Influence of pH on short-cut denitrifying phosphorus removal
2018-05-11WeiLiHuiynZhngHuizhiSunFeiZengYunnGoLeiZhu
Wei Li*,Hui-yn ZhngHui-zhi SunFei ZengYu-nn GoLei Zhu
aSchool of Municipal and Environmental Engineering,Shenyang Jianzhu University,Shenyang 110168,China
bShenyang Academy of Environmental Sciences,Shenyang 110167,China
1.Introduction
Short-cut denitrifying phosphorus removal technology is regarded as a novel hotspot in the field of wastewater treatment with the goals of energy conservation,emission reduction,and environmental protection(Deng,2011;Li et al.,2011;Wang et al.,2011a).In the short-cut denitrifying phosphorus removal process, short-cut denitrifying phosphorus-removing bacteria(SDPB)transform poly-βhydroxy butyrate(PHB)into a potential carbon source under anoxic conditions using nitrite(NO-2)as an electron acceptor rather than O2(Wang et al.,2011b,2014;Zeng et al.,2011;Zhou et al.,2010),thereby solving the conflict of carbon source competition in processes of denitrification and phosphorus removal,achieving double utilization of carbon,and avoiding unnecessary consumptions of oxygen and energy(Wang et al.,2011c;Jabari et al.,2014).
The acetic acid-based phosphorus release reaction equations(Wu,2010)are as follows:

where C6H10O5is glycogen,C4H6O2is PHB,HPO3is polyphosphate(poly-P),and ATP is adenosine triphosphate.
Aerobic phosphorus uptake reaction equations with O2as an electron acceptor are as follows:
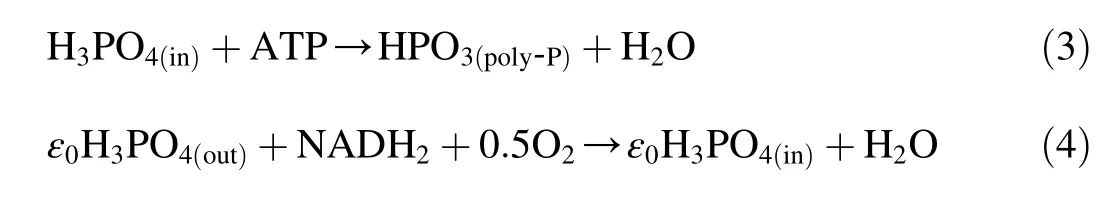
where the subscript(in)means intracellular,the subscript(out)means extracellular,NADH2means coenzyme,and ε0is the amount of phosphorus absorbed by oxidizing NADH2.
As shown in the equations above,the proton transfer resulted in a decrease of pH during anaerobic phosphorus release and an increase of pH in the phase of aerobic/anoxic phosphorus uptake.pH is regarded as a parameter indicating the potential of biological phosphorus removal.It is also an important control parameter that can affect the permeability and surface chargeability of microbial cell membrane and is closely related to the growth and reproduction of microorganisms(Yang et al.,2010;Ding et al.,2010;Za firiadis et al.,2011).
Previous studies(Wang et al.,2005)have shown that,when the influent pH values were8.0±0.1under anaerobic conditions and 7.2±0.1 under anoxic conditions,the optimal performance of the short-cut denitrifying phosphorus removal process was achieved using the sequencing batch reactor(SBR)activated sludge process,while the COD con centration was400mg/Land the NO-2-N concentration was maintained at 35±5 mg/L.Nittami et al.(2010)found that pH has an important impact on acetic acid absorption and anaerobic phosphorus release.When the pH value was in the range of 6.5-8.0,the acetic acid absorption was maintained in a constant range but the phosphorus release amount persistently increased.
In this study,an experiment was designed to investigate the influence of pH on the performance of denitrification and phosphorus removal,explore the correlation of pH with SDPB mechanisms,and provide theoretical evidence and technical support for the application of short-cut denitrifying phosphorus removal technology.
2.Materials and methods
2.1.Experimental equipment
A two-stage SBR installation made of plexiglass,including an anaerobic-anoxic sequencing batch reactor(A2-SBR)and a nitrification sequencing batch reactor(N-SBR),was used in this study(Fig.1).The seeding sludge of A2-SBR was obtained from a sedimentation pond at the Shenyang Northern Sewage Treatment Plant with mixed liquor suspended solids(MLSS)of 3000-4000 mg/L.Wastewater flowed into the two SBRs separately.The short-cut nitrification took place in the N-SBR with an effective volume of 5 L with three cycles per day.The operation parameters of the N-SBR were that MLSS was 3000 mg/L,the solids retention time(SRT)was 14 d,the hydraulic retention time(HRT)was 3 h,and the dissolved oxygen(DO)concentration was 0.3 mg/L.The effluent from the N-SBR was then fed to the 25-L A2-SBR for denitrifying phosphorus uptake under anoxic conditions.The concentration of nitrite as the electron acceptor in the A2-SBR was 18-21 mg/L.The A2-SBR ran three cycles per day,each for 5.5 h,and stood for 2.5 h at the end of each cycle.The daily sewage treatment capacity was 45 L,and the SRT was 20 d.Each operation cycle included an instantaneous filling phase,a 2-h anaerobic phase,a 2.5-hanoxic phase,a 0.5-h settling phase,and a 0.5-h withdrawing phase.
2.2.Experimental procedure

Fig.1.Sketch of SBR installation.
Activated sludge taken from the A2-SBR in a stable stage was used to investigate the effects of pH on denitrifying dephosphatation.Batch experiments were conducted after the SBR circulated steadily and showed a strong performance.The activated sludge was cleaned and put in four 1-L sealed glass bottles separately,domestic sewage was added to the glass bottles as substrate,and the anoxic dosage of nitrite was 20 mg/L.At different pH values(6.5,7.5,8.0,and 8.5),batch experiments were conducted to investigate the effect of pHon the metabolism of SDPB.The operation mode of the batch experiments was in agreement with that of the A2-SBR.The nutrient removal results were checked three times at eachpHvalue to offer the mean value.The slurry mixture was stirred with magnetic stirrer and the MLSS was 3300 mg/L.In the initial anaerobic reaction,nitrogen was injected into the glass bottles to guarantee the anaerobic environment.In the anoxic phase nitrite and nitrate solutions were continuously added to the glass bottles as electron acceptors.
2.3.Wastewater quality
The components of the simulated domestic sewage included anhydrous sodium acetate,ammonium chloride,potassium hydrogen phosphate,calcium chloride,magnesium sulfate,and trace elements.The pH values were adjusted with sodium bicarbonate.The concentrations of the trace elements were as follows:FeCl31.5 g/L,H3BO30.15 g/L,CoCl2⋅7H2O 0.15 g/L,CuSO4⋅5H2O 0.03 g/L, MnCl2⋅4H2O 0.06 g/L,Na2MoO4⋅2H2O 0.06 g/L,ZnSO4⋅7H2O 0.12 g/L,KI 0.18 g/L,and EDTA 10 g/L.The N-SBR in fluent wastewater quality parameters were as follows:COD 20.2-27.5 mg/L,NH+4-N 92.5-103.0 mg/L,total nitrogen(TN)93.0-105.1 mg/L,total phosphorus(TP)0.8-1.2 mg/L,and pH 7.0 to 7.5.The A2-SBR in fluent wastewater quality parameters were as follows:COD152-189mg/L,NH+4-N1.5-2.6mg/L,TN2.9-3.7mg/L,TP 7.1-12.8 mg/L,and pH 6.5-8.5.
2.4.Analysis method
The COD concentration was assessed with rapid digestion spectrophotometry.The-N concentration was measured using N-(1-naphthyl)-ethylenediamine spectrophotometry.The TP concentration and poly-P mass fraction were measured with molybdenum-antimony anti-spectrophotometry.Mixed liquor volatile suspended solids(MLVSS)were determined with the gravimetric method.PHB and pH were measured using gas chromatography and a pH meter,respectively(Li et al.,2006;Li,2006).
3.Results and discussion
The variation of TP concentrations at different pH values is shown in Table 1.When the pH value increased from 6.5 to 8.0,the amount of anaerobic phosphorus release within 120 min increased from 27.48 to 42.65 mg/L.A higher pH value indicated a stronger capacity for phosphorus release.The results show that acetic acid actively diffuses into cell membranes and is transformed into ions and protons within cells.This process consumes bacterial proton motive force,which functions to synthesize ATP via membrane-bound enzyme complexes and transport matrix into cells.The reconstruction or repair of proton motive force in polyphosphateaccumulating organisms(PAOs)needs to disintegrate poly-Pparticles stored in cells,and the disintegrated ions or molecules are transported out of the cells with the proton capacity of transporting ATP,which accordingly leads to phosphorus release and the increase of phosphorus concentration.The increasing pH may decrease proton motive force and PAOs need to disintegrate more poly-P particles,so a higher pH value inducesmore phosphorusrelease (Peng,2011).Smolders et al.(1994)found that increasing pH promoted the energy consumption for the transportation of acetic acid into the cells and conversion of it into PHB,and the consumption was achieved through intracellular poly-P hydrolysis.Therefore,it is speculated that the increment of pH will increase phosphorus release per unit of acetic acid.However,Chua et al.(2013)found that during sludge acclimatization,the PHB production capability of sludge was not affected by pH in the range of 7.0-8.0.The phenomenon may be the presence of other microorganisms containing PHB in the cell.When the pH value was elevated to 8.5,the amount of released phosphorus decreased to 28.65 mg/L.This possibly resulted from phosphate precipitation:some precipitated phosphate adhered to the zooglea surface and prevented the carbon absorption and phosphate release of PAOs(Guo et al.,2005b).
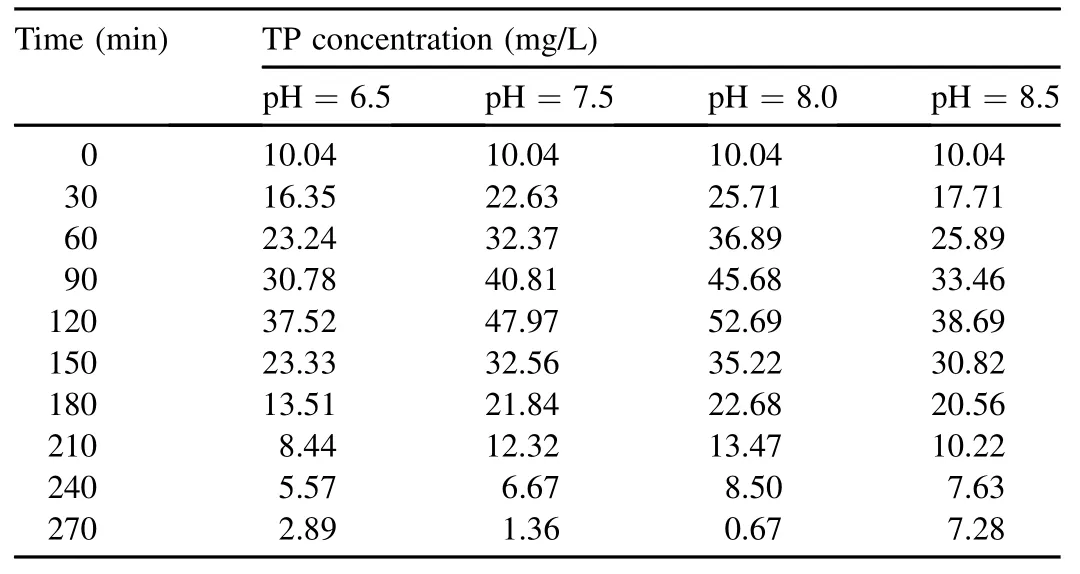
Table 1TP concentration at different pH values.
The anoxic phosphorus uptake capacity also changed at the pH range of 6.5-8.5.When the pH value was 6.5,the sludge had a lower phosphorus uptake capacity and the uptake amount was 34.63 mg/L within 150 min of anoxic reaction.A low pH value influenced the SDPB capacity of releasing phosphorus,affected the synthesis of PHB,and inhibited subsequent anoxic phosphorus uptake.As pH increased,anoxic phosphorus uptake also increased;when the pH values were 7.5 and 8.0,the anoxic phosphorus uptake amounts were 46.61 and 52.02 mg/L,respectively.However,the uptake amount decreased to 31.41 mg/L at pH=8.5.A high pH value may have caused an invalid release of phosphorus,which had no impact on the absorption of organics,adversely affected the synthesis of PHB,and lowered the amount of hypoxia electron donors for phosphorus uptake,showing a negative influence on the whole uptake process.
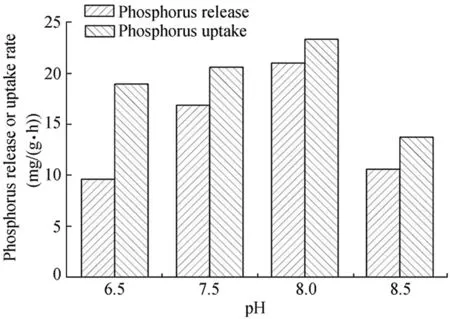
Fig.2.Influence of pH on phosphorus release and uptake rates.
Fig.2 shows the anaerobic phosphorus release rate and anoxic phosphorus uptake rate at different pH values.When pH values were between 6.5 and 8.0,the anaerobic phosphorus release and anoxic phosphorus uptake rates were positively correlated with pH,showing an increasing trend with pH values.The anaerobic phosphorus release rate increased from 9.62 to 20.95 mg/(g·h)when the pH value increased from 6.5 to 8.0;a pH value above 8.0 was prone to induce phosphorus precipitation and the phosphorus release rate decreased.The anaerobic phosphorus release rate decreased to 10.59 mg/(g·h)under the condition of pH=8.5.When pH values were 6.5,7.5,8.0,and 8.5,the anoxic phosphorus uptake rates were 18.92,20.55,23.29,and 13.73 mg/(g·h),respectively.Results demonstrated that the phosphorus release rate was superior to the phosphorus uptake rate in response to the change of pH value,and the operation stability,anaerobic phosphorus release rate,and anoxic phosphorus uptake rate were affected by pH values in the range of 6.5-8.0.
Fig.3 shows the anaerobic acetic acid absorption,PHB synthesis,and poly-P disintegration at different pH values.Anaerobic acetic acid absorption and PHB synthesis were positively correlated with pH values in a range of 6.5-8.0.When the pH value was 6.5,the mass fractions of acetic acid absorbed and PHB synthesized per unit of sludge were 67.13 and 41.47 mg/g,respectively,and the acetic acid absorption rate was 40.28%.When the pH value was 8.0,the mass fractions of acetic acid absorbed and PHB synthesized per unit of sludge were 84.27 and 62.87 mg/g,respectively,and the acetic acid absorption rate was 50.56%.These results indicate that pH control is essential in optimizing the acetic acid absorption and PHB synthesis process.Acetic acid absorption and PHB synthesis behaviors are highly sensitive in the pH range of 7.5-8.0;it is suspected that this phenomenon is caused by the undissociated acetic acid.To explain the effects of pH and acetic acid mass fraction on biomass in activated sludge,Fleit(1995)hypothesized that undissociated acetic acid would rapidly diffuse into bacterial cells,then dissociate and impose a proton load on the intracellular milieu,and subsequently lower the pH.The pH decrement could be detrimental to PHB production.Under low pH conditions,acetic acid will remain mostly in an undissociated form so as to maintain the equilibrium.Therefore,acetic acid diffusion into bacterial cells was probably significant in batch experiments with low pH.The other reason is that the differences in the pH gradient and electrical potential on each side of the cell membrane were accordingly increased with pH,and more microorganisms absorbed acetic acid and converted it into PHB.However,the mass fractions of acetic acid absorbed and PHB synthesized per unit of sludge were 63.2 and 40.33 mg/g,respectively,and the acetic acid absorption rate was 37.92%with a pH value of 8.5,which is consistent with findings of Nittami et al.(2010).It is easy to generate phosphate precipitation under high-pH conditions,and the energy produced by PAOs decreases.Therefore,the mass fractions of acetic acid absorbed and PHB synthesized per unit of sludge decreased.In addition,there is an apparent difference in electric potential on each side of the cell membrane and the difference could be overcome with the energy released by decomposing poly-P.Accordingly,acetic acid was transported into cells and the energy for the synthesis of PHB was reduced.
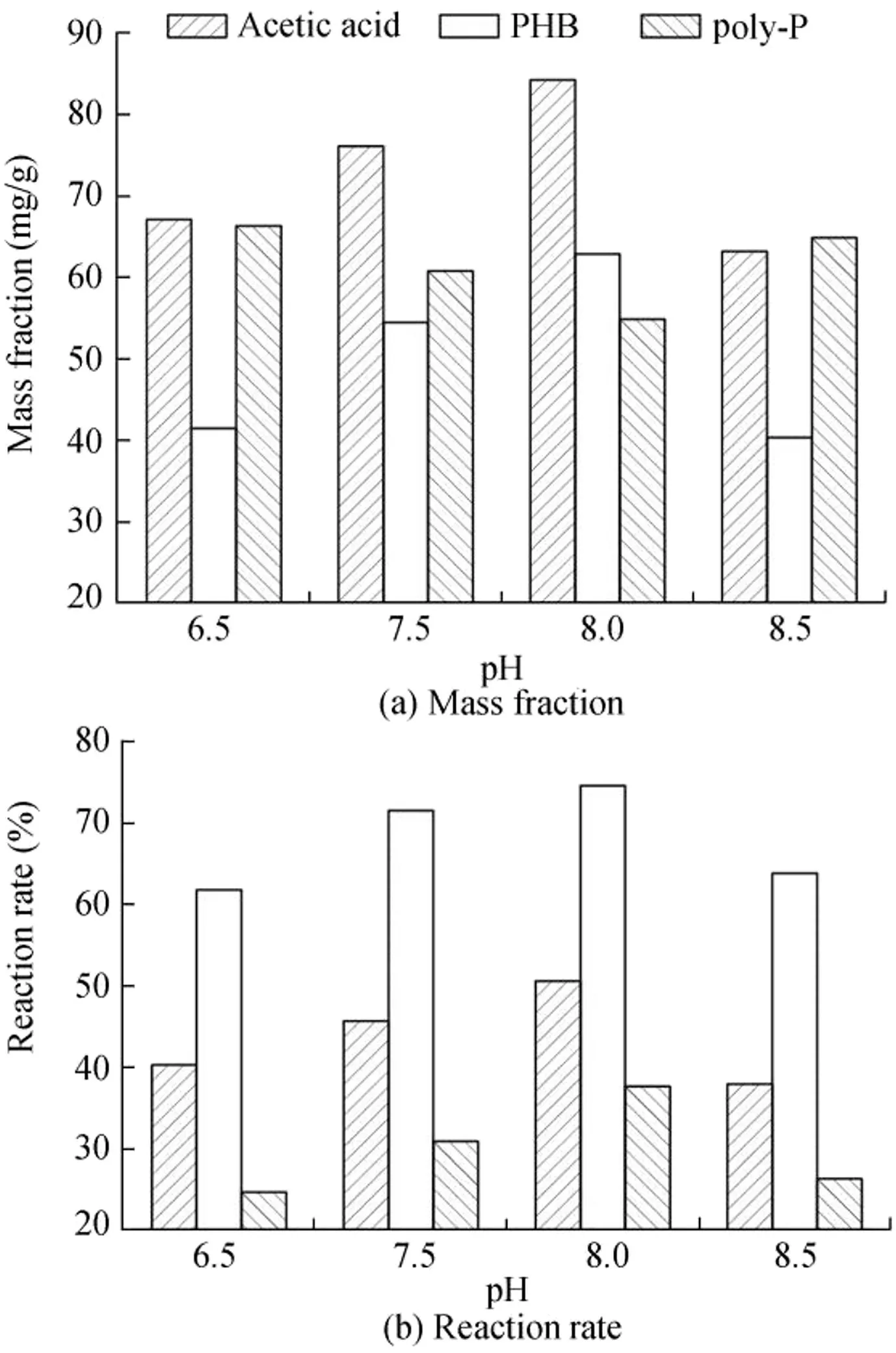
Fig.3.Variations of mass fractions and reaction rates of anaerobic acetic acid,PHB,and poly-P at different pH values.
As the pH value increased from 6.5 to 8.0,the mass fraction of poly-P in sludge gradually decreased and the poly-P decomposition rate gradually increased.Under high-pH conditions,more poly-P was required to provide the energy for acetic acid absorption and PHB synthesis.After the anaerobic reaction was completed,the poly-P mass fraction was 66.32 mg/g,the poly-P decomposition rate was 24.64%at pH=6.5,and the corresponding values were 54.87 mg/g and 37.65%at pH=8.0;a further increment of pH contributed to the formation of phosphate precipitation and affected the decomposition of poly-P.Therefore,when the pH value was 8.5,the poly-P mass fraction increased and the decomposition rate decreased to 26.28%.
Fig.4 illustrates the changes of COD,TP,and-N concentrations and PHB and poly-P mass fractions within a typical cycle.As we can see,the poly-P mass fraction decreased,the poly-P disintegration amount was 33.52 mg/g,and the PHB production amount was 62.87 mg/g in the sludge under anaerobic conditions.The reason for this variation is that PAOs disintegrated poly-P and generated orthophosphate,and the resultant energy was used for absorbing volatile fatty acids in water and storing them in the form of PHB.Thus,the COD concentration decreased while the PHB mass fraction and TP concentration increased along with poly-P disintegration under anaerobic conditions;the TP concentration reached 48.98 mg/L at the end of the anaerobic phase.poly-P gradually accumulated during the anoxic phase and the poly-P mass fraction reached 92.67 mg/g at the end of the anoxic phase;the PHB mass fraction and the-N and TP concentrations decreased,and the effluent TP concentration was 1.47 mg/L,which was in line with Grade I of theDischarge Standard of Pollutants for Municipal Wastewater Treatment Plant(GB 18918-2002).The effluent-N concentration was 1.8 mg/L,indicating that there was a low concentration of nitrite residuals in the reactor,which could not affect anaerobic phosphorus release.Under anoxic conditions,nitrite served as an electron acceptor,PHB was used as a carbon source and as energy,and excessive orthophosphate was transformed into poly-P,which then accumulated within cells(Li,2010).Furthermore,the COD concentration showed a decreasing trend during the reaction;COD was mainly removed under anaerobic conditions,with a removal rate of 78.84%,and the effluent COD concentration was 33.93 mg/L.This finding was consistent with the results of Zhou et al.(2007),which showed that COD concentration in the reactor was about 50 mg/L after the anaerobic reaction was finished.The remaining acetic acid did not likely enter the anoxic phase and interfere with PAO uptake.There were excessive amounts of acetic acid and COD produced under anaerobic conditions,which led to anoxic denitrification and competition of-N,and ultimately restricted anoxic phosphorus uptake(Guo et al.,2005a).
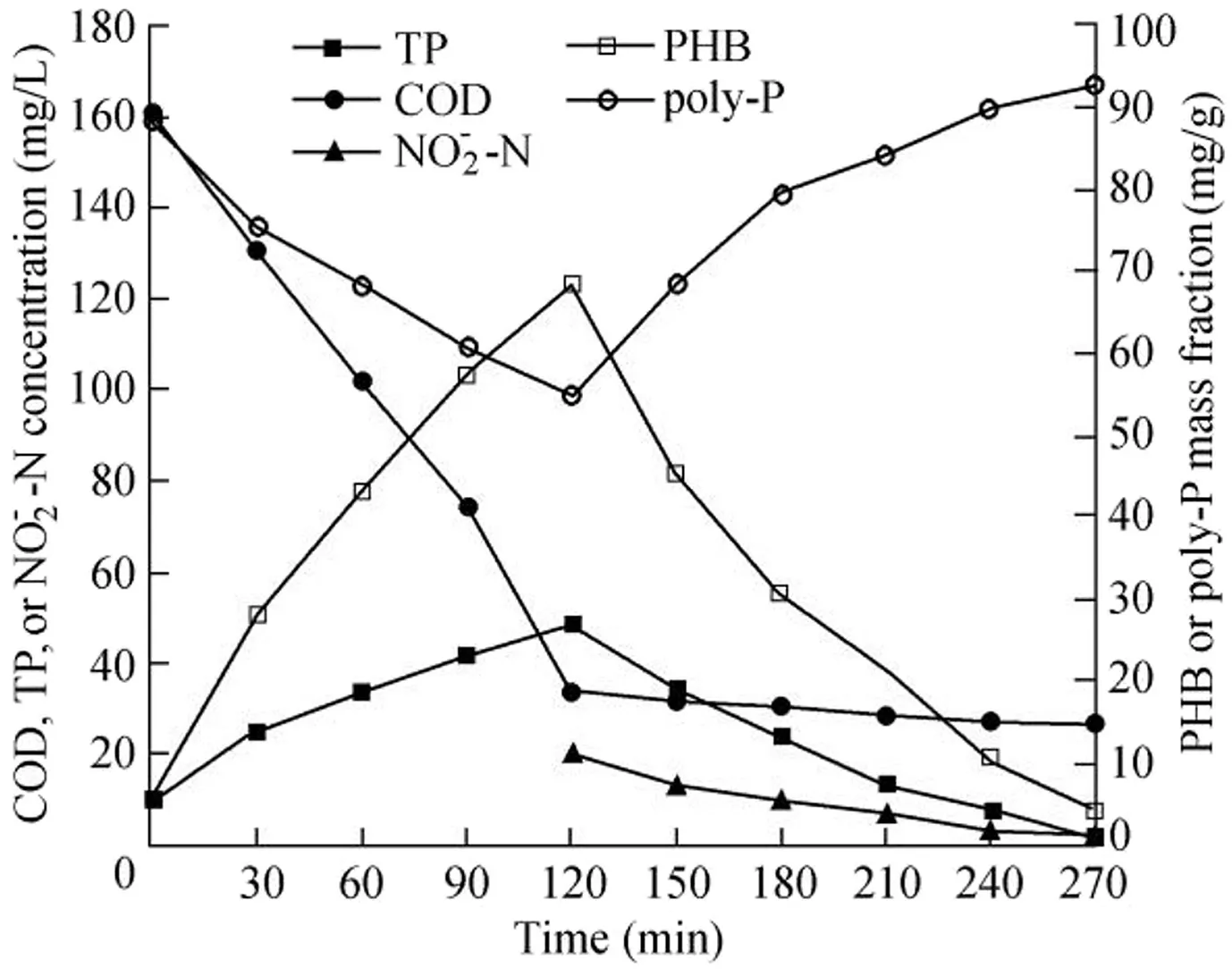
Fig.4.Concentrations of COD,TP,and -N and mass fractions of PHB and poly-P within typical cycle.
4.Conclusions
(1)When the pH value ranged from 6.5 to 8.0,the increment of pH will increase phosphorus release in unit amounts of acetic acid,showing a positive correlation between the TP removal performance and pH.When the pH value was elevated to 8.5,the amount of released phosphorus decreased,possibly resulting from phosphate precipitation.The anoxic effluent concentrations of TP were in the range of 0.67-2.89 mg/L with pH increasing from 6.0 to 8.0.
(2)The phosphorus release rate was superior to the phosphorus uptake rate in response to the change of pH values,and the operation stability,anaerobic phosphorus release rate,and anoxic phosphorus uptake rate were affected by pH values in the range of 6.5-8.0.
(3)The control of pH was essential in optimizing the acetic acid absorption,PHB synthesis,and poly-P disintegration process.Acetic acid absorption and PHB synthesis behaviors were highly sensitive in the pH range of 7.5-8.0,possibly due to the undissociated acetic acid.
(4)The short-cut denitrifying phosphorus removal system was capable of achieving strong removal effects for COD,TP,and-N.
(5)pH control is critical in phosphorus release and uptake,if the pH of a selected active sludge process falls between 6.5 and 8.5.In order to gain more insight into the effects of pH(in the range of 6.5-8.5)on sludge's denitrifying phosphorus removal,more studies are needed.
References
Chua,A.S.M.,Takabatake,H.,Satoh,H.,Mino,T.,2013.Production of poly hy droxyal kanoates(PHA)by activated sludge treating municipal wastewater:Effect of pH,sludge retention time(SRT),and acetate concentration in in fluent.Water Res.37(15),3602-3611.https://doi.org/10.1016/s0043-1354(03)00252-5.
Deng,Q.Q.,2011.Technique of short-cut denitrifying dephosphorization and its study progress.Guangdong Chem.Ind.3,34-35(in Chinese).
Ding,W.C.,Li,Q.,Ji,F.Y.,Tian,X.M.,Wu,D.,Du,Y.,2010.Shortcut nitrification-denitrification and denitrifying phosphorus removal in twosludge SBR system.China Water&Wastewater 26(13),11-14(in Chinese).
Fleit,E.,1995.Intracellular pH regulation in biological excess phosphorous removal systems.Water Res.29(7),87-92.https://doi.org/10.1016/0043-1354(94)00265-9.
Guo,H.J.,Ma,F.,Shen,Y.L.,2005a.Effect of C/N ration on denitrifying dephosphatation.Acta Sci.Circumstantiae 25(3),367-371.https://doi.org/10.3321/j.issn:0253-2468.2005.03.016.
Guo,X.,Meng,Z.H.,Dong,J.H.,2005b.Study on pH value effect on removal of biological phosphorus in anaerobic pool.J.Harbin Univ.of Commerce(Sci. Ed.) 21(3), 292-293. https://doi.org/10.19492/j.cnki.1672-0946.2005.03.009.
Jabari,P.,Munz,G.,Oleszkiewicz,J.A.,2014.Selection of denitrifying phosphorous accumulating organisms in IFAS systems:Comparison of nitrite with nitrate as an electron acceptor.Chemosphere 109,20-27.https://doi.org/10.1016/j.chemosphere.2014.03.002.
Li,J.,Chai,L.Y.,Xiang,R.J.,Cheng,Y.X.,2011.Pilot-scale test on domestic waste water treatment using integrative A/O(anaerobic-aerobic sludge)equipment.J.Cent.S.Univ.:Sci.Technol.42(10),2935-2940(inChinese).
Li,N.,2010.Performance and Removal Approaches of Biological Phosphorus Removal from Wastewater in SBR under Low Temperature.Ph.D.Dissertation.Harbin University of Technology,Harbin(in Chinese).
Li,X.K.,2006.Study on Denitrifying Phosphorus Removal Process and Microbiology Research.Ph.D.Dissertation.Harbin Institute of Technology,Harbin(in Chinese).
Li,X.K.,Zhang,J.,Huang,R.X.,Ma,L.,Bao,R.L.,Jiang,A.X.,2006.Study on characteristic of denitrification phosphorus removal bacteria.China Water&Wastewater 22(3),35-39(in Chinese).
Nittami,T.,Oi,H.,Matsumoto,K.,Seviour,R.J.,2010.Influence of temperature,pH and dissolved oxygen concentration on enhanced biological phosphorus removal under strictly aerobic conditions.J.Biotechnol.150,237.https://doi.org/10.1016/j.jbiotec.2010.09.090.
Peng,Y.Z.,2011.SBR Method for Sewage Biological Phosphorus Removal and Process Control.Science Press,pp.188-190(in Chinese).
Smolders,G.J.F.,van der Meij,J.,van Loosdrecht,M.C.M.,Heijnen,J.J.,1994.Model of the anaerobic metabolism of the biological phosphorus removal process:Stoichiometry and pH influence.Biotechnol.Bioeng.43(6),461-470.https://doi.org/10.1002/bit.260430605.
Wang,A.J.,Wu,L.H.,Ren,N.Q.,Zhao,D.,Yan,M.L.,2005.Feasibility of denitrifying phosphorus removal technique using nitrite as electron acceptor.China Environ.Sci.25(5),515-518(in Chinese).
Wang,J.H.,Peng,Y.Z.,Chen,Y.Z.,2011a.Experimental research on suspended aerobic bio film A2O system for treating domestic wastewater.J.Cent.South Univ.:Sci.Technol.42(12),3918-3922(in Chinese).
Wang,Y.Y.,Geng,J.J.,Ren,Z.J.,He,W.T.,Xing,M.Y.,Wu,M.,Chen,X.W.,2011b.Effect of anaerobic reaction time on denitrifying phosphorus removal and N2O production.Bioresour.Technol.102(10),5674-5684.https://doi.org/10.1016/j.biortech.2011.02.080.
Wang,Y.Y.,Geng,J.J.,Guo,G.,Wang,C.,Liu,S.H.,2011c.N2O production in anaerobic/anoxic denitrifying phosphorus removal process:The effects of carbon sources shock.Chem.Eng.J.172(2-3),999-1007.https://doi.org/10.1016/j.cej.2011.07.014.
Wang,Y.Y.,Zhou,S.,Liu,Y.,Wang,H.,Stephenson,T.,Jiang,X.X.,2014.Nitrite survival and nitrous oxide production of denitrifying phosphorus removal sludges in long-term nitrite/nitrate-fed sequencing batch reactors.Water Res.67,33-45.https://doi.org/10.1016/j.watres.2014.08.052.
Wu,C.Y.,2010.A2/O Denitrifying Phosphorus Process and Its Optimal Control.Ph.D.Dissertation.Harbin University of Technology,Harbin(in Chinese).
Yang,Y.Y.,Zeng,W.,Liu,J.R.,Li,L.,Wang,X.D.,2010.Effect of nitrite on biological phosphorus removal in wastewater.Microbiol.China 37(4),586-593.https://doi.org/10.13344/j.microbiol.china.2010.04.013(in Chinese).
Za firiadis,I.,Ntougias,S.,Nikolaidis,C.,Kapagiannidis,A.G.,Aivasidis,A.,2011.Denitrifying polyphosphate accumulating organisms population and nitrite reductase gene diversity shift in a DEPHANOX-type activated sludge system fed with municipal wastewater.J.Biosci.Bioeng.111(2),185-192.https://doi.org/10.1016/j.jbiosc.2010.09.016.
Zeng,W.,Li,L.,Yang,Y.Y.,Wang,X.D.,Peng,Y.Z.,2011.Denitrifying phosphorus removal and impact of nitrite accumulation on phosphorus removal in a continuous anaerobic-anoxic-aerobic(A2O)process treating domestic wastewater.Enzym.Microb.Technol.48(2),134-142.https://doi.org/10.1016/j.enzmictec.2010.10.010.
Zhou,K.Q.,Liu,H.,Sun,Y.F.,Zhou,Y.P.,Liu,J.P.,2007.Screening and enrichment of denitrifying phosphorus accumulating bacteria using nitrite as electronic acceptor.Environ.Eng.1(8),126-131.https://doi.org/10.3969/j.issn.1673-9108.2007.08.027.
Zhou,S.Q.,Zhang,X.J.,Feng,L.Y.,2010.Effect of different types of electron acceptors on the anoxic phosphorus uptake activity of denitrifying phosphorus removing bacteria.Bioresour.Technol.101(6),1603-1610.https://doi.org/10.1016/j.biortech.2009.09.032.
杂志排行
Water Science and Engineering的其它文章
- Understanding groundwater table using a statistical model
- Application of a water quality model for determining instream aeration station location and operational rules:A case study
- Numerical investigation of pollution transport and environmental improvement measures in a tidal bay based on a Lagrangian particle-tracking model
- Effects of elevated atmospheric CO2and nitrogen fertilization on nitrogen cycling in experimental riparian wetlands
- A simplified physically-based breach model for a high concrete-faced rock fill dam:A case study
- Effect of trapezoidal collars as a scour countermeasure around wing-wall abutments
