非酒精性脂肪性肝病患者血清维生素A水平、肝脏脂肪含量与胰岛素抵抗的相关性分析
2017-12-16丁智勇鲁鸿燕饶正轩
丁智勇, 卜 乐, 鲁鸿燕, 饶正轩
(1 上海市第十人民医院崇明分院 内分泌科, 上海 202157; 2 上海市第十人民医院 内分泌科 200072)
非酒精性脂肪性肝病患者血清维生素A水平、肝脏脂肪含量与胰岛素抵抗的相关性分析
丁智勇1, 卜 乐2, 鲁鸿燕1, 饶正轩1
(1 上海市第十人民医院崇明分院 内分泌科, 上海 202157; 2 上海市第十人民医院 内分泌科 200072)
目的观察和分析非酒精性脂肪性肝病(NAFLD)患者肝脏脂肪含量(LFC)与血清维生素A (VA)水平及胰岛素抵抗(IR)之间的关系。方法征集2016年2月-2017年1月上海市崇明地区初诊NAFLD患者200例和健康志愿者98例。根据口服75 g葡萄糖耐量试验和胰岛素释放试验,将NAFLD患者分为单纯NAFLD组(n=91)、NAFLD合并糖调节受损(IGR)组(n=69),NAFLD合并2型糖尿病(T2DM)组(n=40),另将98例健康志愿者作为健康对照组。用稳态模型评估IR,用高效液相色谱法检测血清VA水平,采用3.0 T质子磁共振波谱进行LFC检测。符合正态分布的计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验;不符合正态分布的计量资料组间比较采用Kruskal-WallisH检验,进一步两两比较采用Mann-WhitmeyU检验。计数资料组间比较采用Pearsonχ2检验。相关性分析采用Spearman相关性分析法。结果健康对照组、单纯NAFLD组、NAFLD合并IGR组和NAFLD合并T2DM组4组间FPG、葡萄糖耐量试验负荷后2 h血糖(2hPG)、糖化血红蛋白(HbA1c)比较差异均有统计学意义(F值分别为303.8、133.1和249.3,P值均<0.01)。FPG、2hPG和HbA1c在单纯NAFLD组、NAFLD合并IGR组、NAFLD合并T2DM组依次上升(P值均<0.01),与单纯NAFLD组、NAFLD合并IGR组、NAFLD合并T2DM组比较,健康对照组BMI、ALT、TG、低密度脂蛋白均明显降低(P值均<0.01)。单纯NAFLD组、NAFLD合并IGR组和NAFLD合并T2DM组患者血清VA、LFC和HOMA2-IR均明显高于健康对照组(F=9.22、H=216.1、H=151.0,P值均<0.01)。在单纯NAFLD组、NAFLD合并IGR组和NAFLD合并T2DM组中,HOMA2-IR、LFC逐步增高(H值分别为26.7、38.6,P值均<0.01)。NAFLD合并IGR组与NAFLD合并T2DM组HOMA2-IR、LFC比较差异均有统计学意义(U值分别为995、800,P值均<0.01);NAFLD合并IGR组和NAFLD合并T2DM组分别与单纯NAFLD组比较,HOMA2-IR、LFC、VA差异均有统计学意义(P值均<0.05)。LFC与VA(R2=0.103,P<0.001) 和HOMA2-IR(R2=0.531,P<0.001)呈正相关关系。结论NAFLD患者LFC增加与高血清VA水平及糖代谢紊乱有关。
非酒精性脂肪性肝病; 维生素A; 肝脏脂肪含量; 胰岛素抵抗
非酒精性脂肪性肝病(NAFLD)目前已成为肝功能异常的最主要病因,其发病率呈增长趋势。上海地区的研究[1]显示在成年人群中NAFLD 患病率已达到了13%,在糖尿病患者中达50%之多。脂肪代谢紊乱和胰岛素抵抗(insulin resistance, IR)是NAFLD的主要危险因素,且在NAFLD患者中,代谢综合征或2型糖尿病(type 2 diabetes mellitus, T2DM)的发病风险明显增加[2-4]。维生素A(vitamin A, VA)是重要的脂源性微量营养素,在肝脏中含量丰富。有研究[5]表明,VA可调节肝脏葡萄糖和脂肪代谢,并与胰岛素分泌有关。但是,在NAFLD患者中,血清VA与肝脏脂肪含量(liver fat content, LFC)及IR的关系还不明确。
1 资料与方法
1.1 研究对象 随机征集2016年2月-2017年1月上海市崇明地区NAFLD患者200例,分为单纯NAFLD组(n=91)、NAFLD合并糖调节受损(Impaired glucose regulation, IGR)组(n=69例),NAFLD合并T2DM组(n=40),另收集健康志愿者98例为健康对照组。
1.2 诊断标准和排除标准 NAFLD诊断标准[6]:(1) 无饮酒史或饮酒折合乙醇量<140 g/周(女性<70 g/周);(2)除外病毒性肝炎、全胃肠外营养等可导致脂肪肝的特定疾病;(3)除原发病临床表现外,可出现乏力、腹胀、肝区隐痛等症状,可伴肝脾肿大;(4)血清转氨酶可升高,并以ALT增加为主,常伴有GGT、TG等水平增高;(5)磁共振及质子磁共振波谱(1H MRS)测定LFC>5%。以上5项标准全部满足即诊断为NAFLD。T2DM和IGR诊断标准参考《中国2型糖尿病防治指南(2013年版)》[7]。T2DM:典型糖尿病症状(多饮、多尿、多食、体质量下降)加上随机血糖检测≥11.1 mmol/L,或加上空腹葡萄糖(fasting plasma glucose,FPG)≥7.0 mmol/L,或加上75 g口服葡萄糖耐量试验负荷后2 h血糖(2hPG)≥11.1 mmol/L。IGR:空腹血糖受损(FPG:6.1~<7.0)和(或)糖耐量减低(2hPG: 7.8~<11.1)。排除标准: (1) 严重心、肾疾病,其他原因引起的肝脏疾病;(2) 既往或正在服用有改变糖类代谢功效的药物(如类固醇激素、β 受体阻滞剂、噻嗪类利尿剂); (3)1型糖尿病、感染、妊娠及哺乳期等;(4) 毒品摄入史,合并精神疾病。检查前告知受试者研究目的并签署知情同意书。
1.3 研究方法 所有受试者详细询问个人史、既往史及家族史。空腹隔夜禁食水12 h,于次日清晨测量身高、体质量,检测肝功能和血脂全套。受试者接受1H MRS检查。肝脏1H MRS诊断脂肪肝分割点为LFC>5%[8]。进行口服葡萄糖耐量试验和胰岛素释放实验,分别测定0、30、60、120 min 血糖(葡萄糖氧化酶法)及胰岛素(放射免疫法)。依据FPG和空腹胰岛素水平计算 HOMA2-IR。采用高效液相色谱法(HPLC)测定各组VA水平。
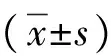
2 结果
2.1 一般资料及生化指标比较 在NAFLD患者中,单纯NAFLD占42.5%,NAFLD合并IGR占37.5%,NAFLD合并T2DM占20.0%。健康对照组、单纯NAFLD组、NAFLD合并IGR组和NAFLD合并T2DM组4组间FPG、2hPG、糖化血红蛋白(HbA1c)比较差异均有统计学意义(F值分别为303.8、133.1和249.3,P值均<0.01)。FPG、2hPG和HbA1c在单纯NAFLD组、NAFLD合并IGR组、NAFLD合并T2DM组依次上升(P值均<0.01)。与单纯NAFLD组、NAFLD合并IGR组、NAFLD合并T2DM组比较,健康对照组BMI、ALT、TG、低密度脂蛋白(LDL)均明显降低(P值均<0.01)(表1)。
单纯NAFLD组、NAFLD合并IGR组和NAFLD合并T2DM组患者血清VA、LFC和HOMA2-IR均明显高于健康对照组(P值均<0.01)。在单纯NAFLD组、NAFLD合并IGR组和NAFLD合并T2DM组中,HOMA2-IR、LFC逐步增高(H值分别为26.7、38.6,P值均<0.01),其中NAFLD合并IGR组与NAFLD合并T2DM组HOMA2-IR、LFC比较差异有统计学意义(U值分别为995、800,P值均<0.01);NAFLD合并IGR组和NAFLD合并T2DM组分别与单纯NAFLD组比较,HOMA2-IR、LFC、VA差异均有统计学意义(P值均<0.05)(表1)。
2.2 血清VA与LFC、IR与LFC、VA与IR间的相关性分析 VA和HOMA2-IR均与LFC呈正相关关系(P值均<0.001),但VA与HOMA2-IR无相关性(P>0.05) (图1) 。
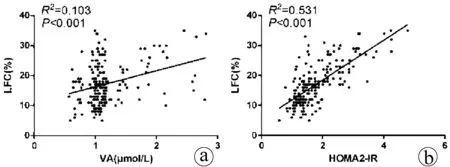

图1 NAFLD患者血清VA、LFC、HOMA2-IR之间的相关性分析a:VA与LFC的相关性; b:HOMA2-IR与LFC的相关性; c:VA与HOMA2-IR的

相关性
注:1)与单纯NAFLD组比较,P<0.05;2)与NAFLD合并IGR组比较,P<0.05
3 讨论
本研究发现,NAFLD患者BMI、VA、TG、LDL、FPG、2hPG、HbA1c、LFC、HOMA2-IR均明显高于健康对照组,表明NAFLD患者存在明显的葡萄糖、脂肪和VA代谢异常;NAFLD患者LFC与血清VA、HOMA2-IR呈线性正相关,而VA与HOMA2-IR无线性相关性,表明VA可促进肝脏脂肪合成,并进一步导致胰岛素抵抗,但VA并不直接导致胰岛素抵抗;通过比较单纯NAFLD组、NAFLD合并IGR组、NAFLD合并T2DM组检测指标,发现NAFLD患者血清VA水平、LFC和HOMA2-IR均与葡萄糖代谢紊乱有关。
NAFLD如不早期预防和治疗,发展为糖尿病、代谢紊乱及心血管并发症的风险则明显增加[3]。笔者团队在前期临床研究[9]中发现,NAFLD是选择性IR(即糖代谢抵抗和脂代谢敏感)在肝脏的表现,NAFLD对糖尿病的发生具有良好的预测作用,及时干预可起到预防糖代谢紊乱的作用。本研究中NAFLD患者T2DM患病率为20.0%,IGR患病率为34.5%。T2DM、肥胖及高脂血症常常被认为与NAFLD有关[10]。人体内游离脂肪酸过多时会在肝脏、骨骼肌、胰腺、心脏等非脂肪组织的器官中异位沉积,其脂毒性会影响这些组织器官的功能[11]。脂肪异位沉积于非脂肪组织的器官被认为是T2DM和IR的主要因素[12-13]。1H-MRS是一种无创、快速、安全、有效的 LFC 定量方法[14],LFC 真实反映了肝脏脂肪异位沉积的程度,而HOMA2-IR主要反映肝脏IR程度和空腹状态下的胰岛素敏感性[15]。脂肪组织IR亦会促进游离脂肪酸在肝细胞中沉积,对肝脏IR一定程度上起正协同作用。
VA是第一个被发现的脂源性维生素,其可调节肝脏糖脂代谢相关基因的表达,并与胰岛素协同促进肝脏脂肪沉积[16]。急性早幼粒细胞白血病患者使用VA类似物全反式维甲酸治疗后体质量增加,血浆TG和胆固醇水平升高[17]。大鼠喂VA缺乏饲料3个月后,血浆TG、胆固醇水平以及肝脏脂肪含量均明显低于VA饲料喂养大鼠[18]。还有研究[19]表明,高浓度VA培养大鼠胰岛细胞可抑制葡萄糖刺激的胰岛素释放。肥胖小鼠,ob/ob和db/db小鼠胰腺B细胞VA水平明显高于正常小鼠[20]。这些研究结果表明VA在糖脂代谢平衡中具有调节作用。
综上所述,LFC与VA和IR密切相关,LFC增加及血清VA过量是NAFLD患者糖代谢紊乱的危险因素。进一步研究VA代谢途径与NAFLD发病的关系可能有助于发现NAFLD治疗新靶点。
[1] FAN JG, ZHU J, LI XJ, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China[J]. J Hepatol, 2005, 43(3): 508-514.
[2] MAILAMUGULI, JIANAERGULI XK, CAI W, et al. Association between non-high-density lipoprotein cholesterol and nonalcoholic fatty liver disease in postmenopausal Uyghur women in Xinjiang, China[J]. J Clin Hepatol, 2016, 32(6): 1155-1159. (in Chinese)
买拉木古丽, 加那尔古丽·夏坎, 蔡雯, 等. 非高密度脂蛋白胆固醇水平与新疆维吾尔族绝经后女性非酒精性脂肪性肝病的关系[J]. 临床肝胆病杂志, 2016, 32(6): 1155-1159.
[3] TILG H, MOSCHEN AR, RODEN M. NAFLD and diabetes mellitus[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(1): 32-42.
[4] TARGHER G, MARCHESINI G, BYRNE CD. Risk of type 2 diabetes in patients with non-alcoholic fatty liver disease: causal association or epiphenomenon?[J]. Diabetes Metab, 2016, 42(3):142-156.
[5] ZHAO S, LI R, LI Y, et al. Roles of vitamin A status and retinoids in glucose and fatty acid metabolism[J]. Biochem Cell Biol, 2012, 90(2): 142-152.
[6] European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease[J]. J Hepatol, 2016, 64(6): 1388-1402.
[7] Chinese Society of Diabetes, Chinese Medical Association. Guidelines for the prevention and treatment of type 2 diabetes in China (version 2013)[J]. Chin J Diabetes, 2014, 22(8): 2-42. (in Chinese)
中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2013年版)[J]. 中国糖尿病杂志, 2014, 22(8): 2-42.
[8] SZCZEPANIAK LS, NURENBERG P, LEONARD D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population[J]. Am J Physiol Endocrinol Metab, 2005, 288(2): 462-468.
[9] BU L, GAO M, QU S, et al. Intraperitoneal injection of clodronate liposomes eliminates visceral adipose macrophages and blocks high-fat diet-induced weight gain and development of insulin resistance[J]. AAPS J, 2013, 15(4): 1001-1011.
[10] ADAMS LA, LYMP JF, ST SAUVER J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study[J]. Gastroenterology, 2005, 129(1) : 113-121.
[11] SUGANAMI T, TANAKA M, OGAWA Y. Adipose tissue inflammation and ectopic lipid accumulation[J]. Endocr J, 2012, 59(10): 849-857.
[12] LAURENS C, MORO C. Intramyocellular fat storage in metabolic diseases[J]. Horm Mol Biol Clin Investig, 2016, 26(1): 43-52.
[13] LEWIS GF, CARPENTIER A, ADELI K, et al. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes[J]. Endocr Rev, 2002, 23(2): 201-229.
[14] WANG Z, HU DY. Research advances in quantitative evaluation of liver fat in patients with nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2013, 29(12): 894-896. (in Chinese)
王梓, 胡道予. 量化评价非酒精性脂肪性肝病患者肝脏脂肪含量的研究进展[J]. 临床肝胆病杂志, 2013, 29(12): 894-896.
[15] KOTRONEN A, JUURINEN L, TIIKKAINEN M, et al. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes[J]. Gastroenterology, 2008, 135(1): 122-130.
[16] LI R, CHEN W, LI Y, et al. Retinoids synergized with insulin to induce Srebp-1c expression and activated its promoter via the two liver X receptor binding sites that mediate insulin action[J]. Biochem Biophys Res Commun, 2011, 406(2): 268-272.
[17] MILLER VA, RIGAS JR, MUINDI JR, et al. Modulation of all-trans retinoic acid pharmacokinetics by liarozole[J]. Cancer Chemother Pharmacol, 1994, 34(6): 522-526.
[18] OLIVEROS LB, DOMENICONI MA, VEGA VA, et al. Vitamin A deficiency modifies lipid metabolism in rat liver[J]. Br J Nutr, 2007, 97(2): 263-272.
[19] CHERTOW BS, GOKING NQ, DRISCOLL HK, et al. Effects of all-trans-retinoic acid (ATRA) and retinoic acid receptor (RAR) expression on secretion, growth, and apoptosis of insulin-secreting RINm5F cells[J]. Pancreas, 1997, 15(2): 122-131.
[20] KANE MA, FOLIAS AE, PINGITORE A, et al. Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion[J]. Proc Nat Acad Sci, 2010, 107(50): 21884-21889.
CorrelationofliverfatcontentwithserumvitaminAlevelandinsulinresistanceinpatientswithnonalcoholicfattyliverdisease
DINGZhiyong,BULe,LUHongyan,etal.
(DepartmentofEndocrinology,ShanghaiTenthPeople'sHospitalChongmingBranch,Shanghai202157,China)
ObjectiveTo investigate the correlation of liver fat content (LFC) with serum vitamin A (VA) level and insulin resistance (IR) in patients with nonalcoholic fatty liver disease (NAFLD).MethodsA total of 200 patients with an initial diagnosis of NAFLD in Shanghai Chongming from February 2016 to January 2017 were enrolled. According to the results of oral glucose tolerance test with 75 g glucose and insulin releasing test, NAFLD patients were divided into simple NAFLD group with 91 patients, NAFLD-impaired glucose regulation (IGR) group with 69 patients, and NAFLD-type 2 diabetes mellitus (T2DM) group with 40 patients. A total of 98 healthy volunteers were enrolled as healthy control group. The homeostasis model was used to evaluate IR, high-performance liquid chromatography was used to measure serum VA level, and 3.0 T1H-magnetic resonance spectroscopy was used to measure LFC. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the LSD-t-test was used for further comparison between two groups; the Kruskal-WallisHtest was used for comparison of non-normally distributed continuous data between multiple groups, and the Mann-WhitmeyUtest was used for further comparison between two groups. The Pearson′s chi-squared test was used for comparison of categorical data between groups. A Spearman correlation analysis was also performed.ResultsThere were significant differences in fasting plasma glucose (FPG), 2-hour postprandial glucose (2hPG), and HbAlc between the healthy control group, simple NAFLD group, NAFLD-IGR group, and NAFLD-T2DM group (F=303.8,133.1, and 249.3, allP<0.01). The simple NAFLD group, NAFLD-IGR group, and NAFLD-T2DM group had significant increases in FPG, 2hPG, and HbAlc (allP<0.01), and compared with the simple NAFLD group, NAFLD-IGR group, and NAFLD-T2DM group, the healthy control group had significant reductions in body mass index, alanine aminotransferase, triglyceride, and low-density lipoprotein (allP<0.01). The simple NAFLD group, NAFLD-IGR group, and NAFLD-T2DM group had significantly higher serum VA level, LFC, and HOMA2-IR than the healthy control group (F=9.2,H=216.1, andH=151.0, allP<0.01). HOMA2-IR and LFC gradually increased in the simple NAFLD group, NAFLD-IGR group, and NAFLD-T2DM group (H=26.7 and 38.6, bothP<0.01). There were significant differences in HOMA2-IR and LFC between the NAFLD-IGR group and the NAFLD-T2DM group (U=995 and 800, bothP<0.01); there were also significant differences in HOMA2-IR, LFC, and VA between the NAFLD-IGR group and the simple NAFLD group, as well as between the NAFLD-T2DM group and the simple NAFLD group (allP<0.05). LFC was positively correlated with VA (R2=0.103,P<0.001) and HOMA2-IR (R2=0.531,P<0.001).ConclusionThe increase in LFC is closely associated with high serum VA level and disorder of glucose metabolism in patients with NAFLD.
nonalcoholic fatty liver disease; vitamin A; liver fat content; insulin resistance
575.5
A
1001-5256(2017)12-2361-05
10.3969/j.issn.1001-5256.2017.12.021
2017-07-07;修回日期:2017-07-24。 基金项目:崇明县科学技术委员会科技项目(cky2017-21) 作者简介:丁智勇(1974-),男,主治医师,主要从事糖脂代谢疾病的相关研究。
引证本文:DING ZY, BU L, LU HY, et al. Correlation of liver fat content with serum vitamin A level and insulin resistance in patients with nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2017, 33(12): 2361-2365. (in Chinese)
丁智勇, 卜乐, 鲁鸿燕, 等. 非酒精性脂肪性肝病患者血清维生素A水平、肝脏脂肪含量与胰岛素抵抗的相关性分析[J]. 临床肝胆病杂志, 2017, 33(12): 2361-2365.
(本文编辑:林 姣)
