胰腺导管腺癌局部治疗联合免疫治疗研究进展
2018-03-22
() ()
(1复旦大学附属中山医院介入治疗科 上海 200032; 2上海市影像医学研究所 上海 200032)
胰腺导管腺癌(pancreatic ductal adeno-carcinoma,PDAC)是我国发病率排行前十位的恶性肿瘤,其死亡率与发病率之比大于85%,且发病率呈现上升趋势[1]。目前外科手术切除是唯一可能治愈PDAC的治疗方式,但80%以上的患者在确诊时已经失去手术机会。放疗及以吉西他滨为主的化疗是不可手术切除的PDAC患者的主要治疗方式。尽管PDAC诊疗技术不断提高,患者5年生存率仍然不超过5%[2]。传统的放疗是局部进展期PDAC的主要治疗方式之一[3]。目前包括立体定向放射治疗(stereotactic body radiotherapy,SBRT)、质子放疗、放射性粒子植入、射频消融治疗、电穿孔治疗(irreversible electroporation,IRE)、高强聚焦超声(high intensity focused ultrasound,HIFU)在内的局部治疗有望成为PDAC患者的新选择。虽然免疫治疗已经在血液系统肿瘤、黑色素瘤、非小细胞肺癌、膀胱癌等实体瘤的治疗中取得令人瞩目的进展,但是PDAC的免疫治疗还处于探索阶段。本文主要聚焦不可手术切除的PDAC局部治疗联合免疫治疗的基础及临床研究进展。
PDAC局部治疗研究概况放疗是局部进展期PDAC的主要治疗方式之一。近年来,随着影像导引技术的提高,高剂量且更加精准的SBRT为PDAC的放疗提供了新的手段[4-7]。质子放疗通过不同能量的带电粒子Bragg峰展宽得到扩展Bragg峰(spread out bragg peak,SOBP),使病灶位于SOBP峰区,从而获得靶区内高剂量。研究表明,质子放疗联合卡培他滨作为胰腺癌新辅助放化疗安全、可行,并可以达到良好的肿瘤局部控制率[8-10]。质子放疗可以覆盖高危淋巴结而减少损伤周围正常组织[11]。与调强适形放疗(intensity-modulated radiation therapy,IMRT)及三维适型放疗(three dimensional conformal radiation therapy,3DCRT)比较,质子放疗较少损伤周围正常组织[12]。目前由于质子放疗资源紧张,其大规模的推广应用受限。通过术中或者影像导引下经皮穿刺植入放射性粒子,这种组织内放疗可提高肿瘤局部的控制率[13-16]。早期报道放射源的植入多通过开腹途径,随着CT导引的应用,经皮穿刺途径具有更高的安全性及微创性。同样,新器械的出现、CT等导引系统的应用突破了原来被认为是“穿刺禁区”的胰腺,使经皮射频/微波消融治疗PDAC的安全性进一步提高[17-20]。2016年,复旦大学附属中山医院率先以21G经皮穿刺针穿刺PDAC,引入Habib消融导丝进行PDAC射频消融治疗(图1)。该穿刺系统较传统穿刺系统直径更小,提高了穿刺的安全性。首例患者30天内未见严重不良反应,但是否能够获得生存获益需要进一步临床试验证实。相比较于射频消融,IRE对于结缔组织的损伤较小且无热沉效应,理论上更加适用于周围血管较多的胰腺。2012年,Bagla等[21]首次报道1例IRE治疗侵犯腹腔动脉及肠系膜上动脉的局部进展期PDAC患者,初步显示出其可行性及良好的安全性。一项多中心临床试验表明IRE治疗局部进展期PDAC安全可行,与传统放疗、化疗比较IRE可提高患者的肿瘤局部/远处无进展时间(14个月/6个月,15个月/9个月)及生存时间(20个月/13个月)[22]。目前最大样本量的研究是Martin等[23]于2015年报道的200例局部进展期PDAC患者在传统治疗的基础上增加IRE,在29个月的中位随访时间中只有 6 例(3%)出现局部复发,患者的中位生存期为24.9个月。该研究中IRE在开腹后超声导引下进行。初步临床研究表明超声或CT导引下经皮穿刺IRE安全可行[24-25]。磁共振导引下HIFU治疗不可手术切除PDAC的初步临床研究表明,这种无创的局部治疗可能在控制肿瘤发展的同时减轻患者疼痛[26-27]。PDAC的局部治疗从原来的开腹巨创到现在的微创,未来正向更加精准、超微创甚至无创的方向发展。
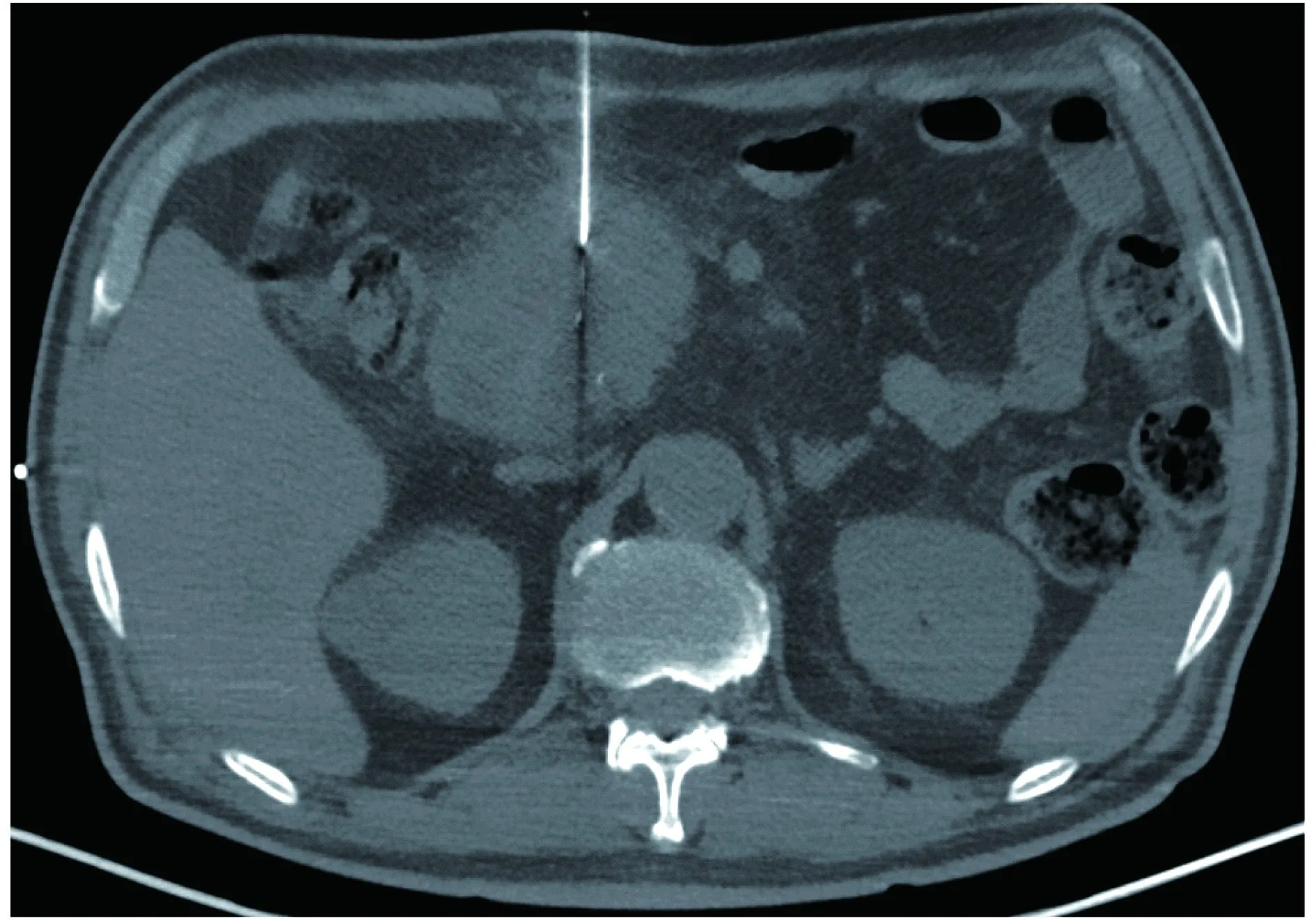
图1 CT导引下经皮穿刺PDAC射频消融术Fig 1 CT-guided percutaneous radiofrequency ablation for PDAC
PDAC免疫治疗概况自19世纪90年代用Coley毒素治疗肿瘤开始,人们不断探索各种免疫疗法用于治疗肿瘤。目前肿瘤的免疫治疗主要包括:免疫检查点阻断治疗(immune checkpoint blockade,ICB)、基因改造T细胞治疗、肿瘤疫苗、免疫刺激因子、溶瘤病毒及基因治疗。近年来ICB已在黑色素瘤、非小细胞肺癌、肾癌、非霍奇金淋巴瘤的治疗中取得重大突破。
ICB的疗效与肿瘤自身的免疫微环境有关。基因改造的PDAC小鼠模型中,肿瘤恶性程度越高,CD4+Treg细胞比例越高。这表明细胞毒T淋巴细胞相关抗原4 (cytotoxic T lymphocyte-associated antigen 4,CTLA-4)抗体可能对PDAC有效[28]。然而,Ipilimumab (CTLA-4抗体) 3 mg/kg单药治疗局部进展期或转移性PDACⅡ期临床试验未见提高肿瘤缓解率。但是,值得注意的是研究中1例患者具有较长的生存时间,说明在特殊情况下CTLA-4单抗可能有效[29]。而另一方面,程序性死亡配体1(programmed death-ligand 1,PD-L1)阳性的PDAC患者的预后显著差于PD-L1阴性患者,这说明阻断PD-1/PD-L1通路可能使PDAC患者获益[30]。令人沮丧的是PD-L1抗体的Ⅰ期临床试验中,14例PDAC患者并没有出现肿瘤缓解[31]。这给ICB治疗PDAC的前景蒙上了一层阴影。究其原因,PDAC免疫阻断治疗的抵抗性可能与PDAC基质丰富及肿瘤内自身CD8+T细胞数量少有关。动物实验表明去除纤维细胞活化蛋白(fibroblast activation protein,FAP)阳性的癌相关成纤维细胞能增加PD-L1抗体的疗效[32]。抑制黏附激酶(focal adhesion kinase,FAK)的同时应用吉西他滨、PD-1及CTLA-4抗体能显著减小肿瘤负荷、延长小鼠生存时间[33]。目前联合FAK抑制剂及PD-1抗体治疗包括PDAC在内的实体性肿瘤的临床试验正在进行中(NCT0275858)。放疗、化疗、射频消融可以引起具有免疫反应的肿瘤细胞死亡(immunogenic cell death,ICD),释放损伤相关模式分子(damage-associated molecular pattern,DAMP),增加肿瘤特异性抗原(tumor-associated antigen,TAA)的暴露,加强DC细胞及T细胞的浸润,从而使原来对ICB无效的肿瘤重新变得敏感[34-36]。这种使得原来免疫“冷”瘤重燃为免疫“热”瘤是局部治疗联合免疫肿瘤的重要理论,具体于下文详述。Jaffee等[38]于2001年报道了粒细胞-巨噬细胞集落刺激因子疫苗(又称为GVAX疫苗)治疗PDAC的Ⅰ期临床试验,证实了该疫苗的安全性[37]。2015年的Ⅱ期临床试验结果显示,Cy/GVAX+CRS270显著提高转移性PDAC患者的中位生存时间(试验组vs.对照组:9.7个月vs.4.6个月)。以腺病毒为载体表达HSV-tk基因治疗PDAC安全可行,并可提高CD8+T细胞浸润及PD-L1的表达[39]。溶瘤病毒治疗有望成为晚期PDAC的治疗方式,但是目前尚处于基础研究阶段[40]。基因改造T细胞能够靶向杀伤肿瘤细胞,而较少杀伤正常组织。以癌胚抗原(carcino-embryonic antigen,CEA)、黏蛋白(mucoprotein 1,MUC1)、间皮素(Mesothelin)为靶点基因改造T细胞治疗PDAC在动物试验中显示出良好的安全性及有效性[41-43]。目前针对CEA、人表皮生长因子受体2(human epidermal growth factor receptor 2,Her-2)、前列腺干细胞抗原(prostate stem cell antigen,PSCA)、间皮素、MUC1、CD-133靶点的基因改造T细胞治疗的临床试验正在进行中(如NCT02349724、NCT02713984、NCT02744287、NCT01897415、NCT02706782、NCT02587689、NCT02541370),包括PDAC在内的多种实体肿瘤。瘤外靶向损伤及细胞因子风暴(cytokine release syndrome,CRS)是基因改造T细胞治疗的主要不良反应[44]。其中CRS指的是T细胞输入后释放IFN-γ、IL-6等,产生高热、低血压、低氧、心衰、肾衰、电解质紊乱等临床症状。双靶点、抑制性因子的敲入、自杀基因的敲入可以增加细胞治疗的精准性及可控性。另外采用局部注射的方式有望在产生局部及全身疗效的同时减少细胞的用量,从而减少不良反应。如何增加输注的基因改造T细胞定植、减少肿瘤微环境对其的抑制是细胞治疗的另一个重要方面。局部治疗可释放CCL4等细胞趋化因子,在理论上可以增加T细胞定植[45]。联合ICB治疗可能进一步提高基因改造T细胞治疗的疗效。在单种细胞因子治疗PDAC方面,Ⅲ期临床实验表明TNFerade不能提高局部进展期PDAC患者的生存时间,而且与经皮穿刺途径比较,通过内镜经食道途径是无疾病进展时间较短的风险因素[46]。由此提示,细胞因子对肿瘤细胞、机体整体及其不同浓度细胞因子对肿瘤的作用有待进一步研究。
总之,新兴的局部治疗的临床有效性需要进一步的临床试验来证明。虽然GVAX疫苗治疗晚期PDAC已取得重要成就,但是免疫治疗单独应用于PDAC的治疗效果较差。如何打破PDAC免疫治疗抵抗需要进一步的研究。
局部治疗后PDAC免疫微环境的改变及联合治疗探索PDAC组织内CD8+T细胞稀少,而调节性T细胞(Treg)及骨髓源性抑制细胞(myeloid-derived suppressor cells,MDSC)丰富[28,30]。丰富的基质减少了癌细胞与T细胞的接触[32]。肿瘤免疫治疗的作用主要取决于:(1)肿瘤特异性抗原的表达;(2)有效的抗原呈递;(3)与肿瘤细胞接触的功能完整的T细胞。从这些角度看,PDAC属于免疫“冷”瘤,单独针对某一环节的免疫治疗难以起效。如何打破肿瘤“冷”的格局是免疫治疗成功的关键。PDAC的局部治疗主要通过物理作用(如辐射、热、电穿孔)直接杀伤肿瘤细胞。与机体默认无免疫反应的程序性凋亡不同,这些局部治疗可以导致ICD。
Filatenkov等[47]研究发现高剂量的放疗后DC细胞活化,释放INFγ,增加CD8+T细胞的浸润,同时还可以减少MDSC,重新塑造肠癌的肿瘤微环境。DC细胞的活化主要通过2条通路:(1)放疗后HMGB1释放,通过Toll样受体4 (toll-like receptor 4,TLR-4)、髓样分化因子(myeloid differentiation factor 88,MYD-88)激活DC细胞从而影响疗效[48];(2) DC细胞感受外来双链DNA的干扰素刺激基因(stimulator of interferon genes,STING)途径,释放Ⅰ型干扰素,从而增强T细胞的活化[49-50]。理论上,放疗可以增加细胞质中的双链DNA,再者氧自由基的作用可减少双链DNA的降解。但这种双链DNA如何进入DC细胞的细胞质需要进一步的研究。PDAC小鼠皮下瘤模型中,放疗联合局部注射环二核苷酸(cyclic dinucleotide,CDN)类似物进一步激活STING通路,导致T细胞依赖的肿瘤坏死[51]。单次高剂量放疗可通过激活T细胞杀伤肿瘤,传统的分次放疗则有可能抑制这种放疗后的抗肿瘤免疫。且联合ad-LIGHT免疫治疗可进一步提高疗效[52]。碘-125粒子能够长期释放低剂量的射线,其是否能够通过类似的途径增强细胞免疫尚需进一步研究。SBRT联合CTLA-4抗体的Ⅰ期临床试验研究中纳入22例患者,根据实体瘤疗效评估标准(response evaluation criteria in solid tumor,RECIST),有18%的患者肿瘤部分缓解,此后的动物试验也只能达到类似的结果。RNA测序分析结果显示,治疗后PD-L1明显升高。联合PD-1抗体后的动物试验显示出令人震惊的疗效(总体生存率100%),其中机制为SBRT增加T细胞抗原受体(T cell receptor,TCR)的多样性、抗CTLA-4减少Treg细胞、抗PD-1/PD-L1逆转T细胞失能三者相辅相成[53]。另外,联合PD-1抗体、TIM-3抗体及放疗能够使小鼠神经胶质瘤体积缩小,这表明联合多通路的ICB治疗有望进一步提高疗效[54]。Zheng等[55]建立表达SIY抗原的Pan02PDAC小鼠模型,放疗联合疫苗(SIY多肽+TLR-3激动剂Poly I:C)能够增加CD8+T细胞浸润;联合PD-L1抗体后显著增加肿瘤的缓解率,延长小鼠生存期。
热、冷、电穿孔等局部治疗也可导致ICD。其潜在机制为术后DNA、RNA、高迁移率族蛋白1(high mobility group box-1 protein,HMGB1)、热休克蛋白70等升高,促进DC细胞、T细胞活化。再者,大量的肿瘤细胞坏死、TAA暴露,相当于在肿瘤原位建立类似于肿瘤疫苗的肿瘤抗原库。由于热消融、冷消融、电穿孔导致细胞损伤的方式不同,术后细胞因子的释放、细胞抗原的保存不尽相同[56]。动物实验表明射频消融术后凝固性坏死区周围可见大量的中性粒细胞、DC细胞浸润,能促进针对肿瘤的抗原特异性免疫[35,57]。HIFU治疗后肿瘤特异性T淋巴细胞、DC细胞增加[58-59]。Zerbini等[60]研究发现,22例接受射频消融的肝癌患者外周血单核细胞能激活患者自身消融术后获取的肿瘤组织并释放INF-γ,但是这种反应并未有效抑制肿瘤的复发。消融术后可以增加T细胞、DC细胞浸润来增强免疫,但另一方面消融术后肿瘤免疫微环境的改变可能导致的免疫抑制会影响这种肿瘤特异性免疫的持续性。Zeng等[61]研究发现肝癌患者消融术后PD-1、PD-L1升高,且与患者预后相关。消融术后抗原持续暴露、缺氧、前列腺素E2 (prostaglandin E2,PGE2)、腺苷(adenosine)及PD-1/PD-L1升高,肿瘤发生EMT,从而导致T细胞失能、肿瘤免疫逃逸。联合ICB治疗逆转消融术后的T细胞失能,有可能提高疗效。Waitz等[62]报道冷冻消融联合CTLA-4抗体能够产生局部及远处病灶CD8+T细胞依赖的肿瘤特异性免疫。Shi等[63]报道肠癌肝转移患者转移灶射频消融术后,原发病灶切除标本CD8/4+T细胞增加,同时PD-L1升高。进一步动物试验表明,射频消融处理后远处的病灶也可观察到这种现象。射频消融联合PD-L1抗体能通过CD8+T细胞依赖的途径显著延长小鼠的生存时间[63]。这表明局部治疗在控制局部肿瘤的同时改变了局部及远处病灶的免疫微环境,联合ICB治疗不仅增强局部的疗效,而且对远处病灶产生T细胞依赖的肿瘤杀伤作用。这种消融术后对于远处病灶免疫微环境的“远隔效应”格外引人注目,有病例报道消融术后远处病灶自行缓解甚至消失[64-65]。这使得人们对于局部治疗联合免疫治疗肿瘤的未来充满期待。目前一项冷冻、射频消融或者经动脉化疗栓塞联合PD-1抗体治疗肝细胞肝癌的临床试验正在进行中(NCT02821754)。近年来局部治疗联合免疫治疗PDAC的临床研究逐渐增加。大部分的临床试验为放疗联合免疫治疗,最常用的联合模式是放疗联合ICB(表1)。
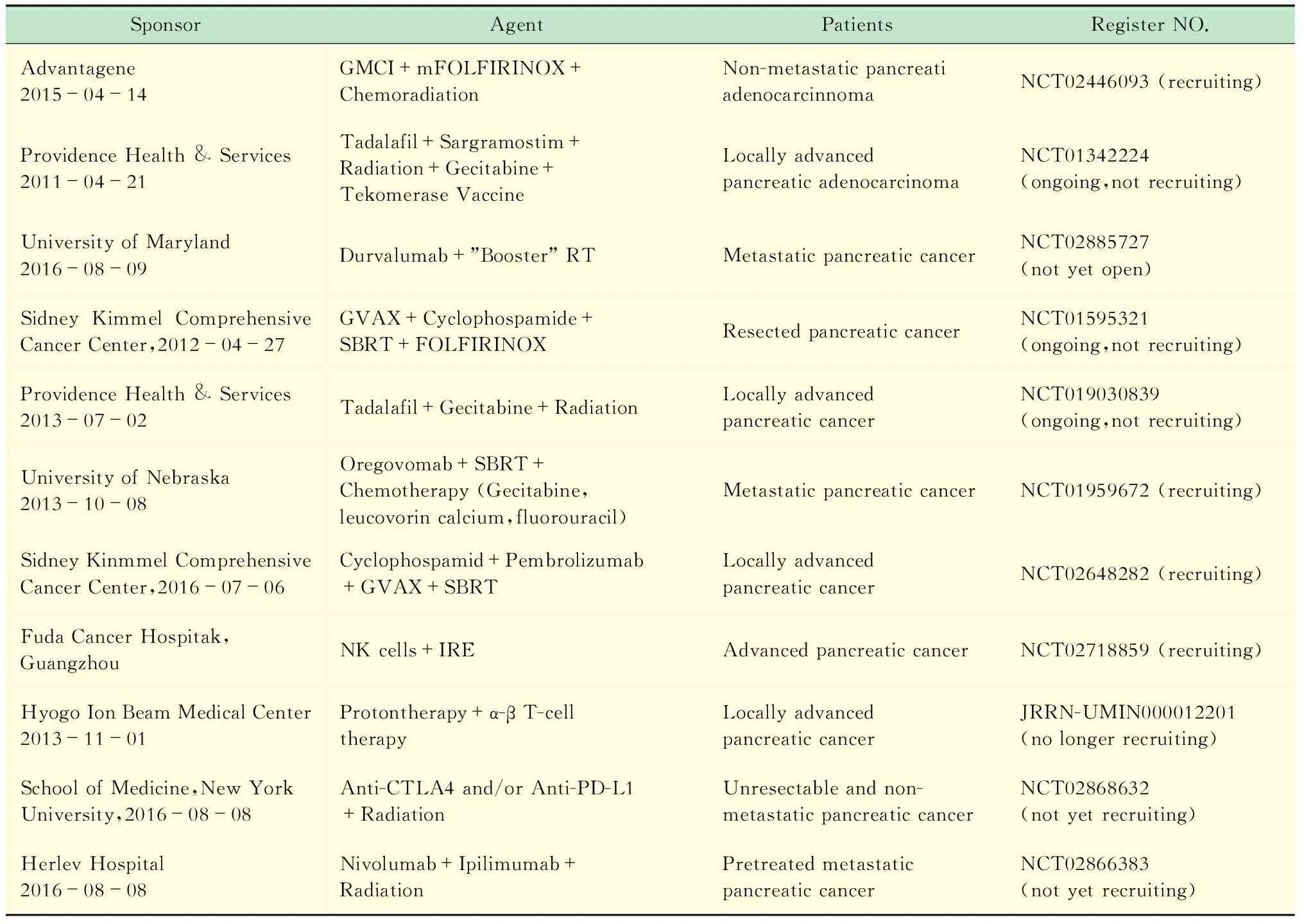
表1 进行中的局部治疗联合免疫治疗PDAC的临床研究Tab 1 Ongoing clinical trials of immunotherapy plus loco-regional therapy for PDAC
GMCI:Gene-mediated cytotoxic immunotherapy;adenovirus expressHSV-tkgene; Tadalafil:PDE5 inhibitor.
结语PDAC免疫治疗的研究在不断探索中前行,局部治疗显示出良好的安全性及有效性。局部治疗能够改变肿瘤局部及远处转移灶的免疫微环境,增加免疫治疗的敏感性,有望减少免疫治疗的不良反应。联合治疗的理论及基础研究为其未来的临床转化提供了充分的证据,近年来注册的临床研究结果值得期待,尤其是放疗联合ICB治疗的临床研究。目前胰腺局部消融治疗联合局部免疫治疗尚无相关研究报道,其机制仍不明确且缺乏相应的循证医学证据。消融、IRE等联合ICB治疗PDAC值得进一步研究。目前尚不清楚局部治疗能否促进基因修饰T细胞的定植、扩增并加强其细胞毒作用。本文未就溶瘤病毒、多肽疫苗、细菌治疗等其他免疫疗法展开阐述。总之,局部治疗与免疫治疗相互结合,可能达到1+1>2的效果。
[1] CHEN W,ZHENG R,BAADE PD,etal.Cancer statistics in China,2015 [J].CACancerJClin,2016,66(2):115-132.
[2] VINCENT A,HERMAN J,SCHULICK R,etal.Pancreatic cancer [J].Lancet,2011,378 (9791):607-620.
[3] WERNER J,COMBS SE,SPRINGFELD C,etal.Advanced-stage pancreatic cancer:therapy options [J].NatRevClinOncol,2013,10(6):323-333.
[4] GURKA MK,KIM C,HE AR,etal.Stereotactic body radiation therapy (SBRT) combined with chemotherapy for unresected pancreatic adenocarcinoma [J].AmJClinOncol,2017,40(2):152-157.
[5] TIMMERMAN RD,KAVANAGH BD,CHO LC,etal.Stereotactic body radiation therapy in multiple organ sites [J].JClinOncol,2007,25(8):947-952.
[6] HERMAN JM,CHANG DT,GOODMAN KA,etal.Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma [J].Cancer,2015,121(7):1128-1137.
[7] KOONG AC,LE QT,HO A,etal.Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer [J].IntJRadiatOncolBiolPhys,2004,58 (4):1017-1021.
[8] HONG TS,RYAN DP,BORGER DR,etal.A phase 1/2 and biomarker study of preoperative short course chemoradiation with proton beam therapy and capecitabine followed by early surgery for resectable pancreatic ductal adenocarcinoma [J].IntJRadiatOncolBiolPhys,2014,89(4):830-838.
[9] KOZAK KR,KACHNIC LA,ADAMS J,etal.Dosimetric feasibility of hypofractionated proton radiotherapy for neoadjuvant pancreatic cancer treatment [J].IntJRadiatOncolBiolPhys,2007,68(5):1557-1566.
[10] HONG TS,RYAN DP,BLASZKOWSKY LS,etal.Phase I study of preoperative short-course chemoradiation with proton beam therapy and capecitabine for resectable pancreatic ductal adenocarcinoma of the head [J].IntJRadiatOncolBiolPhys,2011,79(1):151-157.
[11] LEE RY,NICHOLS RC JR,HUH SN,etal.Proton therapy may allow for comprehensive elective nodal coverage for patients receiving neoadjuvant radiotherapy for localized pancreatic head cancers [J].JGastrointestOncol,2013,4(4):374-379.
[12] LING TC,SLATER JM,MIFFLIN R,etal.Evaluation of normal tissue exposure in patients receiving radiotherapy for pancreatic cancer based on RTOG 0848 [J].JGastrointestOncol,2015,6(2):108-114.
[13] ZHONGMIN W,YU L,FENJU L,etal.Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer [J].EurRadiol,2010,20(7):1786-1791.
[14] YU YP,YU Q,GUO JM,etal.Effectiveness and security of CT-guided percutaneous implantation of (125)I seeds in pancreatic carcinoma [J].BrJRadiol,2014,87(1039):20130642.
[15] SUN S,XU H,XIN J,etal.Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer:results of a pilot trial [J].Endoscopy,2006,38(4):399-403.
[16] LIU B,ZHOU T,GENG J,etal.Percutaneous computed tomography-guided iodine-125 seeds implantation for unresectable pancreatic cancer [J].IndianJCancer,2015,52(Suppl 2):e69-e74.
[17] PAIELLA S,SALVIA R,RAMERA M,etal.Localablative strategies for ductal pancreatic cancer (radiofrequency ablation,irreversible electroporation):a review [J].GastroenterolResPract,2016,2016:4508376.
[18] PANDYA GJ,SHELAT VG.Radiofrequency ablation of pancreatic ductal adenocarcinoma:the past,the present and the future [J].WorldJGastrointestOncol,2015,7(2):6-11.
[19] FEGRACHI S,BESSELINK MG,VAN SANTVOORT HC,etal.Radiofrequency ablation for unresectable locally advanced pancreatic cancer:a systematic review [J].HPB(Oxford),2014,16(2):119-123.
[20] CARRAFIELLO G,IERARDI AM,FONTANA F,etal.Microwave ablation of pancreatic head cancer:safety and efficacy [J].JVascIntervRadiol,2013,24(10):1513-1520.
[21] BAGLA S,PAPADOURIS D.Percutaneous irreversible electroporation of surgically unresectable pancreatic cancer:a case report [J].JVascIntervRadiol,2012,23(1):142-145.
[22] MARTIN RC 2ND,MCFARLAND K,ELLIS S,etal.Irreversible electroporation in locally advanced pancreatic cancer:potential improved overall survival [J].AnnSurgOncol,2013,20(Suppl 3):S443-S449.
[23] MARTIN RC 2ND,KWON D,CHALIKONDA S,etal.Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation:safety and efficacy [J].AnnSurg,2015,262(3):486-494.
[24] SCHEFFER HJ,VROOMEN LG,DE JONG MC,etal.Ablation oflocally advanced pancreatic cancer with percutaneous irreversible electroporation:results of the Phase I/II PANFIRE Study [J].Radiology,2017,282(2):585-597.
[25] MANSSON C,BERGENFELDT M,BRAHMSTAEDT R,etal.Safety and preliminary efficacy of ultrasound-guided percutaneous irreversible electroporation for treatment of localized pancreatic cancer [J].AnticancerRes,2014,34(1):289-293.
[26] ANZIDEI M,NAPOLI A,SANDOLO F,etal.Magnetic resonance-guided focused ultrasound ablation in abdominal moving organs:a feasibility study in selected cases of pancreatic and liver cancer [J].CardiovascInterventRadiol,2014,37(6):1611-1617.
[27] MARINOVA M,RAUCH M,MUCKE M,etal.High-intensity focused ultrasound (HIFU) for pancreatic carcinoma:evaluation of feasibility,reduction of tumour volume and pain intensity [J].EurRadiol,2016,26(11):4047-4056.
[28] CLARK CE,HINGORANI SR,MICK R,etal.Dynamics of the immune reaction to pancreatic cancer from inception to invasion [J].CancerRes,2007,67(19):9518-9527.
[29] ROYAL RE,LEVY C,TURNER K,etal.Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma [J].JImmunother,2010,33(8):828-833.
[30] NOMI T,SHO M,AKAHORI T,etal.Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer [J].ClinCancerRes,2007,13(7):2151-2157.
[31] BRAHMER JR,TYKODI SS,CHOW LQ,etal.Safety and activity of anti-PD-L1 antibody in patients with advanced cancer [J].NEnglJMed,2012,366(26):2455-2465.
[32] FEIG C,JONES JO,KRAMAN M,etal.Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer [J].ProcNatlAcadSciUSA,2013,110(50):20212-20217.
[33] JIANG H,HEGDE S,KNOLHOFF BL,etal.Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy [J].NatMed,2016,22(8):851-860.
[34] GAMEIRO SR,JAMMEH ML,WATTENBERG MM,etal.Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure,resulting in enhanced T-cell killing [J].Oncotarget,2014,5(2):403-416.
[35] DROMI SA,WALSH MP,HERBY S,etal.Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity [J].Radiology,2009,251(1):58-66.
[36] PFIRSCHKE C,ENGBLOM C,RICKELT S,etal.Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy [J].Immunity,2016,44(2):343-354.
[37] JAFFEE EM,HRUBAN RH,BIEDRZYCKI B,etal.Novel allogeneic granulocyte-macrophagecolony-stimulating factor-secreting tumor vaccine for pancreatic cancer:a phase I trial of safety and immune activation [J].JClinOncol,2001,19(1):145-156.
[38] LE DT,WANG-GILLAM A,PICOZZI V,etal.Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer [J].JClinOncol,2015,33(12):1325-1333.
[39] AGUILAR LK,SHIRLEY LA,CHUNG VM,etal.Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma [J].CancerImmunolImmunother,2015,64(6):727-736.
[40] VASSAUX G,ANGELOVA A,BARIL P,etal.Thepromise of gene therapy for pancreatic cancer [J].HumGeneTher,2016,27(2):127-133.
[41] POSEY AD JR,SCHWAB RD,BOESTEANU AC,etal.Engineered CAR T cells targeting the cancer-associated tn-glycoform of the membrane mucin MUC1 control adenocarcinoma [J].Immunity,2016,44(6):1444-1454.
[42] STROMNES IM,SCHMITT TM,HULBERT A,etal.T cells engineered against a native antigen can surmount immunologic and physical barriers to treat pancreatic ductal adenocarcinoma [J].CancerCell,2015,28(5):638-652.
[43] CHMIELEWSKI M,HAHN O,RAPPL G,etal.T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice [J].Gastroenterology,2012,143(4):1095-1107.
[44] BRUDNO JN,KOCHENDERFER JN.Toxicities of chimeric antigen receptor T cells:recognition and management [J].Blood,2016,127(26):3321-3330.
[45] IIDA N,NAKAMOTO Y,BABA T,etal.Antitumor effect after radiofrequency ablation of murine hepatoma is augmented by an active variant of CC Chemokine ligand 3/macrophage inflammatory protein-1alpha [J].CancerRes,2010,70(16):6556-6565.
[46] HERMAN JM,WILD AT,WANG H,etal.Randomized phase Ⅲ multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer:final results [J].JClinOncol,2013,31(7):886-894.
[47] FILATENKOV A,BAKER J,MUELLER AM,etal.Ablativetumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions [J].ClinCancerRes,2015,21(16):3727-3739.
[48] APETOH L,GHIRINGHELLI F,TESNIERE A,etal.Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy [J].NatMed,2007,13(9):1050-1059.
[49] DENG L,LIANG H,XU M,etal.STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors [J].Immunity,2014,41(5):843-852.
[50] BURNETTE BC,LIANG H,LEE Y,etal.The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity [J].CancerRes,2011,71(7):2488-2496.
[51] BAIRD JR,FRIEDMAN D,COTTAM B,etal.Radiotherapy combined with novel STING-targeting oligonucleotides results in regression of established tumors [J].CancerRes,2016,76(1):50-61.
[52] LEE Y,AUH SL,WANG Y,etal.Therapeutic effects of ablative radiation on local tumor require CD8+T cells:changing strategies for cancer treatment [J].Blood,2009,114(3):589-595.
[53] TWYMAN-SAINT VICTOR C,RECH AJ,MAITY A,etal.Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer [J].Nature,2015,520(7547):373-377.
[54] KIM JE,PATEL MA,MANGRAVITI A,etal.Combination therapy with anti-PD-1,anti-TIM-3,and focal radiation results in regression of murine gliomas [J].ClinCancerRes,2017,23(1):124-136.
[55] ZHENG W,SKOWRON KB,NAMM JP,etal.Combination of radiotherapy and vaccination overcome checkpoint blockade resistance [J].Oncotarget,2016,7(28):43039-43051.
[56] CHU KF,DUPUY DE.Thermal ablation of tumours:biological mechanisms and advances in therapy [J].NatRevCancer,2014,14(3):199-208.
[57] ROZENBLUM N,ZEIRA E,BULVIK B,etal.Radiofrequency ablation:inflammatory changes in the periablative zone can induce global organ effects,including liver regeneration [J].Radiology,2015,276(2):416-425.
[58] XIA JZ,XIE FL,RAN LF,etal.High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes [J].UltrasoundMedBiol,2012,38(8):1363-1371.
[59] HU Z,YANG XY,LIU Y,etal.Investigation of HIFU-induced anti-tumor immunity in a murine tumor model [J].JTranslMed,2007,5:34.
[60] ZERBINI A,PILLI M,PENNA A,etal.Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses [J].CancerRes,2006,66(2):1139-1146.
[61] ZENG Z,SHI F,ZHOU L,etal.Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma [J].PLoSOne,2011,6(9):e23621.
[62] WAITZ R,SOLOMON SB,PETRE EN,etal.Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy [J].CancerRes,2012,72(2):430-439.
[63] SHI L,CHEN L,WU C,etal.PD-1 Blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor [J].ClinCancerRes,2016,22(5):1173-1184.
[64] RAO P,ESCUDIER B,DE BAERE T.Spontaneous regression of multiple pulmonary metastases after radiofrequency ablation of a single metastasis [J].CardiovascInterventRadiol,2011,34(2):424-430.
[65] KIM H,PARK BK,KIM CK.Spontaneous regression of pulmonary and adrenal metastases following percutaneous radiofrequency ablation of a recurrent renal cell carcinoma [J].KoreanJRadiol,2008,9(5):470-472.
