression[J].Mol Ther,2013,21(3):610-619.
[11] 邹 燕,戴盛明.长链非编码RNA MEG3调控作用相关的信号通路研究进展[J].中国免疫学杂志,2017,33(11):1741-1743.
Zou Y,Dai SM.The research progress of long non-coding RNA MEG3 on regulatory effects-related signaling pathway[J].Chin J Immunol,2017,33(11):1741-1743.
[12] Huang J,Zhou N,Watabe K,etal.Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27(Kip1)[J].Cell Death Dis,2014,5(1):e1008.
[13] Tao K,Jing Y,Hu Y,etal.Clinical significance of urothelial carcinoma associated 1 in colon cancer[J].Int J Clin Exp Med,2015,8(11):21854-21860.
[14] Shang C,Guo Y,Zhang J,etal.Silence of long noncoding RNA UCA1 inhibits malignant proliferation and chemotherapy resistance to adriamycin in gastric cancer[J].Cancer Chemother Pharmacol,2016,77(5):1061-1067.
[15] Wang F,Ying HQ,He BS,etal.Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway[J].Oncotarget,2015,6(10):7899-7917.
[16] Zhen S,Hua L,Liu YH,etal.Inhibition of long non-coding RNA UCA1 by CRISPR/Cas9 attenuated malignant phenotypes of bladder cancer[J].Oncotarget,2017,8(6):9634-9646.
[17] Pan J,Li X,Wu W,etal.Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells[J].Cancer Lett,2016,382(1):64-76.
[18] Salmena L,Poliseno L,Tay Y,etal.A ceRNA hypothesis:the Rosetta stone of a hidden RNA language?[J].Cell,2011,146(3):353-358.
[19] Cesana M,Cacchiarelli D,Legnini I,etal.A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA[J].Cell,2011,147(2):358-369.
[20] Kallen A,Zhou XB,Xu J,etal.The Imprinted H19 LncRNA Antagonizes Let-7 MicroRNAs[J].Mol Cell,2013,52(1):101-112.
[21] Xue M,Xu LI,Wu WJ,etal.Upregulation of long non-coding RNA urothelial carcinoma associated 1 by CCAAT/enhancer binding protein α contributes to bladder cancer cell growth and reduced apoptosis[J].Oncol Rep,2014,31(5):1993-2000.
[22] Warren S,Gates O.Lung cancer and metastasis [J].Arch of Pathol,1964,78(7):467-473.
[23] Whyte F.Metastasis:the deadly part of cancer[J].Br J Nurs,1996,5(9):535-538.
[24] Decaestecker K.Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer:a systematic review of the literature[J].Eur Urol,2015,67(5):852-863.
[25] Kitamura H.Patterns of metastasis and recurrence of prostate cancer[J].Nihon Rinsho,2016,74(Suppl 3):140-144.
[26] Wang ZQ,He CY,Hu L,etal.Long noncoding RNA UCA1 promotes tumour metastasis by inducing GRK2 degradation in gastric cancer[J].Cancer Lett,2017,408:10-21.
[27] Qian Y,Liu D,Cao S,etal.Upregulation of the long noncoding RNA UCA1 affects the proliferation,invasion,and survival of hypopharyngeal carcinoma[J].Mol Cancer,2017,16(1):68.
[28] Sun XD,Huan C,Qiu W,etal.Clinical significance of UCA1 to predict metastasis and poor prognosis of digestive system malignancies:a meta-analysis[J].Gastroenterol Resand Pract,2016,2016(1):3729830.
[29] Ding G,Yu S,Sheng C,etal.Androgen receptor(AR)promotes male bladder cancer cell proliferation and migration via regulating CD24 and VEGF[J].Am J Transl Res,2016,8(2):578-587.