胰腺癌分子靶向治疗的研究进展
2018-03-22
() ()
(1复旦大学附属中山医院介入科 上海 200032;2上海市影像医学研究所 上海 200032)
作为致死率位居美国第四、世界第七的恶性肿瘤,胰腺癌因85%~95%的转移率导致其5年生存率仅为1.2%~6%,全球每年因此病死亡约33万人[1-2]。外科手术切除是胰腺癌最有效的治疗手段,但因术后远处转移和局部复发,其5年生存率仍低于20%[3]。
吉西他滨化疗是胰腺癌非手术治疗的首选方法,但效果差强人意。临床研究[4]表明:Folforinox和吉西他滨联合应用比吉西他滨单药效果显著。2013年批准上市的白蛋白紫杉醇也与吉西他滨联合应用于胰腺癌化疗[5]。但是,上述方法对于延长晚期胰腺癌患者的生存期极其有限,且化疗的毒性作用和不良反应也明显提高[6]。因此,人们迫切需要更加行之有效的治疗方法。
随着肿瘤分子机制逐渐被了解,一批针对实体肿瘤的分子靶向药物得以开发并取得一定效果,包括靶向血管生成通路、表皮生长因子受体(epidermal growth factor receptor,EGFR)、MEK通路、成纤维细胞生长因子受体(fibroblast growth factor receptor,FGFR)、PI3K/mTOR通路和肿瘤干细胞等,都使得胰腺癌治疗取得进展。本文将回顾这些分子靶向药物在胰腺癌临床及实验研究方面的现状,并讨论其潜在的治疗前景。
胰腺癌的基因突变和分子病理学研究表明[7],胰腺癌平均存在63个基因突变,因此想找到有效的治疗手段需明确胰腺癌复杂的基因突变和病理学特征。胰腺癌的分子病理学分析显示一些常见的肿瘤基因和信号通路参与其中。KRAS基因编码小分子GTP酶,起调节生长因子受体下游信号的肿瘤突变基因的作用,多数晚期胰腺癌中都可发现[8]。KRAS基因簇的特定点位存在错义突变(多为密码子12)[9],而KRAS基因突变是胰腺上皮内瘤变(PanIN)过程最早的基因事件之一[10]。除了KRAS基因的突变,胰腺癌也常发生抑癌基因如INK4A、BRCA2和LKB1的突变。超过90%的胰腺癌编码细胞周期调节蛋白的抑癌基因P16/CDKN2A处于失活状态[11]。P53突变与导致细胞周期停滞和凋亡的毒性压力应答密切相关[12],75%的胰腺癌存在P53突变,其中又有很高比例伴随杂合性丢失的小的基因内突变[7]。55%的胰腺癌存在编码转化生长因子β (transforming growth factor β,TGFβ)信号通路的抑癌基因——SMAD4的错义突变[13],因其与预后不良和广泛转移关系密切,SMAD4突变被认为是潜在的临床观察指标[14]。错配修复基因MLH1和阳离子胰蛋白酶原基因PRSS1的突变也可见于胰腺癌[15]。
一些胰腺癌存在BRAF突变而不是KRAS突变[16]。BRAF编码RAF,后者属于MEK家族的丝氨酸/苏氨酸激酶,MEK再激活ERK形成MAPK信号通路。MAPK信号的激活不仅见于良性病变,也见于胰腺癌[17]。KRAS和BRAF突变的激活最终导致MAPK信号通路的触发,而后者是胰腺癌发展的关键。过表达的MAPK信号通过一系列RAF的活化形式促使胰腺上皮内瘤变(PanIN)/胰腺导管腺癌形成;与此相反,沉默MAPK信号能抑制肿瘤的发生[18-19]。PI3K信号通路是另一个与胰腺癌变关系密切的重要信号通路[20]。PI3K信号通过下游底物例如Akt、p70-S6k和mTOR调节细胞生长和存活。而生长因子受体如血管内皮生长因子(vascular endothelial growth factor,VEGF)和胰岛素样生长因子1受体(insulin-like growth factor-1 receptor,IGF1R)在胰腺癌中也异常表达[21]。这些通路调节一系列重要细胞功能的基因,包括生长、凋亡、分化、转移等。
治疗胰腺癌的分子靶向药物近年来,已有多种分子靶向药物单药或与细胞毒药物多药联合用于治疗胰腺癌,除了靶向上述信号通路外,还包括可能促进肿瘤干细胞信号和肿瘤发生诸多旁分泌信号通路,如Hedgehog、Wnt、Notch和TGFβ等[22]。图1、2总结了当前已知的有关胰腺癌的信号通路和靶向药物的使用情况[23]。表1概括了多种分子靶向药物的临床试验[23]。
血管生成通路 血管新生对于恶性肿瘤的生长至关重要。抗血管生成类的分子靶向药物在治疗肾癌、结肠癌、肺癌、恶性胶质瘤和卵巢癌等方面取得了良好的效果[24]。VEGF是血管发生、生成过程中重要的调控因子,特异性作用于血管内皮细胞,增强血管的渗透性,诱导血管发生、生成和内皮细胞生长,促进细胞迁移、抑制细胞凋亡等,从而促进恶性肿瘤的生长和转移[25]。超过90%的胰腺癌VEGF呈高表达[26],因此以VEGF为靶点进行治疗具有充足的依据。但与此前的Ⅱ期临床试验[27]结果相反,一项Ⅲ期临床试验(CALGB 80303)[23]发现联合应用贝伐珠单抗(一种VEGF单抗)并没有较吉西他滨单药更好。同样,其他类似分子靶向药物如索拉菲尼和阿西替尼效果也一般[28-30]。另一项Ⅱ期临床试验应用TL-118(一种新型VEGF靶向药物)联合吉西他滨治疗合并远处转移的胰腺癌(NCT01509911)仍在进行[23]。阿柏西普(Aflibercept)是一种能抑制VEGF配体依赖型信号进程的重组功能蛋白[31],虽然在细胞株和移植瘤上表现出良好的抗瘤活性,但Ⅲ期临床试验联合吉西他滨对远处转移的胰腺癌总生存期并无获益[32]。Foretinib是一种新型VEGF抑制剂,它竞争性抑制ATP端受体酪氨酸激酶,发挥抗VEGFRs、RON、c-Met、c-KIT、FLT-3和血小板源生长因子受体(platelet-derived growth factor receptors,PDGFRs)作用[33]。由于肝细胞生长因子(hepatocyte growth factor,HGF)和c-MET在胰腺癌中也常常呈高表达[34],所以针对这些通路的治疗方法同样引起了关注。Foretinib不仅能抑制胰腺癌荷瘤动物的VEGFR-2、VEGFR-3和TIE-2信号通路,还能抑制c-MET信号通路,发挥抑制肿瘤生长、血管生成和淋巴管生成的作用,说明多重激酶抑制剂能发挥同步抑制效果抑制胰腺肿瘤生长[35]。
EGFR通路 EGFR是ErbB家族的一种跨膜酪氨酸激酶受体,在肿瘤细胞生物学行为方面发挥着重要作用。异常活化的EGFR导致受体二聚化并随之激活其下游通路,包括RAS和PI3K/Akt/mTOR信号通路成员[36]。90%的胰腺癌高表达EGFR[37],在胰腺癌细胞株中EGFR持续激活,加入EGFR抑制剂能抑制其增殖[38]。但临床试验中应用EGFR和ErbB2抗体效果都不佳[39-40]。另一种EGFR单抗——尼妥珠单抗联合吉西他滨治疗局限性胰腺癌取得生存期获益,毒性作用与不良反应也在可忍受范围内[23]。另一组EGFR抑制剂——厄洛替尼的大样本Ⅲ期临床试验显示[41],联合应用吉西他滨较吉西他滨单药在中位总生存期(P=0.038)和无进展生存期(P=0.004)均有明显改善。推测可能存在KRAS基因突变或EGFR激活是厄洛替尼治疗胰腺癌疗效的预测指标[42]。虽然中位总生存期仅延长2周,但此项试验还是意义非凡,因为这是仅有的吉西他滨/厄洛替尼能改善已发生远处转移的胰腺癌生存期的临床试验。当然,另一方面还需要平衡厄洛替尼带来的潜在致死性并发症的风险[43]。一项Ⅱ期临床试验[44]结果显示,吉非替尼(易瑞沙)联合吉西他滨治疗已发生远处转移的胰腺癌的中位总生存期为7.3个月,无进展生存期为4.1个月,其联合吉西他滨治疗胰腺癌缺乏证据支持。
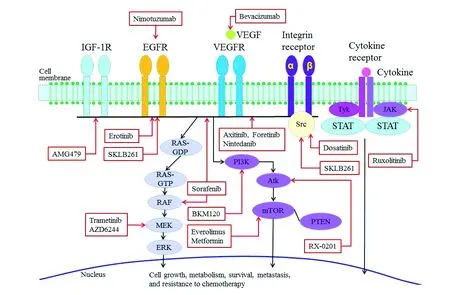
Reproduced by permission from Baishideng Publishing Group Inc:WordJGastroenterol[23],copyright 2016.
图1胰腺癌的分子信号通路及靶向药物作用位点
Fig1Molecularsignalingpathwaysofpancreaticcancerandtargetsiteoftargeted-drugs
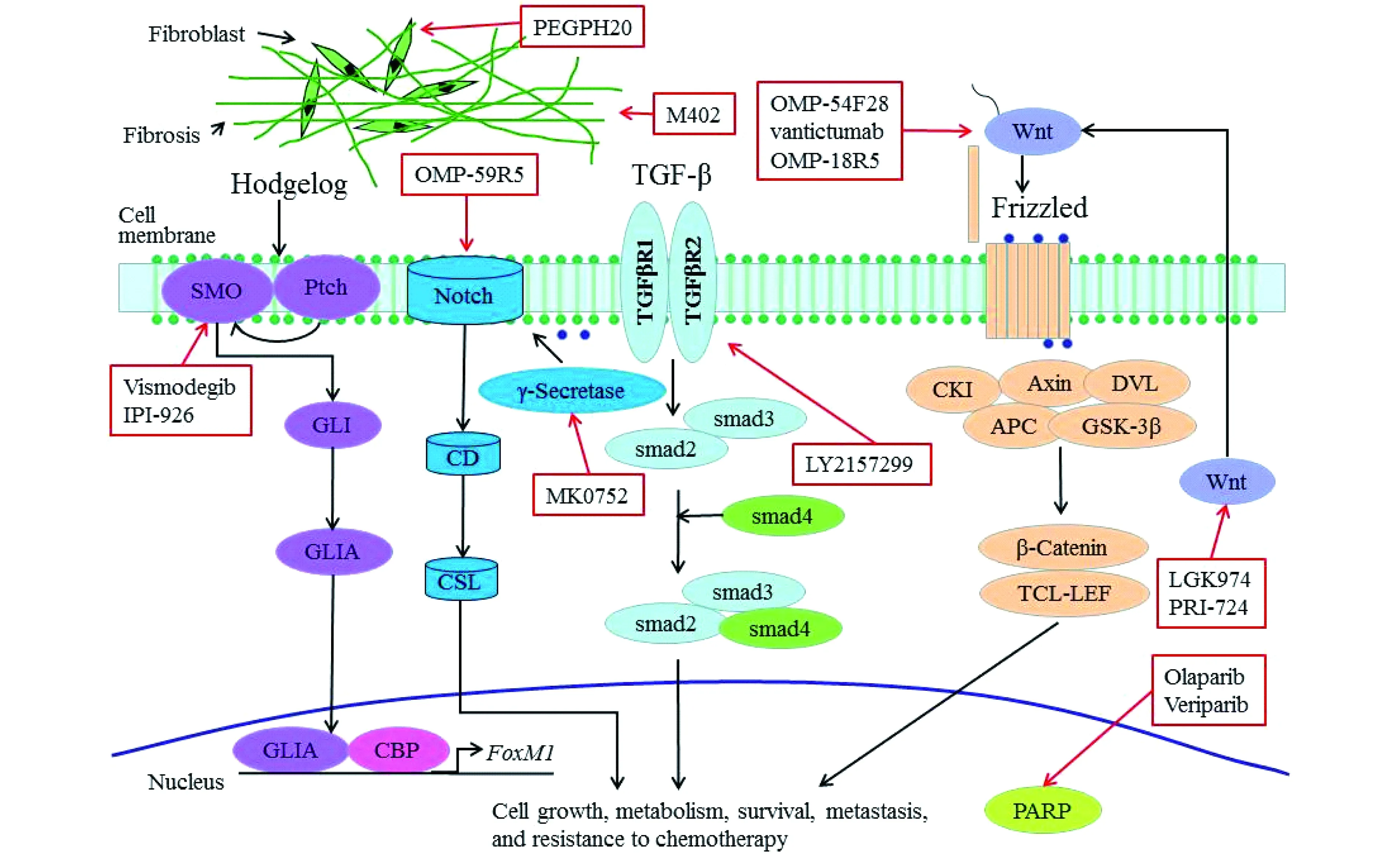
Reproduced by permission from Baishideng Publishing Group Inc:WordJGastroenterol[23],copyright 2016.

图2 胰腺癌的治疗性抑制剂Fig 2 Therapeutic inhibitors for pancreatic cancer
Reproduced by permission from Baishideng Publishing Group Inc:WordJGastroenterol[23],copyright 2016.
IGF1R信号通路 IGF1R属于胰岛素受体家族,在胰腺癌中呈高表达,激活IGF1R能引起信号级联放大进而触发ERK和PI3K/Akt/mTOR信号通路[45]。抑制IGF1R信号能提高吉西他滨对胰腺癌移植瘤的细胞毒作用[46]。但临床试验[23]显示IGF1R抑制剂AMG-479与其单抗(西妥木单抗)并不能让胰腺癌患者获益。但同时阻滞IGF1R和EGFR/Her-2能协同抑制胰腺癌的生长并使IRS-1、Akt完全灭活,阻止MAPK磷酸化[23],证明这两种抑制剂的联合应用能有效避免单药耐药的出现[47],同时将IGF1R、ErbB作为靶点进行双重靶向治疗似乎是一个克服胰腺癌耐药的策略。
RAS信号通路 RAS/RAF/MEK/ERK(MAPK)生长信号能激活相应信号通路的受体(例如EGFR),这些通路对调节肿瘤细胞的增殖和生存至关重要[48]。曲美替尼(MEK抑制剂)联合吉西他滨治疗晚期胰腺癌并无生存期获益[23]。AZD6244(MEK抑制剂)联合厄洛替尼作为二线化疗方案治疗晚期胰腺癌的临床试验仍在进行(NCT01222689)中[23]。
PI3K/Akt/mTOR信号通路 被RAS或者EGFR激活后,PI3K进一步激活Akt,随后触发多个下游目标如mTOR,导致其对细胞进程进行必要的调节,包括肿瘤细胞的生长、代谢、生存、转移和耐药[49]。59%的胰腺癌存在PI3K-Akt信号通路激活[50],该信号通路常表现异常,即PTEN(同源性磷酸酶-张力蛋白,PI3K的天然拮抗剂)的缺失或低表达[51]。一项Ⅱ期临床试验正评估RX-0201(Akt的一种反义寡核苷酸)联合吉西他滨治疗已发生远处转移的胰腺癌(NCT01028495)[23]。依维莫斯(mTOR抑制剂)在联合其他抗肿瘤药物时,表现出抗肿瘤协同效应[52]。一项Ⅱ期临床试验[53]显示,依维莫斯联合卡培他滨治疗晚期胰腺癌,缓解率达6.5%、整体生存期为8.9个月,联合用药能增强卡培他滨的疗效。索拉非尼是一种靶向Raf-1、BRaf、VEGFR1、VEGFR2、VEGFR3和PDGFRβ的多重酶抑制剂,对晚期肝细胞肝癌有效[54]。一项Ⅰ/Ⅱ期索拉非尼联合依维莫斯治疗晚期胰腺癌的临床试验已近尾声,但结果尚未发表(NCT00981162)[23]。二甲双胍用于治疗糖尿病,能通过直接影响代谢激活抑癌基因TSC2来抑制mTOR信号通路。一项Ⅱ期临床试验(NCT01210911)正在探索二甲双胍和厄洛替尼联合吉西他滨治疗已发生远处转移的胰腺癌的安全性和有效性[23]。
Src信号通路 Src属于原癌非受体蛋白酪氨酸激酶家族,通过一系列蛋白间的相互作用调控多个信号转导通路,包括受体酪氨酸激酶和G-蛋白连接受体。约70%的胰腺癌可见C-Src高表达[55]。达沙替尼[Dasatinib,Bcr-Abl及SRC激酶家族(SRC、LCK、YES、FYN)、c-KIT、EPHA2和PDGFR-B等多种激酶抑制剂]是一种与塞卡替尼有关的化合物,一项Ⅱ期临床试验正评估其与5-FU、亚叶酸、奥沙利铂联合治疗已发生远处转移的胰腺癌的效果(FOLFOX-D、NCT01652976)[23]。
JAK/STAT信号通路 很多肿瘤都能发现Janus激酶/信号传感和转录(JAK/STAT)信号通路激活[56]。JAK/STAT信号通路的异常直接促使细胞内转化,增强肿瘤细胞的增殖、抑制凋亡和促进血管生成。胰腺癌存在JAK突变和STAT活化[57-58]。一项Ⅱ期临床试验(NCT01423604)发现卡培他滨联合鲁索利替尼(Ruxolitinib,JAK1和JAK2酪氨酸激酶抑制剂)能改善已发生远处转移的胰腺癌生存期。另一项吉西他滨、鲁索利替尼联合或不联合白蛋白紫杉醇的临床试验(NCT01822756)仍在进行[23]。
肿瘤干细胞胰腺癌干细胞是一小群对化疗和放疗都能耐受的细胞,是癌变、进展和转移的主因[59]。Notch、Hedgehog、Wnt在胰腺癌干细胞发展过程中起关键作用[60]。随着对于胰腺癌干细胞了解的深入,这些都成为胰腺癌靶向治疗的新热点。
Notch信号通路 Notch参与许多器官的发育和功能,调控胰腺祖细胞发育成胰腺和调节胰腺导管细胞分化[61],也参与了肿瘤的增殖和生存[62],在胰腺癌组织中Notch配体和受体均呈高表达[63]。研究表明耐药的胰腺癌干细胞与Notch信号通路激活有关[64]。一项Ⅰb期的临床试验发现,OMP-59R5(一种抗Notch2/3抑制剂)联合白蛋白紫杉醇和吉西他滨治疗已发生远处转移的胰腺癌,显示了良好的耐受性和缓解率(部分缓解46%,持续完全缓解77%)[23]。另一种类似抑制剂(MK0752)联合吉西他滨治疗晚期胰腺癌的临床试验也在进行中(NCT01098344)[23]。
Hedgehog信号通路 Hedgehog发挥调控胚胎发育的作用,在正常胰腺组织中没有表达。Hedgehog与胞外受体和转录的目标基因——Patched(Ptch)结合,后者进一步释放SMO,导致SMO异位到细胞表面,引起GLI转录因子活化和包括GLI和Ptch1在内的Hedgehog靶基因顺向感应。Hedgehog在胰腺癌组织内呈明显高表达[65]。早期的胰腺癌瘤组织内可发现Hedgehog磷酸化,晚期其间质内也可高表达[66]。Hedgehog与KRAS突变关系密切,两者共同促进胰腺瘤变发展为早期胰腺癌[67]。Hedgehog局限于胰腺癌组织基质池内并维持微环境,如使音猬因子(sonic Hedgehog)高表达会诱导转基因小鼠正常胰腺发生癌前病变[68];相反,如减少音猬因子的表达,则能促使吉西他滨递送到荷瘤鼠胰腺癌模型病灶部位,引起基质消散和肿瘤血管化[69],这表明Hedgehog信号通路是药物靶向递送发展的一个方向。GDC-0449(类似维莫德吉的小分子化合物,SMO小分子拮抗剂)能抑制Hedgehog信号通路,但联合吉西他滨治疗已发生远处转移的胰腺癌并没有优于吉西他滨单药[70],但可单药用于外科手术后的辅助化疗(NCT01064622、NCT01096732)。而维莫德吉(Vismodegib)联合吉西他滨治疗复发或已转移的胰腺癌结果令人失望(NCT01064622)。SMO的另一种小分子拮抗剂IPI-926联合吉西他滨的临床试验(NCT01130142)[23]刚刚结束,结果令人期待。
Wnt信号通路 Wnt信号通路发挥调控干细胞的重要作用[71-72],也在胰腺癌肿瘤生物学和发病机制方面发挥重要作用,并且很可能与胰腺癌耐药密切相关[73]。Wnt信号抑制剂如LGK974(NCT01302405)和PRI-724(NCT01351103)还在Ⅰ期临床试验评估中[23]。
TGFβ TGFβ密切参与了胰腺癌细胞的一系列生理过程,包括细胞的分化、内稳态和上皮细胞间质转化(epithelial-mesenchymal transition,EMT)[74]。一项Ⅱ期临床试验(NCT01373164)正在评估LY2157299(TGFβ-1受体特异性抑制剂)联合吉西他滨的治疗效果[23]。
基质环境胰腺癌化疗效果不佳的一个重要原因就是细胞外基质(extracellular matrix,ECM)和基质细胞[75]。胰腺癌拥有丰富的肿瘤基质微环境[76],被认为是唯一的富基质肿瘤,其丰富的基质阻碍了化疗药物到达肿瘤部位。因此,消除基质例如降解透明质酸等可能成为一个潜在的有效递送化疗药物的策略[69]。一项Ⅱ期临床试验(NCT01839487)正在评估PEGPH20(聚乙二醇化重组人透明质酸酶)联合白蛋白紫杉醇和吉西他滨的效果[23]。另一项Ⅰ/Ⅱ期临床试验(NCT01959139)也在评估改良FOLFIRINOX方案联合PEGPH20治疗新诊断的已发生转移的胰腺癌[23]。硫酸乙酰肝素蛋白多糖(heparan sulfate proteoglycans,HSPGs)是一种多糖,也是细胞间质的重要组成部分,与胶原蛋白一起维持间质结构的稳定,参与调节肿瘤生物学行为的多个方面(如肿瘤的发生、发展和转移)[77]。M402(一种硫酸乙酰肝素的类似物)能阻断硫酸乙酰肝素与肿瘤间的相互作用[77]。一项Ⅱ期临床试验(NCT01621243)正在评估M402联合标准化疗方案[23]。基质金属蛋白酶(matrix metalloproteinases,MMPs)是一类蛋白水解酶家族,可降解结缔组织蛋白,在胰腺癌中经常异常表达。马马司他(Marimastat)作为其抑制剂在Ⅲ期临床试验中联合吉西他滨并没有表现出令人满意的结果[78]。
选择性多聚ADP核糖聚合酶通路通过BRCA1和BRCA2蛋白介导的同源重组可以修复DNA双链的断裂以维持基因稳定性或细胞死亡。当BRCA发生障碍,多聚ADP核糖聚合酶通路则在DNA修复中发挥作用[79]。多聚ADP核糖聚合酶蛋白(poly ADP-ribose polymerase,PRAP)在包括DNA转录、DNA损伤应答、基因组稳定性维护和细胞周期调控等方面发挥重要作用。多聚ADP核糖聚合酶抑制剂能导致致死性的肿瘤DNA修复失败或同源性修复缺陷。有5%~7%的胰腺癌伴肿瘤细胞DNA修复不良导致BRCA1和BRCA2的胚系突变,而多聚ADP核糖聚合酶抑制剂能对此产生积极的临床效果。Ⅱ期临床试验(NCT01585805)应用Veliparib(多聚ADP核糖聚合酶的选择性抑制剂)联合顺铂和吉西他滨治疗晚期或伴转移的胰腺癌正在进行。另一项Ⅰ/Ⅱ期临床试验(NCT01296763)应用奥拉帕尼(Olaparib,多聚ADP核糖聚合酶抑制剂,也作用于BRCA1或BRCA2突变)联合伊立替康、顺铂和丝裂霉素C治疗晚期胰腺癌也在进行中[23]。
近期的有关胰腺癌治疗的实验研究尽管很多临床试验在验证已开发的靶向药物,但相较于其他肿瘤,在胰腺癌治疗方面进展并不大。因此,此处汇报最新的实验室研究,希望能为找新的治疗胰腺癌策略提供方向。
一些新颖的酪氨酸激酶抑制剂可以靶向多个不同的信号通路来发挥抗癌效果。多重激酶抑制剂Foretinib能针对胰腺癌发挥多重抑制效果[35]。通过先导化合物优化而获得的SKLB261发挥抗EGFR、Src和 VEGFR2活性。对于胰腺癌移植瘤鼠[80],SKLB261不仅比吉西他滨单药治疗组,同时也比达沙替尼、吉西他滨或厄洛替尼联合治疗组拥有更强大的抗肿瘤效果,明显延长了荷瘤鼠生存期。尼达尼布(Nintedanib)是一种靶向VEGFR1/2/3、FGFR1/2/3和 PDGFRα/β信号通路的三重靶向血管激酶抑制剂[81-82],对胰腺癌细胞株拥有强大的抗肿瘤活性,也增强了吉西他滨的抑癌作用[81,83]。尼达尼布能诱导胰腺癌细胞和间质细胞凋亡,这为临床上联合传统细胞毒药物治疗胰腺癌提供了强大的理论支持[83]。马赛替尼(Masitinib)也是一种多重靶向的酪氨酸激酶抑制剂,能选择性绑定并抑制干细胞因子受体突变体(c-Kit、SCFR)和PDGFR、FGFR3。一项Ⅲ期临床试验发现,马赛替尼联合吉西他滨能延长ACOX1高表达组生存期,其中位整体生存期达11.7个月(95%CI:8.3~19.9),而吉西他滨+安慰剂中位整体生存期仅5.6个月(95%CI:3.7~12.9)[84]。
缺氧诱导因子-1 (hypoxia inducible factor-1,HIF-1)是细胞适应乏氧环境的主要介质,88%的胰腺癌都有表达[85]。PX-478是HIF-1抑制剂,通过促进胰腺癌细胞免疫源性死亡来增强吉西他滨的抗肿瘤效果[86]。
糖原合成酶激酶-3β (glycogen synthase kinase-3β,GSK3β)与胰腺癌细胞增殖密切相关,这为以GSK3β为靶点治疗胰腺癌提供了论据支持[87]。GSK3β抑制剂能通过包括JNK-cJUN活化的机制诱导肿瘤细胞凋亡[88]。CXCR4 (CXC趋化因子受体-4)通过调控GSK3β表达和Akt信号来增强胰腺癌细胞的侵袭力,因此CXCR4抑制剂能应用于胰腺癌的靶向治疗[89]。
FOXM1(forkhead box M1)是FOX蛋白家族的转录因子[90],是调控一系列肿瘤进程(包括肿瘤细胞的存活、增殖和上皮细胞间质转化)的关键基因,在胰腺癌发展过程中起着重要的作用[91]。研究报道[92]FOXM1通过增强uPAR基因转录和随后的肿瘤上皮细胞间质转化来促进胰腺癌发展。这都表明抑制FOXM1信号通路可能成为胰腺癌靶向治疗的一个新方向。
结语胰腺癌具有基因异质性和分子信号通路串扰的复杂性,这直接导致现有治疗的失败。迄今,只有很少的分子靶向药物,例如厄洛替尼,取得了生存期延长。
针对单个信号通路的靶向药物的联合应用对抗肿瘤疗效至关重要,例如抑制PI3K/Akt /mTOR信号通路,肿瘤通过MAPK信号通路能加强不同信号通路间的串扰,产生逃逸效果,最终减弱抑瘤效果。研究发现[93],以PI3K抑制剂ZSTK474和Raf/MEK抑制剂RO5126766为原型的双效药物在体外细胞实验中能高效的抑制PI3K和MEK1,起到降低胰腺癌细胞株生存能力的效果。另一项研究表明[94],Dinaciclib(周期蛋白依赖性激酶抑制剂)联合MK-2206(Akt抑制剂)能明显抑制8种胰腺癌模型的肿瘤生长和转移,取得完全缓解的效果,表明同时阻滞RAF和KRAS下游通路能显著提高治疗效果。临床前研究也表明EGFR和其他通路抑制剂联合应用具有抗胰腺癌移植瘤前景:双抗EGFR和HER2的曲美替尼能显著增强MEK1/2抑制剂对胰腺癌移植瘤的抑瘤效果[95],这表明针对胰腺癌的治疗既需要靶向EGFR-HER2,也需要靶向KRAS以达到疗效最大化[96]。
新的靶向药物既能针对肿瘤细胞,又能兼顾肿瘤基质或许更有意义。越来越多的证据显示基质细胞分泌的蛋白质(Cox-2、基质衍生因子、整合素、分泌蛋白的酸性蛋白、丰富的半胱氨酸)与放、化疗耐受导致的预后不良密切相关[97]。
迄今,绝大多数靶向药物的胰腺癌临床试验并未取得满意效果;动物和细胞实验虽有进展,结果还需观察、验证。分子靶向药物在不断取得进展的同时,在胰腺癌面前却无能为力,这也证明胰腺癌的生物学行为还有待更多揭示。
[1] SIEGEL R,NAISHADHAM D,JEMAL A.Cancer statistics,2012[J].CACancerJClin,2012,62(1):10-29.
[2] SIEGEL RL,MILLER KD,JEMAL A.Cancer statistics,2015[J].CACancerJClin,2015,65(1):5-29.
[3] OETTLE H,POST S,NEUHAUS P,etal.Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer:a randomized controlled trial[J].JAMA,2007,297(3):267-277.
[4] CONROY T,DESSEIGNE F,YCHOU M,etal.FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer[J].NEnglJMed,2011,364(19):1817-1825.
[5] BORAZANCI E,VON HOFF DD.Nab-paclitaxel and gemcitabine for the treatment of patients with metastatic pancreatic cancer[J].ExpertRevGastroenterolHepatol,2014,8(7):739-747.
[6] THOTA R,PAUFF JM,BERLIN JD.Treatment of metastatic pancreatic adenocarcinoma:a review[J].Oncology,2014,28(1):70-74.
[7] JONES S,ZHANG X,PARSONS DW,etal.Core signaling pathways in human pancreatic cancers revealed by global genomic analyses[J].Science,2008,321(5897):1801-1806.
[8] ROZENBLUM E,SCHUTTE M,GOGGINS M,etal.Tumor-suppressive pathways in pancreatic carcinoma[J].CancerRes,1997,57(9):1731-1734.
[9] YASHIRO M,CARETHERS JM,LAGHI L,etal.Genetic pathways in the evolution of morphologically distinct colorectal neoplasms[J].CancerRes,2001,61(6):2676-2683.
[10] MOSKALUK CAHRUBAN RH,KERN SE.p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma[J].CancerRes,1997,57(11):2140-2143.
[11] SCHUTTE M,HRUBAN RH,GERADTS J,etal.Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas[J].CancerRes,1997,57(15):3126-3130.
[12] MULLER PA,VOUSDEN KH.p53 mutations in cancer[J].NatCellBiol,2013,15(1):2-8.
[13] IACOBUZIO-DONAHUE CA,SONG J,PARMIAGIANI G,etal.Missense mutations of MADH4:characterization of the mutational hot spot and functional consequences in human tumors[J].ClinCancerRes,2004,10(5):1597-1604.
[14] BLACKFORD A,SERRANO OK,WOLFGANG CL,etal.SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer[J].ClinCancerRes,2009,15(14):4674-4679.
[15] HEZEL AF,KIMMELMAN AC,STANGER BZ,etal.Genetics and biology of pancreatic ductal adenocarcinoma[J].GenesDev,2006,20(10):1218-1249.
[16] CALHOUN ES,JONES JB,ASHFAQ R,etal.BRAF and FBXW7 (CDC4,FBW7,AGO,SEL10) mutations in distinct subsets of pancreatic cancer:potential therapeutic targets[J].AmJPathol,2003,163(4):1255-1260.
[17] HINGORANI SR,PETRICOIN EF,MAITRA A,etal.Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse[J].CancerCell,2003,4(6):437-450.
[18] ARDITO CM,GRUNER BM,TAKEUCHI KK,etal.EGF receptor is required for KRAS-induced pancreatic tumorigenesis[J].CancerCell,2012,22(3):304-317.
[19] COLLINS MA,BEDNAR F,ZHANG Y,etal.Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice[J].JClinInvest,2012,122(2):639-653.
[20] CANTLEY LC.The phosphoinositide 3-kinase pathway [J].Science,2002,296(5573):1655-1657.
[21] HIRAKAWA T,YASHIRO M,MURATA A,etal.IGF-1 receptor and IGF binding protein-3 might predict prognosis of patients with resectable pancreatic cancer[J].BMCCancer,2013,13(21):392.
[22] NEESSE A,MICHL P,FRESE KK,etal.Stromal biology and therapy in pancreatic cancer[J].Gut,2011,60(6):861-868.
[23] MATSUOKA T,YASHIRO M.Molecular targets for the treatment of pancreatic cancer:Clinical and experimental studies[J].WorldJGastroenterol,2016,22(2):776-789.
[24] WHIPPLE C,KORC M.Targeting angiogenesis in pancreatic cancer:rationale and pitfalls[J].LangenbecksArchSurg,2008,393(6):901-910.
[25] KOCH S,CLAESSON-WELSH L.Signal transduction by vascular endothelial growth factor receptors[J].ColdSpringHarbPerspectMed,2012,2(7):a006502.
[26] SEO Y,BABA H,FUKUDA T,etal.High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma[J].Cancer,2000,88(10):2239-2245.
[27] KINDLER HL,NIEDZWIECKI D,HOLLIS D,etal.Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer:phase III trial of the Cancer and Leukemia Group B (CALGB 80303)[J].JClinOncol,2010,28(22):3617-3622.
[28] CARDIN DB,GOFF L,LI CI,etal.Phase II trial of sorafenib and erlotinib in advanced pancreatic cancer[J].CancerMed,2014,3(3):572-579.
[29] SAIF MW.Pancreatic cancer:Sorafenib:no effect on efficacy of chemotherapy in pancreatic cancer[J].NatRevGastroenterolHepatol,2014,11(1):8-9.
[30] IOKA T,OKUSAKA T,OHKAWA S,etal.Efficacy and safety of axitinib in combination with gemcitabine in advanced pancreatic cancer:subgroup analyses by region,including Japan,from the global randomized Phase III trial[J].JpnJClinOncol,2015,45(5):439-448.
[31] KINDLER HL,IOKA T,RICHEL DJ,etal.Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma:a double-blind randomised phase 3 study[J].LancetOncol,2011,12(3):256-262.
[32] ROUGIER P,RIESS H,MANGES R,etal.Randomised,placebo-controlled,double-blind,parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer[J].EurJCancer,2013,49(12):2633-2642.
[33] KATAOKA Y,MUKOHARA T,TOMIOKA H,etal.Foretinib (GSK1363089),a multi-kinase inhibitor of MET and VEGFRs,inhibits growth of gastric cancer cell lines by blocking inter-receptor tyrosine kinase networks[J].InvestNewDrugs,2012,30(4):1352-1360.
[34] KIEHNE K,HERZIG KH,FOLSCH UR.c-met expression in pancreatic cancer and effects of hepatocyte growth factor on pancreatic cancer cell growth[J].Pancreas,1997,15(1):35-40.
[35] CHEN HM,TSAI CH,HUNG WC.Foretinib inhibits angiogenesis,lymphangiogenesis and tumor growth of pancreatic cancerinvivoby decreasing VEGFR-2/3 and TIE-2 signaling[J].Oncotarget,2015,6(17):14940-14952.
[36] VOLDBORG BR,DAMSTRUP L,SPANG-THOMSEN M,etal.Epidermal growth factor receptor (EGFR) and EGFR mutations,function and possible role in clinical trials[J].AnnOncol,1997,8(12):1197-1206.
[37] TOBITA K,KIJIMA H,DOWAKI S,etal.Epidermal growth factor receptor expression in human pancreatic cancer:Significance for liver metastasis[J].IntJMolMed,2003,11(3):305-309.
[38] YAMANAKA Y,FRIESS H,KOBRIN MS,etal.Coexpression of epidermal growth factor receptor and ligands in human pancreatic cancer is associated with enhanced tumor aggressiveness[J].AnticancerRes,1993,13(3):565-569.
[39] SAFRAN H,IANNITTI D,RAMANATHAN R,etal.Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu[J].CancerInvest,2004,22(5):706-712.
[40] PHILIP PA,BENEDETTI J,CORLESS CL,etal.Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma:Southwest Oncology Group-directed intergroup trial S0205[J].JClinOncol,2010,28(22):3605-3610.
[41] MOORE MJ,GOLDSTEIN D,HAMM J,etal.Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer:a phase III trial of the National Cancer Institute of Canada Clinical Trials Group[J].JClinOncol,2007,25(15):1960-1966.
[42] DA CUNHA SANTOS G,DHANI N,TU D,etal.Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer:National Cancer Institute of Canada Clinical Trials Group Study PA.3[J].Cancer,2010,116(24):5599-5607.
[43] TOGASHI Y,HAYASHI H,NAKAGAWA K,etal.Clinical utility of erlotinib for the treatment of non-small-cell lung cancer in Japanese patients:current evidence[J].DrugDesDevelTher,2014,8(31):1037-1046.
[44] FOUNTZILAS G,BOBOS M,KALOGERA-FOUNTZILA A,etal.Gemcitabine combined with gefitinib in patients with inoperable or metastatic pancreatic cancer:a phase II Study of the Hellenic Cooperative Oncology Group with biomarker evaluation[J].CancerInvest,2008,26(8):784-793.
[45] SACHDEV D,YEE D.Disrupting insulin-like growth factor signaling as a potential cancer therapy[J].MolCancerTher,2007,6(1):1-12.
[46] POLLAK MN,SCHERNHAMMER ES,HANKINSON SE.Insulin-like growth factors and neoplasia[J].NatRevCancer,2004,4(7):505-518.
[47] URTASUN N,VIDAL-PLA A,PEREZ-TORRAS S,etal.Human pancreatic cancer stem cells are sensitive to dual inhibition of IGF-IR and ErbB receptors[J].BMCCancer,2015,15:223.
[48] DE LUCA A,MAIELLO MR,D′ALESSIO A,etal.The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways:role in cancer pathogenesis and implications for therapeutic approaches[J].ExpertOpinTherTargets,2012,16(Suppl 2):S17-S27.
[49] WILLEMS L,TAMBURINI J,CHAPUIS N,etal.PI3K and mTOR signaling pathways in cancer:new data on targeted therapies [J].CurrOncolRep,2012,14(2):129-138.
[50] SCHLIEMAN MG,FAHY BN,RAMSAMOOJ R,etal.Incidence,mechanism and prognostic value of activated AKT in pancreas cancer[J].BrJCancer,2003,89(11):2110-2115.
[51] ASANO T,YAO Y,ZHU J,etal.The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells[J].Oncogene,2004,23(53):8571-8580.
[52] MATSUZAKI T,YASHIRO M,KAIZAKI R,etal.Synergistic antiproliferative effect of mTOR inhibitors in combination with 5-fluorouracil in scirrhous gastric cancer[J].CancerSci,2009,100(12):2402-2410.
[53] KORDES S,KLUMPEN HJ,WETERMAN MJ,etal.Phase II study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced pancreatic cancer[J].CancerChemotherPharmacol,2015,75(6):1135-1141.
[54] LLOVET JM,RICCI S,MAZZAFERRO V,etal.Sorafenib in advanced hepatocellular carcinoma[J].NEnglJMed,2008,359(4):378-390.
[55] THOMAS SM,BRUGGE JS.Cellular functions regulated by Src family kinases[J].AnnuRevCellDevBiol,1997,13:513-609.
[56] JATIANI SS,BAKER SJ,SILVERMAN LR,etal.Jak/STAT pathways in cytokine signaling and myeloproliferative disorders:approaches for targeted therapies[J].GenesCancer,2010,1(10):979-993.
[57] MULLER S,RAULEFS S,BRUNS P,etal.Next-generation sequencing reveals novel differentially regulated mRNAs,lncRNAs,miRNAs,sdRNAs and a piRNA in pancreatic cancer[J].MolCancer,2015,14:94.
[58] LILI LN,MATYUNINA LV,WALKER LD,etal.Evidence for the importance of personalized molecular profiling in pancreatic cancer[J].Pancreas,2014,43(2):198-211.
[59] RAO CV,MOHAMMED A.New insights into pancreatic cancer stem cells[J].WorldJStemCells,2015,7(3):547-555.
[60] CASTELLANOS JA,MERCHANT NB,NAGATHIHALLI NS.Emerging targets in pancreatic cancer:epithelial-mesenchymal transition and cancer stem cells[J].OncoTargetsTher,2013,6:1261-1267.
[61] AVILA JL,KISSIL JL.Notch signaling in pancreatic cancer:oncogene or tumor suppressor?[J].TrendsMolMed,2013,19(5):320-327.
[62] LEACH SD.Epithelial differentiation in pancreatic development and neoplasia:new niches for nestin and Notch[J].JClinGastroenterol,2005,39(4 Suppl 2):S78-S82.
[63] RISTORCELLI E,LOMBARDO D.Targeting Notch signaling in pancreatic cancer[J].ExpertOpinTherTargets,2010,14(5):541-552.
[64] WANG Z,AHMAD A,LI Y,etal.Targeting notch to eradicate pancreatic cancer stem cells for cancer therapy[J].AnticancerRes,2011,31(4):1105-1113.
[65] CHEN JK,TAIPALE J,YOUNG KE,etal.Small molecule modulation of Smoothened activity[J].ProcNatlAcadSciUSA,2002,99(22):14071-14076.
[66] THAYER SP,DI MAGLIANO MP,HEISER PW,etal.Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis[J].Nature,2003,425(6960):851-856.
[67] PASCA DI MAGLIANO M,SEKINE S,ERMILOV A,etal.Hedgehog/Ras interactions regulate early stages of pancreatic cancer[J].GenesDev,2006,20(22):3161-3173.
[68] TANAKA S.Cancer stem cells as therapeutic targets of hepato-biliary-pancreatic cancers[J].JHepatobiliaryPancreatSci,2015,22(7):531-537.
[69] OLIVE KP,JACOBETZ MA,DAVIDSON CJ,etal.Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer[J].Science,2009,324(5933):1457-1461.
[70] KIM EJ,SAHAI V,ABEL EV,etal.Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma[J].ClinCancerRes,2014,20(23):5937-5945.
[71] ESPADA J,CALVO MB,DIAZ-PRADO S,etal.Wnt signalling and cancer stem cells[J].ClinTranslOncol,2009,11(7):411-427.
[72] BAARSMA HA,KONIGSHOFF M,GOSENS R.The WNT signaling pathway from ligand secretion to gene transcription:molecular mechanisms and pharmacological targets[J].PharmacolTher,2013,138(1):66-83.
[73] CUI J,JIANG W,WANG S,etal.Role of Wnt/beta-catenin signaling in drug resistance of pancreatic cancer[J].CurrPharmDes,2012,18(17):2464-2471.
[74] FUXE J,KARLSSON MC.TGF-beta-induced epithelial-mesenchymal transition:a link between cancer and inflammation[J].SeminCancerBiol,2012,22(5-6):455-461.
[75] OBERSTEIN PE,OLIVE KP.Pancreatic cancer:why is it so hard to treat?[J].TherapAdvGastroenterol,2013,6(4):321-337.
[76] RUCKI AA,ZHENG L.Pancreatic cancer stroma:understanding biology leads to new therapeutic strategies[J].WorldJGastroenterol,2014,20(9):2237-2246.
[77] ZHOU H,ROY S,COCHRAN E,etal.M402,a novel heparan sulfate mimetic,targets multiple pathways implicated in tumor progression and metastasis[J].PLoSOne,2011,6(6):e21106.
[78] BRAMHALL SR,SCHULZ J,NEMUNAITIS J,etal.A double-blind placebo-controlled,randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer[J].BrJCancer,2002,87(2):161-167.
[79] HELLEDAY T,BRYANT HE,SCHULTZ N.Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy[J].CellCycle,2005,4(9):1176-1178.
[80] PAN Y,ZHENG M,ZHONG L,etal.A preclinical evaluation of SKLB261,a multikinase inhibitor of EGFR/Src/VEGFR2,as a therapeutic agent against pancreatic cancer[J].MolCancerTher,2015,14(2):407-418.
[81] KUTLUK CENIK B,OSTAPOFF KT,GERBER DE,etal.BIBF 1120 (nintedanib),a triple angiokinase inhibitor,induces hypoxia but not EMT and blocks progression of preclinical models of lung and pancreatic cancer[J].MolCancerTher,2013,12(6):992-1001.
[82] ROLFO C,RAEZ LE,BRONTE G,etal.BIBF 1120/ nintedanib :a new triple angiokinase inhibitor-directed therapy in patients with non-small cell lung cancer[J].ExpertOpinInvestigDrugs,2013,22(8):1081-1088.
[83] AWASTHI N,HINZ S,BREKKEN RA,etal.Nintedanib,a triple angiokinase inhibitor,enhances cytotoxic therapy response in pancreatic cancer[J].CancerLett,2015,358(1):59-66.
[84] DEPLANQUE G,DEMARCHI M,HEBBAR M,etal.A randomized,placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer[J].AnnOncol,2015,26(6):1194-1200.
[85] SHIBAJI T,NAGAO M,IKEDA N,etal.Prognostic significance of HIF-1 alpha overexpression in human pancreatic cancer[J].AnticancerRes,2003,23(6C):4721-4727.
[86] ZHAO T,REN H,JIA L,etal.Inhibition of HIF-1alpha by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma[J].Oncotarget,2015,6(4):2250-2262.
[87] MIYASHITA K,NAKADA M,SHAKOORI A,etal.An emerging strategy for cancer treatment targeting aberrant glycogen synthase kinase 3 beta[J].AnticancerAgentsMedChem,2009,9(10):1114-1122.
[88] MARCHAND B,TREMBLAY I,CAGNOL S,etal.Inhibition of glycogen synthase kinase-3 activity triggers an apoptotic response in pancreatic cancer cells through JNK-dependent mechanisms[J].Carcinogenesis,2012,33(3):529-537.
[89] MA S,LI Q,PAN F.CXCR4 promotes GSK3beta expression in pancreatic cancer cells via the Akt pathway[J].IntJClinOncol,2015,20(3):525-530.
[90] CLARK KL,HALAY ED,LAI E,etal.Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5[J].Nature,1993,364(6436):412-420.
[91] WANG Z,BANERJEE S,KONG D,etal.Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells[J].CancerRes,2007,67(17):8293-8300.
[92] HUANG C,XIE D,CUI J,etal.FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal transition and metastasis via upregulation of expression of the urokinase plasminogen activator system[J].ClinCancerRes,2014,20(6):1477-1488.
[93] VAN DORT ME,GALBAN S,WANG H,etal.Dual inhibition of allosteric mitogen-activated protein kinase (MEK) and phosphatidylinositol 3-kinase (PI3K) oncogenic targets with a bifunctional inhibitor[J].BioorgMedChem,2015,23(7):1386-1394.
[94] HU C,DADON T,CHENNA V,etal.Combined inhibition of cyclin-dependent kinases (Dinaciclib) and AKT (MK-2206) blocks pancreatic tumor growth and metastases in patient-derived xenograft models[J].MolCancerTher,2015,14(7):1532-1539.
[95] NEWHOOK TE,LINDBERG JM,ADAIR SJ,etal.Adjuvant trametinib delays the outgrowth of occult pancreatic cancer in a mouse model of patient-derived liver metastasis[J].AnnSurgOncol,2016,23(6):1993-2000.
[96] LINDBERG JM,NEWHOOK TE,ADAIR SJ,etal.Co-treatment with panitumumab and trastuzumab augments response to the MEK inhibitor trametinib in a patient-derived xenograft model of pancreatic cancer[J].Neoplasia,2014,16(7):562-571.
[97] TANG SC,CHEN YC.Novel therapeutic targets for pancreatic cancer[J].WorldJGastroenterol,2014,20(31):10825-10844.
