二苯乙烯苷对神经细胞淀粉样肽前体蛋白表达的影响及其与CREB调节相关的作用机制
2017-12-19尹晓敏戴伍飞钱嘉逸
尹晓敏 徐 婷 戴伍飞 钱嘉逸 陈 晨 张 兰 李 林*
(1.首都医科大学宣武医院药物研究室 北京市神经药物工程技术研究中心,北京 100053;2.南通大学医学院生物化学与分子生物学系,江苏南通 226001)
·神经系统疾病的基础和临床研究·
二苯乙烯苷对神经细胞淀粉样肽前体蛋白表达的影响及其与CREB调节相关的作用机制
尹晓敏1,2徐 婷2戴伍飞2钱嘉逸2陈 晨1张 兰1李 林1*
(1.首都医科大学宣武医院药物研究室 北京市神经药物工程技术研究中心,北京 100053;2.南通大学医学院生物化学与分子生物学系,江苏南通 226001)
目的探讨二苯乙烯苷(tetrahydroxystilbene glucoside,TSG)对培养神经细胞淀粉样肽前体蛋白(amyloid precursor protein,APP)表达的影响及其作用机制。方法应用Western blotting法检测APP和cAMP效应元件结合蛋白(cAMP responsive element-binding protein,CREB)的表达。用Matinspector软件分析APP启动子区CRE顺式反应元件;构建不同长度APP启动子区荧光素酶表达质粒;用双荧光素酶报告基因系统检测细胞中荧光素酶活性。结果由Forskolin激活SH-SY5Y细胞中CREB的同时可下调APP表达,成功构建了含有CRE元件的APP启动子区荧光素酶表达质粒。TSG能够抑制SH-SY5Y细胞内APP表达,并对APP启动子转染细胞内含有CRE1和2的APP荧光素酶报告基因质粒表达有显著抑制作用。结论TSG能够下调神经细胞中APP的表达,其机制可能与影响CREB与CRE元件的结合有关。
二苯乙烯苷;淀粉样肽前体蛋白;cAMP效应元件结合蛋白;阿尔茨海默病
阿尔茨海默病(Alzheimer’s disease,AD)是老年人中最常见的神经退行性疾病,其患者脑内主要病理表现为细胞内大量的神经原纤维缠结的形成以及细胞外大量β-淀粉样肽(β-amyloid,Aβ)聚集形成的老年斑[1]。其中,Aβ是由淀粉样肽前体蛋白(amyloid precursor protein,APP)经成淀粉样肽途径生成的小分子毒性片段[2]。有文献[3]显示,APP表达水平与可溶性Aβ水平增高呈正相关。
cAMP效应元件结合蛋白(cAMP responsive element-binding protein,CREB)在真核生物正常的生理调节下可调控其靶基因的转录。CREB可以特异性地结合到cAMP效应元件(cAMP responsive element,CRE)上。CRE广泛存在于真核生物基因启动子区,CREB通过与CRE结合,可以影响下游靶基因的转录效率。CREB在神经元生成、突触可塑性以及学习记忆等方面都具有非常重要的调节作用[4-6],可作为神经系统疾病的潜在药物靶点。本研究中笔者证实了CREB对APP表达的调节作用。
二苯乙烯苷(2,3,5,4′-tetrahydroxystilbene-2-O-b-D-glucoside,TSG)是中药何首乌的主要有效成分和标志成分。本课题组前期在APP转基因小鼠模型中,发现TSG能够改善模型鼠的学习记忆能力,减少脑颞叶皮质淀粉样斑块的数目和面积,减低皮质和海马Aβ的量[7-9]。但是TSG对APP表达的影响及其作用机制尚未见报道。本文通过构建APP启动子区双荧光素酶报告基因质粒,研究TSG对神经细胞内APP蛋白表达的影响,并探讨其与CREB调节相关的分子机制。
1 材料与方法
1.1 药物与试剂
TSG购自中国食物与药物研究所,纯度>98%;Forskolin购自美国Sigma-aldrich公司,纯度>98%。抗APP抗体购自德国Merck-Millipore公司,CREB及磷酸化CREB抗体购自美国Cell signaling公司,GAPDH抗体购自美国Santa cruz公司。蛋白标准品购自美国Bio-rad公司;DNA标准品购自北京全式金生物技术有限公司。
1.2 细胞培养
人神经母细胞瘤细胞系SH-SY5Y细胞培养在含10%(体积分数)胎牛血清的DMEM/F12培养基中,置37 ℃含5%(体积分数)CO2的培养箱内。人胚胎肾细胞系HEK-293FT细胞培养在含10%(体积分数)胎牛血清的高糖DMEM培养基中,置37 ℃含5%(体积分数)CO2的培养箱,每两天换一次培养基。
1.3 Western blotting分析
细胞裂解后,细胞总蛋白经聚丙烯酰胺凝胶电泳(SDS-PAGE)分离,然后电转至聚偏氟乙烯(PVDF)膜。用5%(质量分数)脱脂牛奶封闭30 min,一抗(抗APP、CREB或p-CREB抗体)在室温下孵育过夜。然后用含0.5% (体积分数)Tween-20的Tris-HCl缓冲液(TBST)洗涤,与辣根过氧化物酶标记的二抗在室温下孵育2 h。再经TBST洗涤后,加入ECL化学发光试剂(美国Thermo Fisher公司)进行胶片曝光显影。
1.4 质粒构建
荧光素酶报告基因质粒pGL3/APP的构建:通过分析APP启动子区的评分较高的CRE元件的位置,笔者构建了3种不同长度的APP启动子荧光素酶报告基因质粒pGL3/APP,其中APP1含有顺式作用元件CRE 1~4,APP2含有CRE 3~4,APP3只含有CRE4。
1.5 聚合酶链式反应(PCR)
以人cDNA为模板进行PCR扩增反应,APP所用的引物为:上游,5′-CAC TTT GTG ATT CCC TAC CGC-3′,下游,5′-CAC CAG ACA TCC GAG TCA TCC-3′;GAPDH所用的引物为:上游,5′-TCA ACG GAT TTG GTC GTA TT-3′,下游,5′-CTG TGG TCA TGA GTC CTT CC-3′。各APP启动子区的引物分别是:APP1上游引物,5′-GGG GTA CCT GGG CCT CCT AAA GTG CTG-3′;APP2上游引物,5′-GGG GTA CCA GGC ACC CTT GTC AGC G-3′;APP3上游引物,5′-GGG GTA CCA ACC CAA GCC CAG AAC C-3′;所有的下游引物均为5′-GAA GAT CTA GGG CTG GGC CGA AAG-3′。反应条件设定为:95 ℃ 3 min;95 ℃ 30 s,60 ℃ 30 s,72 ℃ 30 s,35个循环;72 ℃ 5 min。PCR产物用1.5%(质量分数)琼脂糖凝胶电泳分离。
1.6 细胞转染
转染前一天将HEK-293FT细胞以50%密度接种于细胞培养板,转染当天待细胞密度约80%左右,按照Lipofectamine 3000(Invitrogen公司,美国)说明书进行操作,将荧光素酶报告基因质粒pGL3/APP转染入细胞。
1.7 双荧光素酶报告基因系统分析
根据双荧光素酶报告基因检测系统(购自美国Promega公司)说明书进行操作,测定荧光素酶激发所释放的荧光值(A1),样品中内参质粒(pRL-TL)所携带的海肾荧光素酶激发底物所释放荧光值(A2)。每一个样品的A1/A2比值即为该质粒转染细胞后所表达的荧光素酶的相对活性。
1.8 统计学方法
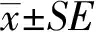
2 结果
2.1 TSG对神经细胞内APP表达的影响
TSG(100 μmol/L)与人神经母细胞瘤细胞系SH-SY5Y细胞孵育48 h,然后应用Western blotting和RT-PCR方法检测内源性APP蛋白表达量的变化,结果显示无论在蛋白水平还是mRNA水平,TSG可明显降低神经细胞内APP的表达水平(图1A,1.000±0.043vs0.612±0.044,P<0.001;图1B,1.000±0.032vs0.750±0.047,P<0.01)。
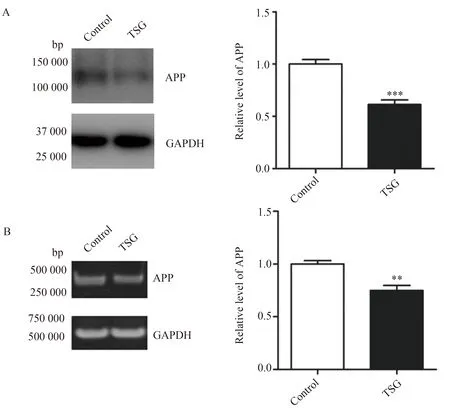
图1 TSG对SH-SY5Y细胞内APP表达的影响Fig.1 Effect of TSG on APP expression in SH-SY5Y cells
SH-SY5Y cells were incubated with 100 μmol/L TSG for 48 h.The endogenous APP level was determined by Western blotting (A) and RT-PCR (B),and GAPDH was used as a reference.Data were presented as mean ±SE,n=4.**P<0.01***P<0.001vscontrol group;TSG:tetrahydroxystilbene glucoside;APP:amyloid precursor protein.
2.2 CREB对SH-SY5Y细胞内APP表达的影响
笔者在上述研究中发现,TSG对SH-SY5Y细胞内CREB磷酸化水平有一定升高作用(图2A),提示其对CREB有一定激活作用。为了进一步研究CREB对APP表达的影响,笔者用PKA激活剂Forskolin(10 μmol/L)与SH-SY5Y细胞孵育12 h,以此来活化CREB。通过Western blotting分析,笔者检测到磷酸化CREB(p-CREB)表达明显升高(图2B,1.000±0.042vs1.423±0.063,P<0.01),表明CREB被激活,同时发现APP表达明显降低(图2B,1.000±0.057vs0.684±0.043,P<0.01)。这些结果提示CREB通过与CRE元件结合,对APP的表达有下调作用。
2.3 APP启动子区CRE元件分析及双荧光素酶报告基因质粒的构建
为了研究人APP的表达调控,笔者由转录起始点开始,分析APP转录起始位点上游2 000 bp左右的启动子区,用Matinspector软件分析了该区域的CRE元件,筛选出4个评分较高的CRE元件,分别位于 -1 972~-1 952(CRE1),-1 886~-1 866(CRE2),-1 031~-1 011(CRE3),-711~-691(CRE4)(图3A)。按照其分布,笔者构建了3个不同长度的APP启动子截断体,命名为APP 1~3,其中APP 1含有顺式作用元件CRE 1~4;APP 2含有CRE 3~4;APP3只含有CRE4(图3B)。然后将它们分别连接到pGL3载体中,对pGL3/APP质粒进行酶切鉴定,琼脂糖凝胶电泳结果显示酶切的条带长度均符合预期(图3C),进一步的测序鉴定结果也提示荧光素酶报告基因质粒构建成功。
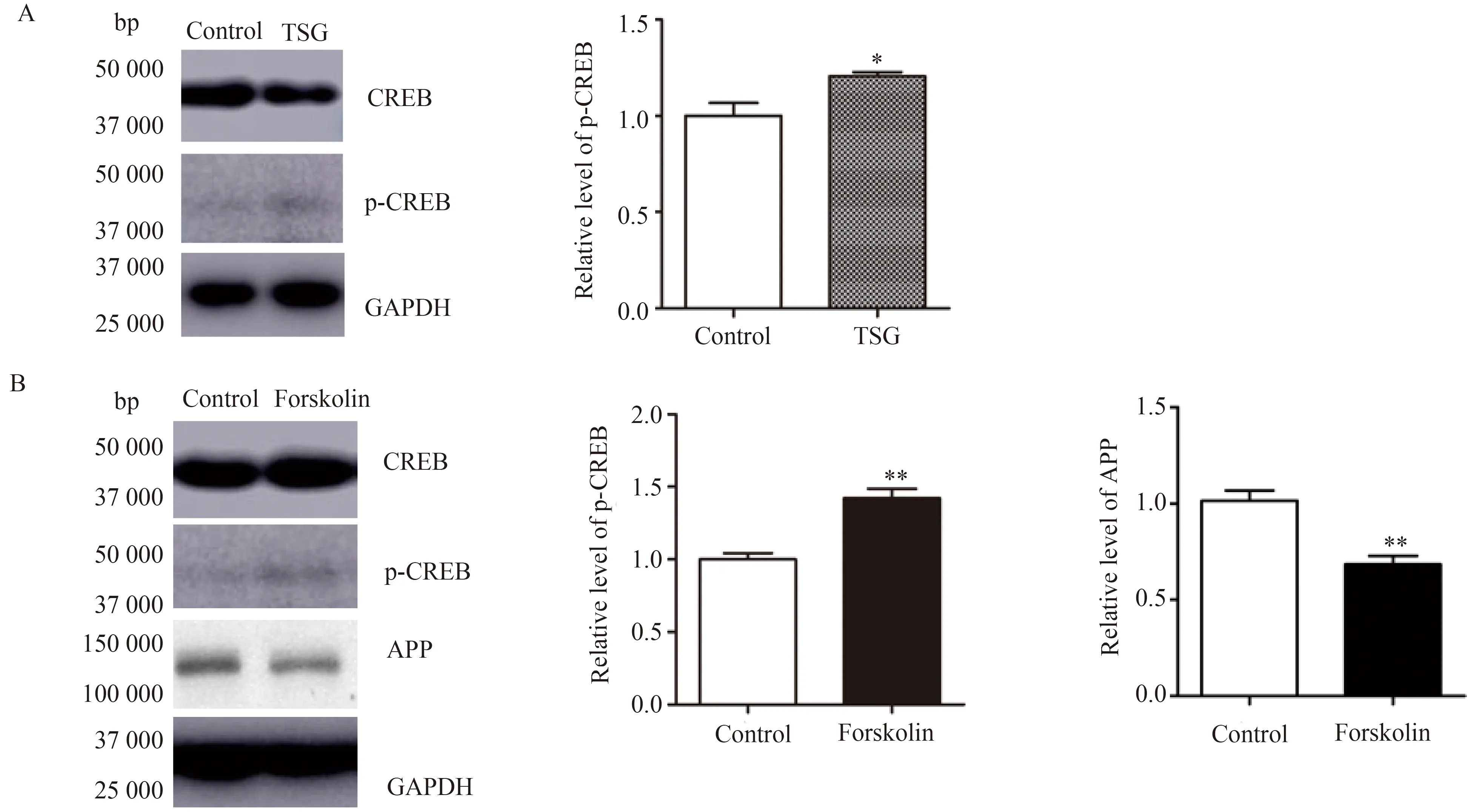
图2 CREB对SH-SY5Y细胞内APP表达的影响Fig.2 Effect of CREB on APP expression in SH-SY5Y cells
A:SH-SY5Y cells were incubated with 100 μmol/L TSG for 48 h.The endogenous CREB,phosphorylated CREB (p-CREB) and GAPDH protein levels were determined by Western blotting.The data are presented as mean ±SE,n=4.*P<0.05vscontrol group;B: SH-SY5Y cells were incubated with 10 μmol/L Forskolin (a PKA activator) for 12 h.The endogenous p-CREB,CREB,APP and GAPDH levels were determined by Western blotting assay.Quantitative data are presented as mean ±SE,n=4.**P<0.01vscontrol group;CREB:cAMP responsive element-binding protein;APP:amyloid precursor protein.
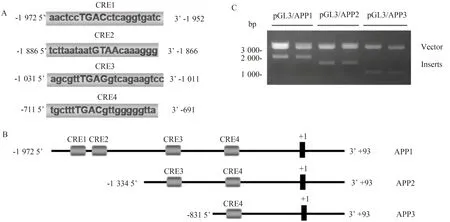
图3 APP启动子区CRE元件的分析及双荧光素酶报告基因质粒的构建Fig.3 Analysis of CRE elements in APP promoter and construction of luciferase double reporter gene plasmids
A:The promoter of human APP gene was analyzed by matinspector software.There were four highly ranked CRE elements named as CRE 1 to 4 listed in the panel;B:According to their positions,we generated three deletion mutant forms of APP promoter;C:The APP promoter deletion mutants was amplified by PCR and cloned into pGL3 vectors.The plasmids were digested by restriction enzymes and subjected for agarose gel electrophoresis;APP:amyloid precursor protein;CRE:cAMP responsive element.
2.4 TSG对APP启动子质粒转染细胞中APP双荧光素酶报告基因表达的影响
将pGL3/APP1~3质粒分别和pRL-TK质粒(作为内参)共同转染至HEK-293FT细胞中,48 h后用荧光双报告基因检测系统分析APP启动子区各截断体中荧光素酶表达的情况。结果显示,与对照组(pGL3空载体)相比,pGL3/APP1~3的荧光素酶表达量有不同程度的增高,其中含有CRE数目最多的APP1荧光素酶表达最高,表明含有CRE的APP启动子区可以很好地驱动荧光素酶基因的表达,并且与CRE的量呈正相关(图4)。TSG(100 μmol/L)与pGL3/APP1~3转染细胞孵育48 h后,APP启动子区驱动荧光素酶表达的能力有所下降,尤其是TSG对APP1组的抑制作用最为明显,与未处理组相比差异有统计学意义(235.5±2.50vs177.0 ±4.00,P<0.01;图4),而TSG对APP其他两个截断体2和3无显著影响(P=0.21,P=0.11),结果提示TSG可能影响CREB与CRE1和CRE2元件的结合。
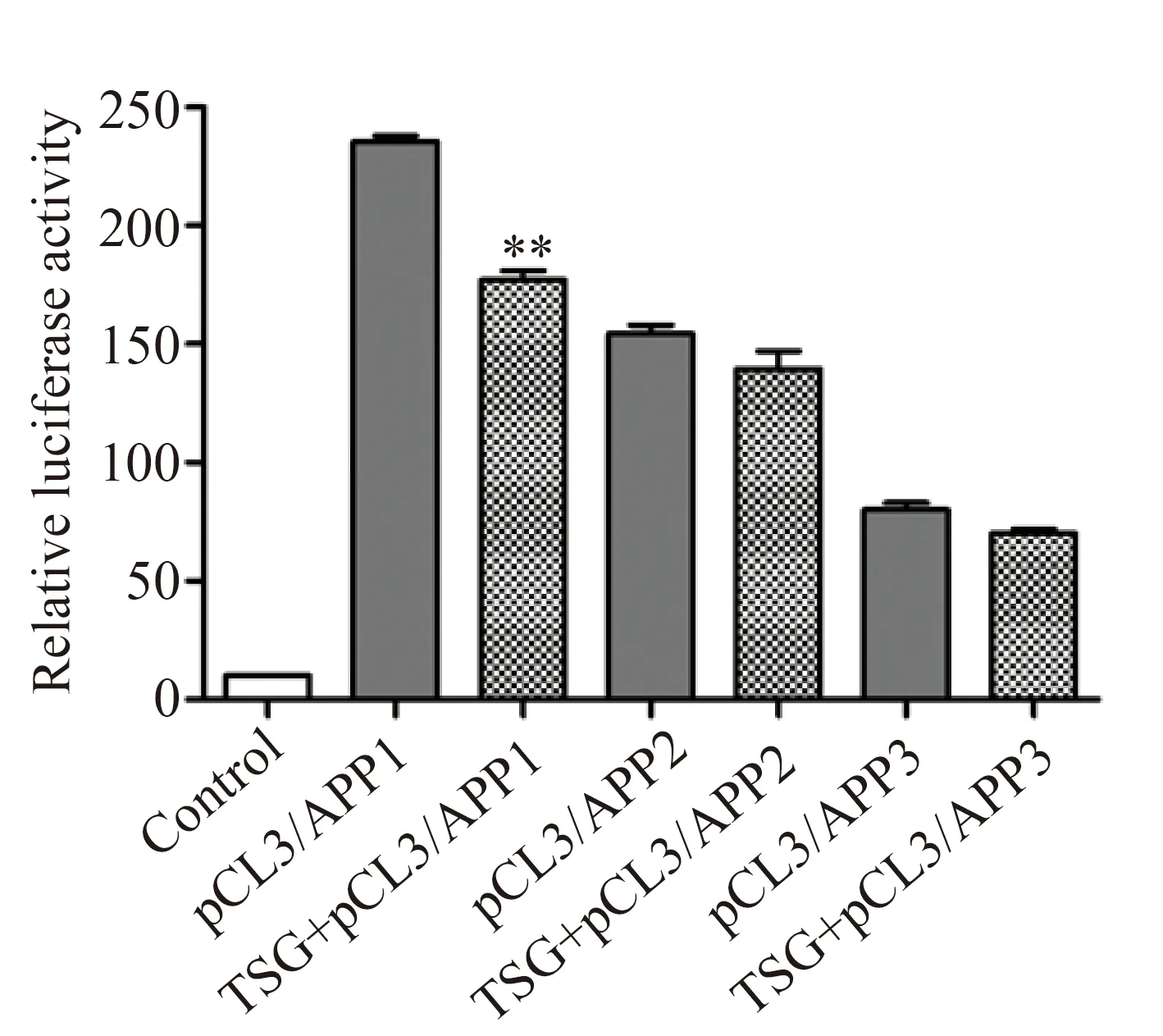
图4 TSG对APP启动子质粒转染细胞中APP双荧光素酶报告基因表达的影响Fig.4 Effect of TSG on luciferase double reporter gene expression of APP in APP promoter plamid-transfected cells
The pGL3/APP or pGL3-basic vectors were transfected into HEK-293FT cells together with pRL-TK (as a reference).The drug treated groups were incubated with 100 μmol/L TSG since transfection.After 48 h,the cells were lysed,and luciferase activity assay was measured by luciferase double reporter gene system.Data were presented as mean ±SE,n=4.**P<0.01vsthe non-TSG treated pGL3/APP1 group.
3 讨论
二苯乙烯苷(TSG)是中药何首乌的主要有效成分。笔者以前的研究[10]结果显示,TSG十二指肠给药可在家兔血浆和脑脊液中检测到,表明TSG能够透过血-脑脊液屏障,这为TSG防治AD提供了可行性。本课题组自行研制的中药5类新药泰思胶囊(二苯乙烯苷)获得国家食品药品监督管理总局(China Food and Drug Administration,CFDA)临床研究批件,已完成治疗AD的Ⅱ期临床试验,证明有效安全,目前正在进行Ⅲ期临床试验。在临床前药效学研究中,本课题组在应用APP转基因小鼠模型的实验中发现,TSG灌胃给药能够改善模型鼠的学习记忆能力,减少脑颞叶皮质淀粉样斑块的数目和面积,减低皮质和海马Aβ量;在体外实验中,TSG能明显抑制β分泌酶活性[7-9]。这些前期研究结果提示TSG与APP-Aβ通路关系密切。因此,在本实验中笔者研究了TSG对神经细胞内APP表达的影响及其作用机制,发现TSG显著抑制神经细胞内APP表达;进一步通过克隆APP启动子区荧光素酶报告基因质粒,发现TSG可能通过影响CREB与CRE元件的结合,从而下调APP的表达。
CREB通过结合启动子从而调节其下游基因的转录。CREB活性受其自身丝氨酸133位点磷酸化调控,该位点的磷酸化是由蛋白激酶A(protein kinase A,PKA)调控的[11]。CREB在神经系统的作用至关重要,可参与学习记忆、细胞周期调控、神经元诱导分化等生理活动,还影响神经退行性疾病的发生发展[12-13]。作为细胞内的重要转录因子之一,已有研究[14-16]报道CREB调节与AD发病相关的多个基因的表达。由此提示,在AD的发病过程中,CREB通路活性的改变可能影响AD患者病理的进展。
本研究中,笔者发现随着APP启动子区CRE数目的减少,荧光素酶质粒的荧光强度也随之下降,说明CRE元件对于APP表达至关重要。此外,TSG处理对于APP1的影响明显大于其他两个截断体,这提示TSG对CRE1和CRE2的影响对APP表达更为重要,笔者推测TSG可能通过调节CREB与CRE元件的结合从而下调APP表达,这也将是笔者下一步需要探讨和证实的问题。
[1] Walsh D M,Selkoe D J.Deciphering the molecular basis of memory failure in Alzheimer’s disease[J].Neuron,2004,44(1): 181-193.
[2] Selkoe D J,Hardy J.The amyloid hypothesis of Alzheimer’s disease at 25 years [J].EMBO Mol Med,2016,8(6):595-608.
[3] Matsui T,Ingelsson M,Fukumoto H,et al.Expression of APP pathway mRNAs and proteins in Alzheimer’s disease [J].Brain Res,2007,1161(1): 116-123.
[4] Kida S,Serita T.Functional roles of CREB as a positive regulator in the formation and enhancement of memory [J].Brain Res Bull,2014,105:17-24.
[5] Flavell S W,Greenberg M E.Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system [J].Annu Rev Neurosci,2008,31:563-590.
[6] Mantamadiotis T,Lemberger T,Bleckmann S C,et al.Disruption of CREB function in brain leads to neurodegeneration [J].Nat Genet,2002,31(1):47-54.
[7] 张兰,邢颖,叶翠飞,等.二苯乙烯苷对不同时程APP 转基因拟痴呆小鼠脑内ERK蛋白表达及磷酸化的影响[J].中国康复理论与实践,2009,15(3):723-727.
[8] 邢颖,张兰,李林.二苯乙烯苷对APP转基因小鼠学习记忆能力和脑内β-淀粉样肽表达的影响[J].中国新药杂志,2006,15(7):510-513.
[9] Zhang L,Xing Y,Ye C F,et al.Learning-memory deficit with aging in APP transgenic mice of Alzheimer’s disease and intervention by using tetrahydroxystilbene glucoside [J].Behav Brain Res,2006,173(2): 246-254.
[10] 王文,王蓉,艾厚喜,等.何首乌二苯乙烯苷在家兔体内的分布和代谢研究[J].中华医学研究与实践,2006,2(4):108-110.
[11] Kandel E R.The molecular biology of memory: cAMP,PKA,CRE,CREB-1,CREB-2,and CPEB [J].Mol Brain,2012,5:14.
[12] Benito E,Barco A.CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models [J].Trends Neurosci,2010,33(5): 230-240.
[13] Sakamoto K,Karelina K,Obrietan K.CREB: a multifaceted regulator of neuronal plasticity and protection [J].J Neurochem,2011,116(1):1-9.
[14] Lonze B E,Ginty D D.Function and regulation of CREB family transcription factors in the nervous system [J].Neuron,2002,35(4):605-623.
[15] Scott Bitner R.Cyclic AMP response element-binding protein(CREB) phosphorylation: a mechanistic marker in the development of memory enhancing Alzheimer’s disease therapeutics[J].Biochem Pharmacol,2012,83(6) : 705-714.
[16] Saura C A.CREB-regulated transcription coactivator 1-dependent transcription in Alzheimer’s disease mice[J].Neurodegener Dis,2012,10(1-4) : 250-252.
EffectsoftetrahydroxystilbeneglucosideonamyloidprecursorproteinexpressioninnervecellsanditsmechanismrelatedtoCREBregulation
Yin Xiaomin1,2,Xu Ting2,Dai Wufei2,Qian Jiayi2,Chen Chen1,Zhang Lan1,Li Lin1*
(1.DepartmentofPharmacology,XuanwuHospital,CapitalMedicalUniversity,BeijingEngineeringResearchCenterforNerveSystemDrugs,Beijing100053,China;2.DepartmentofBiochemistry,MedicalSchoolofNantongUniversity,Nantong226001,JiangsuProvince,China)
ObjectiveTo explore the effects of tetrahydroxystilbene glucoside (TSG) on the expression of amyloid precursor protein (APP) and its mechanism in cultured nerve cells.MethodsThe expression of APP and cAMP responsive element-binding protein (CREB) was detected by Western blotting assay.The CRE cis-elements of APP promoter were analyzed by Matinspector software.The plasmids expressing serial deletion mutants of APP promoter were constructed.The luciferase activity in cells was measured by luciferase double reporter gene system.ResultsActivation of CREB by Forskolin down-regulated APP expression in SH-SY5Y cells.The luciferase expression plasmids of APP promoter containing CRE elements were successfully constructed.TSG treatment decreased APP level in SH-SY5Y cells,and reduced luciferase activity of APP reporter gene containing CRE1 and CRE2 cis-elements in APP promoter plasmids-transfected cells.ConclusionTSG down-regulated APP expression in nerve cells,and its mechanism may be related to affecting the binding of CREB with CRE cis-elements.
tetrahydroxystilbene glucoside;amyloid precursor protein;cAMP responsive element-binding protein;Alzheimer’s disease
国家自然科学基金(81273498,81473373,81503077),国家重大新药创制重大专项(2015ZX09101016001),江苏省大学生创新训练计划项目(201610304095x)。This study was supported by National Natural Science Foundation of China (81273498,81473373,81503077),National Science and Technology Major Project for New Drug Research and Development of China (2015ZX09101-016),Jiangsu Students’ Innovation and Entrepreneurship Training Program (201610304095x).
*Corresponding author,E-mail:linlixw@126.com
时间:2017-12-13 21∶25
http://kns.cnki.net/kcms/detail/11.3662.R.20171213.2125.054.html
10.3969/j.issn.1006-7795.2017.06.012]
R749.1
2017-10-01)
编辑 陈瑞芳
