Effects of elevated atmospheric CO2and nitrogen fertilization on nitrogen cycling in experimental riparian wetlands
2018-05-11JunLiuGloriaAppiahSefahTheresaOtengApreku
Jun Liu*,Gloria Appiah-Sefah,Theresa Oteng Apreku
aCollege of Materials Science and Technology,Nanjing Forestry University,Nanjing 210037,China
bNanjing Technical Vocational College,Nanjing 210019,China
cKey Laboratory of Integrated Regulation and Resource Development on Shallow Lakes of Ministry of Education,Hohai University,Nanjing 210098,China
1.Introduction
The functions of wetlands,including flood control,water purification,and even production offish,cannot be utterly overlooked.However,in some situations,the plants in wetlands tend to become invasive and,in some cases,they have the ability to alter nutrient cycling process,productivity,and food webs in the wetland.On the other hand,when plants are not present,shifts in soil microbial communities have also been found to be capable of altering the ecosystem's nutrient cycling process in a manner similar to that observed when plants are present(Balser and Firestone,2005;Balser et al.,2001),since microorganisms play an important mediating role in biogeochemical cycles.Research by Koizumi et al.(1991)showed that these microbial activities often do not change at low atmospheric CO2concentrations while the activities at high CO2concentrations are still under study.On the contrary,both empirical and modeling studies have shown a possible increase in sensitivity of plant productivity to increasing CO2concentrations(Morgan et al.,2007).However,not all plant species respond in the same manner.Differences in photosynthetic pathways and the ability to fix nitrogen are important species traits in plants,which affect their response(Reich et al.,2001).Both elevated CO2and nitrogen additions have been found to increase plant biomass.However,the effect of the former has been found to be greater with high available nitrogen levels(Martín-Olmedo et al.,2002;Daepp et al.,2000).Both have also been found to alter the carbon-to-nitrogen ratio of plant tissues,which tends to modify the decomposability of plantresidues(van Groenigen et al.,2006).In a spate of climatic changes,CO2concentrations have been rising and are expected to rise further.
Furthermore,elevated CO2can reduce soil nitrogen availability due to a possible increase in microbial growth(Gill et al.,2002;Dίaz et al.,1993),leading to an increase in inorganic nitrogen.Plant nitrogen uptake can further reduce available soil nitrogen in the long term because of increasing storage of nitrogen in long-lived plant biomass and soil organic matter(Reich et al.,2006;Luo et al.,2004).Deiglmayr et al.(2004)and Norby(1994)determined that plants under elevated atmospheric CO2conditions often strongly respond to nitrogen immobilization and reduce nitrification in the soil by increasing the allocation of carbon from root to soil,which subsequently reduces the availability for the nitrifying bacteria,causing a further decrease in soil nitrate concentrations.In systems in which soil nitrogen availability is reduced by elevated CO2,the increases in plant growth may only be possible when plants increase their nitrogen uptake.On the other hand,elevated CO2can significantly increase mineralization and denitrification by soil microorganisms,thereby increasing the amount of available soil nitrogen(Dijkstra et al.,2008).Elevated CO2concentrations also change some microbial functions in the soil,which tends to decrease available soil nitrogen for nitrifiers and also decrease soil inorganic nitrogen concentrations over time(Hungate,1999).
Alternanthera philoxeroides,a C3 herb commonly known as alligator weed,is common in Asia,Australia,South America,and North America.This species is often used in wetlands because of its tolerance to high-temperature,saline,and droughtconditions.However,in many countries,including China,it is considered an invasive species causing both economic and ecological problems(Chen et al.,2013).The effect of increased nitrogen deposition on the species has been studied and found to be capable of altering their population dynamics and structure(Wang et al.,2014).However,possible effects under elevated atmospheric CO2conditions have not been studied yet.
Since the relationship between plant nitrogen content and soil nitrogen reduction under elevated CO2conditions and with nitrogen additions in wetland ecosystems remains unknown,the objective of this study was to assess the interactive effects of elevated CO2and inorganic nitrogen additions on soil nitrogen cycling and plant nitrogen content changes.The effects of these two factors on a cultured riparian wetland dominated by alligator weeds were also studied.
2.Materials and methods
2.1.Experimental site
This study was conducted in the Water Research Greenhouse at the Jiangning Campus of Hohai University in Nanjing,China.The study area is situated at latitude 31°54′57′′N and longitude 118°46′51′′E.
2.2.Materials and experimental setup
Two open top chambers(OTCs),each with a volume of 13.5 m3,were placed in a ventilated greenhouse.The elevated and ambient CO2concentrations in each chamber were 700 μmol/mol and 380 μmol/mol,respectively.The cultured chambers were prepared by adjusting three time pulses of nitrogen fertilization within two weeks.The sediments used in this experiment were collected from Taihu Lake(at latitude 31°25′6′′N and longitude 120°14′45′′E)with an area of approximately 36895 km2in Eastern China.600 kg of air-dried sediments with equal heights of about 13-15 cm were placed in each chamber prior to cultivation of the wetland plants.
Each OTC was separated into four sub-chambers:two with alligator weeds at a low nitrogen((NH4)2SO4)level of 4 mg/L and the other two with alligator weeds at a high nitrogen((NH4)2SO4)level of 6 mg/L.
Alligator weeds with total plant biomass of 1 kg were then cultured in each sub-chamber of OTC.The cultured water surface,40 cm in depth,which was obtained with an amount of approximate 1000 L,was maintained for 15 d.
A plastic gas pipe(with an inside diameter of 0.2 cm)was laid along the inner edges of the elevated CO2chamber with perforations to allow CO2to pass.The desired CO2concentrations were reached within 24 h using a gas flow meter and a gas regulator.CO2concentrations were monitored twice a day(in the morning and evening)using an infrared gas analyzer(TES 1370 NDIR CO2meter).A controllable photoperiod of 12 h(from 9:00 am to 9:00 pm)was maintained with fluorescent lights inside the OTC.An air conditioner was used to control the daily temperature(25 ± 2°C),and the air and CO2were mixed in the OTC.
2.3.Chemical analysis of plant samples
To test for nitrogen content,plant samples were dried at 65°C for 48 h.0.5 g of finely-ground plant samples were digested with H2SO4,H2O2,and lithium sulphate at 300°C for 3 h.The samples were then removed and cooled.Distilled water was added to the digest,placed in a 50-mL volumetric flask,and topped to the 50-mL mark.This was followed by steam distillation of the digested samples using excess NaOH.The distillate was collected in saturated H3BO3and then titrated with 0.01 mol/L of HCl.The percentage of nitrogen(θ)was calculated as shown in Eq.(1):whereais the volume of titer HCl for a sample,bis the volume of titer HCl for blank,Vis the final volume of the digestion,wis the weight of the sample,andaLis the aliquot of the solution taken for analysis.The plant nitrogen content was calculated as the percentage of nitrogen multiplied by the total plant weight.

2.4.Chemical analysis of soil sample extractions
Soil pH was measured at a water-to-soil mass ratio of 2:1.Bulk density was calculated as the oven-dried weight of soil divided by soil volume.Soil texture was determined with the hydrometer method.Initial soil properties were as follows:the total carbon content was 13.12±0.62 mg/g,the total nitrogen content was 0.40±0.04 mg/g,the pH value was 8.0±0.2,the bulk density was 1.11±0.08 g/cm3,and the texture consisted of 48.8%of clay,50.4%of silt,and 0.8%of sand.
Soil samples were collected from the top 0-5-cm layer of the surface soil,because most biological and microbial activities are expected to be concentrated in the upper surface of the sediment(Luo et al.,2005),and submerged soils(0-5 cm)in fresh or salt water systems are more likely to exhibit shortterm changes in response to plants.According to the guidelines of theStandard Method for the Examination of Water and Wastewaterin China,the collected soil samples were dried at 60°C for 10 h.Then,5 g of the soil were ground and passed through a 2-mm sieve.A 2 mol/L of KCl was added,shaken vigorously for 30 min, filtered,and immediately analyzed for soil-N with the Berthelot reaction,for-N with the sodium salicylate method,and for TN concentrations with the Kjeldahl method.An ultraviolet(UV)spectrometer(Shimadzu UV-2450)was used to measure-N and-N concentrations.The dissolved inorganic nitrogen(DIN)content of soil was calculated as the summation of-N and-N contents,whereas the dissolved organic nitrogen(DON)concentration was calculated by subtracting the DIN content from the dissolved total nitrogen(DTN)content.
2.5.Statistical analyses
The effects of the two CO2conditions and the two nitrogen fertilization levels on plant nitrogen contents,soil DIN contents,and soil dissolved organic nitrogen(DON)contents were analyzed with a repeated analysis of variance(ANOVA)measure using the SPSS Statistics 17.0(USA).Linear regression was also carried out using the SPSS Statistics 17.0 to investigate the relationship between the treatment effects.The data generated included mean±standard error,the coefficient of determination(R2),F-distribution under the null hypothesis(F),the significance level(α),and the calculated probability(P)value(sample sizen=11)except for the total plant biomass.Statistical significance was accepted at α=0.05.The relative CO2treatment efficiency(E)was calculated using Eq.(2):

whereCEis the elevated CO2concentration andCAis the ambient CO2concentration.
The gingerbread man came to a river but he could not swim.
The numbers of observations and data points were appropriately treated as independent replicates from the duplicate treatments of each OTC sub-chamber for a stricter evaluation of statistical significance.
3.Results
3.1.Total plant biomass of alligator weeds
The total plant biomass under all conditions was measured at the end of the experiment(Table 1).The total plant biomass of CO2-enriched chambers significantly increased by 30.77%(R2=0.88,P=0.081,andF=42.416)at low nitrogen levels and by 31.37%(R2=0.99,P=0.002,andF=105.051)at high nitrogen levels under both ambient and elevated CO2conditions(Table 2).
3.2.Plant nitrogen content
A decrease in plant nitrogen content under elevated CO2conditions by 6.54%(R2=0.61,F=1.774,andP=0.275)at low nitrogen levels and 8.86%(R2=0.17,F=0.092,andP=0.782)at high nitrogen levels was observed but was statistically insignificant(Table 1).Observed changes over time are shown in Fig.1.
3.3.Elevated CO2and soil nitrogen contents
Under elevated CO2conditions,soil DTN contents slightly decreased by 1.41%(P=0.000 andF=205.974)and 2.14%(P=0.000 andF=190.480)at low and high nitrogen levels,respectively,compared to those under ambient CO2conditions,and this was statistically significant(Table 1).These effects over time are shown in Fig.2.
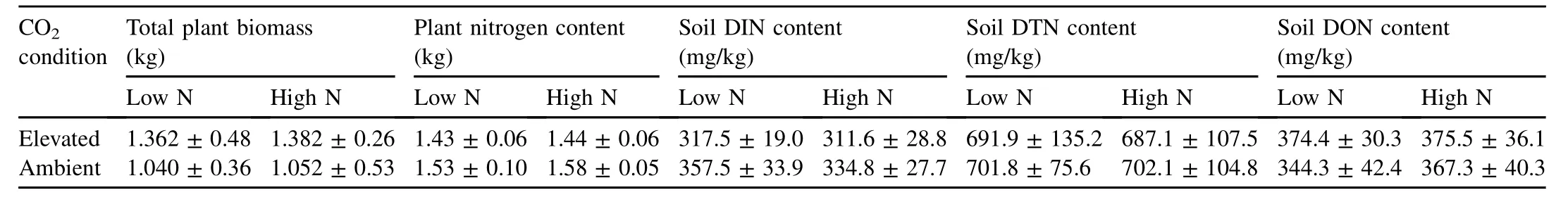
Table 1Comparison of effects of elevated and ambient CO2concentrations and varying nitrogen fertilization levels on total plant biomass,plant nitrogen content,soil DIN,soil DTN,and soil DON contents.

Table 2Analysis results of total plant biomass,plant nitrogen content,soil DIN,soil DTN,and soil DON contents for varying nitrogen fertilization levels.
The DON content was calculated as the difference between the DTN and DIN contents.Soil DON contents significantly increased under elevated CO2conditions by 8.74%(P=0.002 andF=19.070)at low nitrogen levels and by 2.23%(P=0.000 andF=40.280)at high nitrogen levels(Table 1).The effects of elevated CO2concentrations on soil DON contents at low and high nitrogen levels over time are shown in Fig.3(a)and(b),respectively.
On the other hand,the DIN content(the summation of-N and-N concentrations)under elevated CO2conditions,compared to those under ambient CO2conditions,significantly decreased by 11.18%(P=0.003 andF=17.039)and 6.93%(P=0.000 andF=110.854)at low and high nitrogen levels,respectively(Table 1).The changes over time are shown in Fig.4.
4.Discussion
This study investigated the availability of nitrogen and the effects of ambient and elevated CO2concentrations on plant and soil nitrogen contents.The combined use of different CO2concentrations and nitrogen levels provided a unique opportunity to study different plant and soil nitrogen contents.
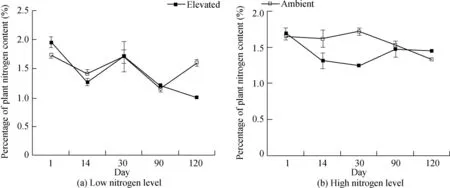
Fig.1.Percentage changes in plant nitrogen content over time at low and high nitrogen levels under ambient and elevated CO2conditions.

Fig.2.Changes in soil DTN contents over time at low and high nitrogen levels under ambient and elevated CO2conditions.
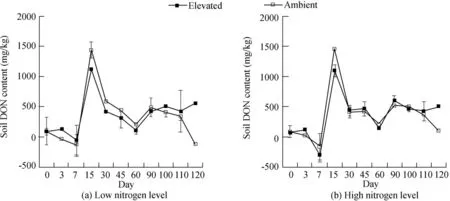
Fig.3.Changes in soil DON contents over time at low and high nitrogen levels under ambient and elevated CO2conditions.
There was a large difference in the total plant biomass in the elevated and ambient CO2chambers after the experimental period.The study by Morgan et al.(2004)revealed that the biomass of specific plant species increased due to the direct effects of elevated CO2concentrations on photosynthesis.However,other studies(van Groenigen et al.,2006;Guo et al.,2010)have pointed out a contrasting dependence on nitrogen availability instead of CO2concentrations.For instance,the grassHesperostipa comataand sub-shrubArtemisia frigidashowed strong increases in above-ground biomass with elevated CO2in a five-year OTC experiment in Colorado,while above-ground biomass of the other plants were not affected(Morgan et al.,2007).Therefore,specific plant species may respond to elevated CO2concentration and nitrogen availability based on different photosynthetic pathways and their ability to fix nitrogen.In some situations,soil resources also compete with variable plant species for carbon and nitrogen.However,in this study,we believe that elevated CO2concentrations had a stronger effect than the nitrogen input since,under low and high nitrogen levels,the differences in the total plant biomass were not large(30.77%and 31.37%,respectively).Hence,the increase in biomass of alligator weeds is largely attributed to the elevated CO2concentrations,which might have caused an increase in plant photosynthesis.
The following are the four well-known mechanisms reported to cause a decrease in plant nitrogen content under elevated CO2conditions:(1)the dilution effect,(2)increased nitrogen use efficiency or increased nitrogen availability in below ground,(3)changed allocation of nitrogen within the plant tissues,and(4)partial stomatal cluster and subsequent decrease in the leaf transpiration rate due to CO2enrichment(Pang et al.,2006;Hagedorn et al.,2008).Based on the results of this study,under elevated CO2conditions,the factors above might have influenced the reduction in plant nitrogen and a subsequent reduction of labile inorganic nitrogen concentrations in the soil(the nitrogen concentration can be diluted by the accumulation of carbohydrates).

Fig.4.Changes in soil DIN contents over time at low and high nitrogen levels under ambient and elevated CO2conditions.
DIN contents in the soil decreased by 11.18%at low nitrogen levels and by 6.93%at high nitrogen levels due to elevated CO2.Thus,at low nitrogen levels the DIN content decreased more significantly than at high nitrogen levels.Berntson and Bazzaz(1998)and Hungate et al.(1996)mentioned that greater rates of microbial immobilization under elevated CO2conditions may reduce soil DIN content.Elevated CO2concentrations may also improve nitrogen use efficiency and thus offset limitations imposed by new nutrient input.In addition,microorganisms,rather than plants,may be in far greater competition for soil nitrogen,which may have been why microorganisms consumed the inorganic nitrogen before the plants could,thereby decreasing plant and soil nitrogen contents.However,soil DON content increased with the elevated CO2concentration.This can be explained by the fact that,with greater plant growth,there is more dissolved organic carbon and DON due to the stimulated microbial metabolism(Hagedorn et al.,2008;Bragazza et al.,2007),and more resulting changes in the sizes of nitrate and ammonium pools in the soil,given that changes in plant growth under elevated CO2conditions are often nitrogen-limited.
Higher soil labile carbon-induced CO2concentrations might have stimulated the growth of carbon-limited microorganisms and led to an increase inimmobilization and hence an increase in soil nitrogen availability as found in a similar study by Guo et al.(2010).Ammonia-oxidizing bacteria sometimes also convert ammonia into nitrous oxide,thereby increasing denitrification rates and acting as the main pathway of nitrogen removalfrom water(Balserand Firestone,2005).After all,for most cases,a rapid denitrificationrate has been observed in alkaline soil(with pH values of 8.0-8.6),such as that used in this study.The denitrification rate increased rapidly with a rise in temperature ranging from 2°C to 25°C.Thus,use of alkaline soil and temperature control were possibly responsible for the fast microbial-driven changes to the nitrogen cycling.Given the results from this study,alkaline soil may play an important role in altering soil nitrogen.The reduction in plant nitrogen content under elevated CO2conditions may also be connected to the reduction of labile inorganic nitrogen concentration in the soil.Sediment is a major source of denitrification as microorganisms can transfer labile nitrogen from the sediments to overlying water bodies,thereby reducing nitrogen concentrations in the soil(Altmann et al.,2004).C16:1ω5c,mycorrhizal fungi biomarkers,dominated the soil in the elevated CO2chamber in this study.Hu et al.(2001)stated that when fungi are inhibited in the soil,denitrifiers or ammonia-oxidizing bacteria produce a large amount of nitrous oxide and increase nitrate availability in the soil.
5.Conclusions
The increase in plant growth in wetlands possibly relies on an increase in soil nitrogen availability with increasing CO2.In this study,R2values for soil nitrogen content increment ranged between 0.81 and 0.96.Reduced plant and soil nitrogen contents are to be expected due to the potential exhaustive use of inorganic nitrogen by soil microorganisms even before it can be made available to the soil and plants.From the results,it can be ascertained that the dilution effect(representing the imbalance between nitrogen absorption and increased plant photosynthesis)changed the allocation of nitrogen within the plant tissues,and a possible decrease in the leaf transpiration rate under CO2enrichment might have resulted in lower plant nitrogen levels in spite of the external inputs.Higher soil labile carbon-induced CO2may stimulate the growth of carbonlimited microorganisms and lead to an increase inimmobilization,and hence a decrease in soil nitrogen availability.Many wetland ecosystems have been damaged while others have been rendered less useful as a result of increasing climatic changes.The results from this study provide important information to managers on future possible effects of such changes on nitrogen cycling in wetland systems.The results obtained on the alligator weed,a C3 herb,can be used to predict possible responses of other C3 herbs under elevated CO2conditions in the future.These can be incorporated in the planning and creation of sustainable management practices.It is recommended that larger-scale field studies be conducted to determine the effects of other factors on the nitrogen cycling in wetland systems.
Acknowledgements
The authors would like to thank Mr.Eyram Norgbey from Hohai University,in Nanjing,and Mr.Philip Nkrumah from The University of Queensland,Australia,for proofreading the manuscript,which helped in improving its quality.
References
Altmann,D.,Stief,P.,Amann,R.,de Beer,D.,2004.Nitrification in freshwater sediments as influenced by insect larvae:Quantification by microsensors and fluorescence in situ hybridization.Microb.Ecol.48(2),145-153.https://doi.org/10.1007/s00248-003-2015-6.
Balser,T.C.,Kinzig,A.P.,Firestone,M.K.,2001.Linking soil microbial communities and ecosystem functioning.In:Kinig,A.,Pacala,A.S.,Tilman,D.,eds.,The Functional Consequences of Biodiversity.Princeton University Press,Princeton,pp.265-293.
Balser,T.C.,Firestone,M.K.,2005.Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest.Biogeochemistry 73(2),395-415.https://doi.org/10.1007/s10533-004-0372-y.
Berntson,G.M.,Bazzaz,F.A.,1998.Regenerating temperate forest microcosms in elevated CO2:Belowground growth and nitrogen cycling.Oecologia 113(1),115-125.https://doi.org/10.1007/s004420050359.
Billings,S.A.,Schaeffer,S.M.,Zitzer,S.,Charlet,T.,Smith,S.D.,Evans,R.D.,2002.Alterations of nitrogen dynamics under elevated CO2in an intact Mojave Desert ecosystem:Evidence from15N natural abundance.Oecologia 131(3),463-467.https://doi.org/10.1007/s00442-002-0898-4.
Bragazza,L.,Freeman,C.,Jones,T.,Rydin,H.,Limpens,J.,Fenner,N.,Ellis,T.,Gerdol,R.,H′ajek,M.,H′ajek,T.,et al.,2007.Atmospheric nitrogen deposition promotes carbon loss from peat bogs.Proc.Natl.Acad.Sci.103(51),19386-19389.https://doi.org/10.1073/pnas.0606629104.
Chen,Y.,Zhou,Y.,Yin,T.F.,Liu,C.X.,Luo,F.L.,2013.The invasive wetland plantAlternanthera philoxeroidesshows a higher tolerance to waterlogging than its native congenerAlternanthera sessilis.PLOS ONE 8(11),e81456.https://doi.org/10.1371/journal.pone.0081456.
Cotrufo,M.F.,Gorissen,A.,1997.Elevated CO2enhances below-ground C allocation in three perennial grass species at different levels of N availability.New Phytol.137(3),421-431.https://doi.org/10.1046/j.1469-8137.1997.00839.x.
Daepp,M.,Suter,D.,Almeida,P.F.,Isopp,H.,Hartwig,U.A.,Frehner,M.,Blum,H.,N¨osberger,J.,Lu¨scher,A.,2000.Yield response ofLolium perenneswards to free air CO2enrichment increased over six years in a high N input system on fertile soil.Global Change Biol.6(7),805-816.https://doi.org/10.1046/j.1365-2486.2000.00359.x.
Deiglmayr,K.,Philippot,L.,Hartwig,U.A.,Kandeler,H.,2004.Structure and activity of the nitrate-reducing community in the rhizosphere ofLolium prenneandTrifolium repensunder long-term elevated atmosphere CO2.FEMS Microbiol. Ecol.49(3), 445-454. https://doi.org/10.1016/j.femsec.2004.04.017.
Dίaz,S.,Grime,J.P.,Harris,J.,Mcpherson,E.,1993.Evidence of a feedback mechanism limiting plant response to elevated carbon dioxide.Nature 364(6438),616-617.https://doi.org/10.1038/364616a0.
Dijkstra,F.A.,Pendall,E.,Mosier,A.R.,King,J.Y.,Milchunas,D.G.,Morgan,J.A.,2008.Long-term enhancement of N availability and plant growth under elevated CO2in a semi-arid grassland.Funct.Ecol.22(6),975-982.https://doi.org/10.1111/j.1365-2435.2008.01398.x.
Gill,R.A.,Polley,H.W.,Johnson,H.B.,Anderson,L.J.,Maherali,H.,Jackson,R.B.,2002.Nonlinear grassland responses to past and future atmospheric CO2.Nature 417(6886),279-282.https://doi.org/10.1038/417279a.
Guo,X.L.,Lu,X.G.,Tian,K.,2010.Nitrogen cycling in plant-soil systems in three freshwater wetland types along a water level gradient in the Sanjiang Plain,Northeast China.Fresenius Environ.Bull.19(4),1-8.
Hagedorn,F.,van Hees,P.A.W.,Handa,I.T.,H¨attenschwiler,S.,2008.Elevated atmospheric CO2fuels leaching of old dissolved organic matter at the alpine treeline.Global Biogeochem.Cycles 22(2).https://doi.org/10.1029/2007GB003026.
Hu,S.,Chapin,F.S.,Firestone,M.K.,Field,C.B.,Chiarello,N.R.,2001.Nitrogen limitation of microbial decomposition in grassland under elevated CO2.Nature 409(6817),188-191.https://doi.org/10.1038/35051576.
Hungate,B.A.,Canadell,J.,Chapin III,F.S.,1996.Plant species mediate changes in soil microbial N in response to elevated CO2.Ecology 77(8),2505-2515.https://doi.org/10.2307/2265749.
Hungate,B.A.,1999.Ecosystems responses to rising atmospheric CO2:Feedbacks through the N cycling.Carbon Dioxide and Environmental Stress.Academic Press,pp.265-285.https://doi.org/10.1016/B978-012460370-7/50011-5.
Koizumi,H.,Nakadai,T.,Usami,Y.,Satoh,M.,Shiyomi,M.,Oikawa,T.,1991.Effect of carbon dioxide concentration on microbial respiration in soil.Ecol.Res.6(3),227-232.https://doi.org/10.1007/BF02347124.
Luo,Q.,Krumholz,L.R.,Najar,F.Z.,Peacock,A.D.,Roe,B.A.,White,D.C.,Elshahed,M.S.,2005.Diversity of the microeukaryotic community in sulfide-rich zodletone spring(Oklahoma).Environ.Microbiol.71(10),6175-6184.https://doi.org/10.1128/AEM.71.10.6175-6184.2005.
Luo,Y.Q.,Su,B.,Currie,W.S.,Dukes,J.S.,Finzi,A.,Hartwig,U.,Hungate,B.,Mcmurtrie,R.E.,Oren,R.,Parton,W.J.,et al.,2004.Progressive nitrogen limitation of ecosystem response to rising atmospheric carbon dioxide.Bioscience 54(8),731-739.https://doi.org/10.1641/0006-3568(2004)054[0731:PNLOER]2.0.CO;2.
Martín-Olmedo,P.,Rees,R.M.,Grace,J.,2002.The in fluence of plants grown under elevated CO2and N fertilization on soil nitrogen dynamics.Global Change Biol.8(7),643-657.https://doi.org/10.1046/j.1365-2486.2002.00499.x.
Morgan,J.A.,Mosier,A.R.,Milchunas,D.G.,Lecain,D.R.,Nelson,J.A.,Parton,W.J.,2004.CO2enhance productivity,alters species composition,and reduces digestibility of shortgrass steppe vegetation.Ecol.Appl.14(1),208-219.https://doi.org/10.1890/02-5213.
Morgan,J.A.,Milchunas,D.G.,Lecain,D.R.,West,M.,Moisier,A.R.,2007.Carbon dioxide enrichment alters plant community structure and accelerates shrub growth in the shortgrass steppe.Proc.Natl.Acad.Sci.USA 104(37),14724-14729.https://doi.org/10.1073/pnas.0703427104.
Norby,R.J.,1994.Issues and perspectives for investigating root responses to elevated atmospheric carbon dioxide.Plant Soil 165(1),9-20.https://doi.org/10.1007/BF00009958.
Pang,J.,Zhu,J.G.,Xie,Z.B.,Liu,G.,Zhang,Y.L.,Chen,G.P.,Zheng,Q.,Cheng,L.,2006.A new explanation of the N concentration decrease in tissue of rice(Oryza sativaL.)exposed to elevated atmosphericpCO2.Environ. Exp. Bot. 57(1-2), 98-105. https://doi.org/10.1016/j.envexpbot.2005.04.004.
Reich,P.B.,Knops,J.,Tilman,D.,Craine,J.,Ellsworth,D.,Tjoelkerd,M.,Lee,T.,Wedin,D.,Naeem,S.,Bahauddin,D.,et al.,2001.Plant diversity enhances ecosystem responses to elevated CO2and N deposition.Nature 410(6830),809-812.https://doi.org/10.1038/35071062.
Reich,P.B.,Hungate,B.A.,Luo,Y.Q.,2006.Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide.Annu.Rev.Ecol.Evol.Syst.37,611-636.https://doi.org/10.1146/annurev.ecolsys.37.091305.110039.
van Groenigen,K.J.,Six,J.,Hungate,B.A.,de Graff,M.,van Breemer,N.,van Kessel,C.,2006.Element interactions limit soil carbon storage.PNAS 103(17),6571-6574.https://doi.org/10.1073/pnas.0509038103.
Wang,A.O.,Jiang,X.X.,Zhang,Q.Q.,Zhou,J.,Li,H.L.,Luo,F.L.,Zhang,M.X.,Yu,F.H.,2014.Nitrogen addition increases intraspecific competition in the invasive wetland plantAlternanthera philoxeroides,but not in its native congenerAlternanthera sessilis.Plant Species Biol.30(3),176-183.https://doi.org/10.1111/1442-1984.12048.
杂志排行
Water Science and Engineering的其它文章
- Understanding groundwater table using a statistical model
- Application of a water quality model for determining instream aeration station location and operational rules:A case study
- Influence of pH on short-cut denitrifying phosphorus removal
- Numerical investigation of pollution transport and environmental improvement measures in a tidal bay based on a Lagrangian particle-tracking model
- A simplified physically-based breach model for a high concrete-faced rock fill dam:A case study
- Effect of trapezoidal collars as a scour countermeasure around wing-wall abutments
