PEG-IFNα-2a与阿德福韦酯的联用方式对HBeAg阳性慢性乙型肝炎疗效的影响
2017-12-16武晓丽
武晓丽
(山东省禹城市人民医院 感染科, 山东 禹城 251200)
论著/病毒性肝炎
PEG-IFNα-2a与阿德福韦酯的联用方式对HBeAg阳性慢性乙型肝炎疗效的影响
武晓丽
(山东省禹城市人民医院 感染科, 山东 禹城 251200)
目的探讨用药顺序对PEG-IFNα-2a联合阿德福韦酯(ADV)治疗HBeAg阳性慢性乙型肝炎患者临床疗效的影响。方法选取2011 年9月1日-2013 年11月1日在山东省禹城市人民医院接受治疗的HBeAg阳性慢性乙型肝炎患者86例。随机分为A组(n=28,后期1例退出)、B组(n=29,后期2例退出)和C组(n=29,后期3例退出),均采用PegIFNα-2a联合ADV治疗。A组同期联合用药;B组先给予PegIFNα-2a治疗24周,再与ADV联用;C组先给予ADV治疗24周,再与PEG-IFNα-2a联用,共治疗60周,停药后随访24周。对比3组的临床疗效(HBeAg消失和血清转换率、HBsAg转阴率、HBV DNA转阴率、ALT复常率)与不良反应。计量资料组间比较采用t检验或方差分析;计数资料组间比较采用行×列表资料的χ2检验。结果治疗60周: HBeAg消失和血清转换率3组间比较差异有统计学意义(85.2% vs 81.5% vs 69.2%,χ2=6.253,P<0.05),A组和B组均高于C组(P值均<0.012 5);HBV DNA转阴率3组间比较差异有统计学意义(81.5% vs 55.6% vs 80.8%,χ2=7.409,P<0.05),A组和C组均高于B组(P值均<0.012 5);ALT复常率3组间比较差异有统计学意义(81.5% vs 80.8% vs 57.7%,χ2=7.425,P<0.05),A组高于C组(P<0.012 5)。停药24周时:HBeAg消失和血清转换率3组间比较差异有统计学意义(81.5% vs 81.5% vs 65.4%,χ2=6.723,P<0.05),A组和B组均高于C组(P值均<0.012 5);ALT复常率3组间比较差异有统计学意义(81.5% vs 74.1% vs 53.8%,χ2=9.690,P<0.05),A组高于C组(P<0.012 5)。不良反应多集中于治疗24周内,主要表现为低热、头痛、肌肉酸痛等流感样症状,多数患者未经干预自行缓解;部分患者发生骨髓抑制,多表现为WBC、中性粒细胞及PLT减少,给予粒细胞集落刺激因子后得以缓解。结论先经ADV 治疗降低HBV DNA水平再予以PEG-IFNα-2a,与二者同时联用效果相仿,可能对降低PEG-IFNα-2a的用药时间和用药量有一定的指导意义。
肝炎, 乙型, 慢性; 干扰素α-2a; 阿德福韦酯
慢性乙型肝炎(CHB)是一个全球性的健康问题,2012年数据[1]调查显示,中国内地现有HBV携带者高达9300 万,CHB患者约为2000万,每年相关死亡人数接近50万。研究[2]表明HBV持续复制导致肝纤维化、肝硬化和肝细胞癌是引发死亡的最主要原因,因此治疗该病的关键在于清除HBV感染。目前,临床治疗HBeAg阳性CHB的有效药物有2大类:核苷类似物和干扰素。前者抗病毒能力强,起效迅速,但HBeAg血清转换率低,且易产生耐药性,停药后易复发;后者通过提高免疫功能发挥抗病毒作用,能够实现较为持久和稳定的应答率,但副作用明显,且价格较高,长期用药加重了部分患者的经济负担[3]。近几年,核苷类似物和干扰素联合给药成为临床研究的热点,其中PEG-IFNα-2a和阿德福韦酯(adefovir dipivoxil,ADV)在HBeAg阳性CHB的临床治疗中显示出较好的应用前景[4]。但目前其联用方式尚不统一,疗程、剂量及应用时机尚需进一步探索,以优化联用效果。本研究重点考察二者应用时间顺序对疗效的影响,为完善其临床联用方案提供参考。
1 资料和方法
1.1 研究对象 选取本院肝病科2011 年9月1日-2013 年11月1日收治的HBeAg阳性CHB患者86例。纳入标准:(1)符合2010 年《慢性乙型肝炎防治指南》[5]中HBeAg阳性CHB的诊断标准,基线包括:血清HBsAg、HBeAg阳性,抗-HBe阴性,HBV DNA阳性,ALT持续或反复升高,或肝组织学检查有肝炎病变;(2)血清HBeAg阳性且抗HBe阴性;(3)血清HBV DNA≥105拷贝/ml;(4)签署知情同意书。排除标准:(1)肝硬化或癌变;(2)合并有其他类型的肝炎病毒;(3)甲状腺功能异常;(4)自身免疫性疾病史;(5)合并神经、精神类疾病;(6)妊娠期或哺乳期;(7)6个月内有激素、实验相关药物应用史;(8)合并肾脏疾病。所有患者按照HBV DNA水平由低到高编号,以随机数字表分为A组(n=28)、B组(n=29)和C组(n=29)。
1.2 治疗方法 基本药物:PEG-IFNα-2a(上海罗氏公司),用量为180 μg/周,皮下注射,1次/周;ADV(葛兰素史克)用量为10 mg/d,口服。用药策略:A组于治疗第1天同时使用PEG-IFNα-2a和ADV,连续使用60周;B组先以PEG-IFNα-2a治疗24周,再与ADV联合应用36周,共治疗60周;C组29例先给予ADV治疗24周,再与PEG-IFNα-2a联合应用36周,共治疗60周。
1.3 观察指标 治疗60周时和停药后24周进行疗效评估:(1)HBeAg消失率和血清转换率:采用ARCHITECT i2000SR化学发光免疫检测仪(美国雅培)和乙型肝炎五项定量检测试剂盒(美国雅培)测定HBeAg和抗-HBe。HBeAg消失率和血清转换率=[HBeAg阴性(≤1.0 s/co)或抗HBe阳性(>1.0 s/co)的患者数量/该组患者数量]×100%。(2)HBsAg转阴率:采用化学发光免疫检测仪和乙型肝炎五项定量检测试剂盒测定HBsAg。HBsAg转阴率=(HBsAg<0.05 IU/ml的患者数量/该组患者数量)×100%。(3)HBV DNA转阴率:采用实时定量PCR仪(Applied BiosystemsTM)和HBV DNA定量试剂盒(上海罗氏公司)检测病毒载量。HBV DNA转阴率=(HBV DNA<15 IU/L的患者数量/该组患者数量)×100%。(4)ALT复常率:采用AU5800全自动生化免疫分析仪(德国贝克曼库尔特公司)和试剂盒测定ALT,测定原理为乳酸脱氨酶法;ALT复常率=(ALT<50 U/L的患者数量/该组患者数量)×100%。

2 结果
2.1 一般资料 86例患者中男47例,女39例,年龄27~73岁,平均(41.2±12.6)岁,A、B、C组间一般资料比较差异均无统计学意义(P值均<0.05)(表1)。
2.2 临床疗效 治疗过程中,A、B、C组分别有1例、2例、3例退出实验。治疗60周:HBeAg消失和血清转换率3组间比较差异有统计学意义(P<0.05),A组和B组均高于C组(P值均<0.012 5);HBV DNA转阴率3组间比较差异有统计学意义(P<0.05),A组和C组均高于B组(P值均<0.012 5);ALT复常率3组间比较差异有统计学意(P<0.05),A组高于C组(P<0.012 5)。停药24周时:HBeAg消失和血清转换率3组间比较差异有统计学意义(P<0.05),A组和B组均高于C组(P值均<0.012 5);ALT复常率3组间比较差异有统计学意义(P<0.05),A组高于C组(P<0.012 5)(表2)。
2.3 不良反应 不良反应多集中于治疗24周内,主要表现为低热、头痛、肌肉酸痛等流感样症状,多数患者未经干预均可自行缓解;部分患者发生骨髓抑制,多表现为WBC、中性粒细胞及PLT减少,给予粒细胞集落刺激因子后得以缓解,未影响用药治疗。各组不良反应发生情况如表3所示,各不良反应组间差异均无统计学意义(P值均>0.05)。

表1 各组患者一般资料基线水平比较
注:与A组比较,1)P<0.012 5;与B组比较,2)P<0.012 5
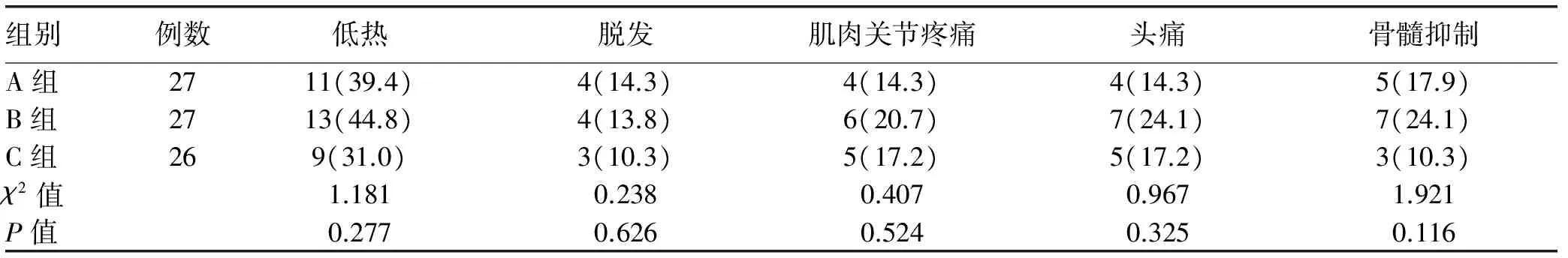
表3 各组不良反应发生率比较[例(%)]
3 讨论
CHB的发病机制十分复杂,现有研究已证实免疫机制占主导作用,患者自身对HBV的特异性免疫反应弱,不足以清除病原体,以致HBV在体内持续复制,诱发肝炎、肝纤维化、肝硬化甚至肝癌[6]。目前,用于CHB临床治疗的药物主要有干扰素及核苷酸类药物,其代表药物分别是PEG-IFNα-2a和ADV。PEG-IFNα-2a具有抗病毒和调节免疫双重作用,是CHB的一线用药,其典型优势在于HBeAg 血清学转换率高,且有良好的后续效应,疗程相对较短,停药后不易复发。但PEG-IFNα-2a并非对所有患者均敏感,且价格昂贵,存在一定的副作用[7-8]。ADV通过竞争性抑制HBV DNA多聚酶活性直接抑制HBV DNA复制,其典型优势是起效迅速、作用强势,但该药疗程较长,期间可能出现耐药问题,停药后复发率也高[9-10]。可见,二者各有利弊,均未达到理想的治疗效果。个体化的用药固然能够事半功倍,但临床并不易实施。近年,越来越多的研究[11-12]支持PEG-IFNα-2a和ADV联合给药的治疗方案,并指出二者的协同作用对于提高早期应答率、缩短疗程具有积极作用。
目前,关于PEG-IFNα-2a和ADV的联用方案尚无确明确规范。安红杰等[13]指出,PEG-IFNα-2a对低病毒载量的CHB应答效果更佳,先以ADV降低HBV DNA水平再给予PEG-IFNα-2a可能取得更好的疗效。与此同时,笔者考虑到先给予一定疗程的PEG-IFNα-2a 促进HBeAg转阴,对降低ADV的用药时间,减少耐药性可能会有一定的作用。基于以上考虑,本研究对PEG-IFNα-2a和ADV的用药时机做适当调整,分析用药时间顺序对疗效和不良反应的影响,以期为临床用药提供依据。
2012年欧洲肝病年会[14]提出了HBeAg阳性CHB的理想终点为持续HBsAg转阴或血清转换,满意终点为持续HBeAg血清转换,考虑到持续HBsAg转阴较为困难,研究以HBeAg血清转换作为治疗终点的衡量标准。研究结果显示治疗60周,A、B组的HBeAg消失和血清转换率高于C组;A、C组的HBV-DNA转阴率高于B组。主要原因存在于ADV通过竞争性抑制HBV DNA多聚酶活性直接抑制HBV-DNA复制而发挥抗病毒作用。既往也有学者[15]对PEG-IFNα-2a和ADV单独治疗CHB的用药效果进行观察,指出ADV对HBV DNA的作用优势明显。随访至24周时,A、B组患者的HBeAg消失和血清转换率高于C组,各组HBV DNA转阴率差异均无统计学意义。这表明A、B组可取得相当的治疗效果,与安红杰等[13]报道结果(先经 ADV 治疗降低 HBV DNA后再联合PEG-IFN α-2a治疗更为经济、有效)稍有差异。本研究未发现B组可获得更高的疗效,但在获得相当疗效时的用药时间和用药量相对较少,可能对降低整体用药费用、减轻患者经济负担有积极意义。由于本研究未做长期观察,尚无法得出确切结论,尚需进一步研究对此加以证实。此外,3组患者的不良反应以低热、头痛、肌肉酸痛等流感样症状和骨髓抑制为主,主要由PEG-IFNα-2a所致,但其组间发生率差异无统计学意义,表明3种方案的安全性相当。
综上所述,PEG-IFNα-2a和ADV用药时间对HBeAg阳性CHB患者的临床疗效有影响,先经ADV 治疗降低HBV DNA水平再予以PEG-IFNα-2a,可取得与二者联用相当的效果,可能对减小PEG-IFN α-2a用药时间和降低用药量有一定的指导意义。
[1] AI-MAHTAB M, BAZINET M, VAILLANT A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive bangladeshi patients with HBeAg+chronic hepatitis B infection[J]. PLoS One, 2016, 11(6): e0156667.
[2] ZHANG Y. A quantitative analysis of serum HBsAg in patients with chronic hepatitis B treated by varying courses of pegylated interferon[J]. Chin Hepatol, 2012, 17(12): 862-864. (in Chinese)
张燕. 不同疗程聚乙二醇干扰素治疗慢性乙型肝炎的血清HBsAg定量分析[J]. 肝脏, 2012, 17(12): 862-864.
[3] WANG JB, KANG HY, CAO XG, et al. The effect of LAM or ADV add-on therapy for HBeAg negative chronic hepatitis B patients with suboptimal response to PEG-IFN[J]. Int J Virol, 2015, 22(5): 310-314. (in Chinese)
王建彬, 康海燕, 曹雪改, 等. 聚乙二醇干扰素应答不佳HBeAg阴性慢性乙肝患者加用拉米夫定或阿德福韦酯疗效观察[J]. 国际病毒学杂志, 2015, 22(5): 310-314.
[4] ZHANG K, CAO H, LIANG J, et al. CONSORT: effects of adding adefovirdipivoxil to peginterferon alfa-2a at different time points on HBeAg-positivepatients: a prospective, randomized study[J]. Medicine, 2016, 95(31): e4471.
[5] JIA JD, LI LJ. The guideline of prevention and treatment for chronic hepatitis B (2010 version)[J]. J Clin Hepatol, 2011, 27(1): 113-128. (in Chinese)
贾继东, 李兰娟. 慢性乙型肝炎防治指南(2010年版)[J]. 临床肝胆病杂志, 2011, 27(1): 113-128.
[6] RAPTI I, HADZIYANNIS S. Risk for hepatocellular carcinoma in the course of chronic hepatitis B virus infection and the protective effect of therapy with nucleos(t)ide analogues[J]. World J Hepatol, 2015, 7(8): 1064-1073.
[7] CHON YE, KIM DJ, KIM SG, et al. An observational, multicenter, cohort study evaluating the antiviral efficacy and safety in korean patients with chronic hepatitis B receiving pegylated interferon-alpha 2a (pegasys): TRACES Study[J]. Medicine, 2016, 95(14): e3026.
[8] YANG L, YANG Y, JIANG XH, et al. Efficacy of peginterferon α-2a in treatment of chronic hepatitis B resistant to multiple nucleos(t) ide analogues[J]. J Clin Hepatol, 2016, 32(4): 691-694. (in Chinese)
杨龙, 杨阳, 蒋雪花, 等. 聚乙二醇干扰素α-2a治疗多种核苷和核苷酸类药物耐药慢性乙型肝炎的效果观察[J]. 临床肝胆病杂志, 2016, 32(4): 691-694.
[9] YANG S, XING HC, YAO YY, et al. Genotype resistance profile in chronic hepatitis B patients with suboptimal virological response to adefovir dipivoxil[J]. Chin J Exp Clin Infect Dis: Electronic Edition, 2015, 9(1): 10-13. (in Chinese)
杨松, 邢卉春, 姚永远, 等. 阿德福韦酯治疗应答不佳的慢性乙型肝炎患者的耐药分析J]. 中华实验和临床感染病杂志: 电子版, 2015, 9(1): 10-13.
[10] KARAYIANNIS P. Direct acting antivirals for the treatment of chronic viral hepatitis[J]. Scientifica, 2012, 2012: 478631.
[11] YOU J, CHEN Q, YE QX, et al. Efficacy of add - on adefovir dipivoxil at week 12 /24 in HBeAg - positive chronic hepatitis B patients during PEG-IFNα therapy[J]. J Clin Hepatol, 2016, 32(4): 687-690. (in Chinese)
游佳, 陈靖, 叶巧霞, 等. 聚乙二醇干扰素α加用阿德福韦酯治疗HBeAg阳性慢性乙型肝炎的效果观察[J]. 临床肝胆病杂志, 2016, 32(4): 687-690.
[12] BARONE M, IANNONE A, di LEO A. HBsAg clearance by peg-interferon addition to a long-term nucleos(t)ide analogue therapy[J]. WJG, 2014, 20(26): 8722-8725.
[13] AN HJ, HE WY, ZHAO CS, et al. De novo or at time of decreased serum viral load combination of pegylated interferon α- 2a and adevir dipivoxil in treatment of patients with HBeAg positive and high HBV DNA levels[J]. J Prac Hepatol, 2015, 18(5): 534-535. (in Chinese)
安红杰, 何文艳, 赵崇山, 等. 阿德福韦酯在不同时间联合聚乙二醇干扰素α-2a治疗HBeAg阳性慢性乙型肝炎患者疗效的比较[J]. 实用肝脏病杂志, 2015, 18(5): 534-535.
[14] WANG L, LIU YD. An interpretation of clinical practice guidelines for the management of chronic HBV infection by European Association for the Study of the Liver in 2012[J/CD]. Chin J Front Med Sci: Electronic Version, 2012, 4(7): 56-59. (in Chinese)
王磊, 刘友德. 2012年欧洲肝病学会慢性乙型肝炎病毒感染管理临床实践指南解读[J/CD]. 中国医学前沿杂志: 电子版, 2012, 4(7): 56-59.
[15] LIU TY, ZHANG LY, LI YR, et al. Prospective cohort observation of patients with hepatitis B e antigen-positive chronic hepatitis B treated by adefovir dipivoxil and interferon-α[J]. J Shandong Univ: Health Sci, 2014, 52(6): 72-77. (in Chinese)
刘同燕, 张龙跃, 李月荣, 等. 干扰素α与阿德福韦酯治疗HBeAg阳性慢性乙型肝炎前瞻性队列观察[J]. 山东大学学报: 医学版, 2014, 52(6): 72-77.
InfluenceofcombinationmodeofPEG-IFNα-2aandadefovirdipivoxilonoutcomeofpatientswithHBeAg-positivechronichepatitisB
WUXioali.
(DepartmentofInfectiousDiseases,YuchengPeople′sHospital,Yucheng,Shandong251200,China)
ObjectiveTo investigate the influence of the sequence of PEG-IFNα-2a and adefovir dipivoxil (ADV) on the clinical outcome of patients with HBeAg-positive chronic hepatitis B (CHB).MethodsA total of 86 patients with HBeAg-positive CHB who were treated in Yucheng People’s Hospital from September 1, 2011 to November 1, 2013 were enrolled and randomly divided into groups A (28 patients, among whom one dropped out in the late stage), B (29 patients, among whom two dropped out in the late stage), and C (29 patients, among whom three dropped out in the late stage). All patients were treated with PEG-IFNα-2a combined with ADV; the patients in group A were given PEG-IFNα-2a and ADV concurrently, those in group B were given PEG-IFNα-2a for 24 weeks, followed by PEG-IFNα-2a combined with ADV, and those in group C were given ADV for 24 weeks, followed by PEG-IFNα-2a combined with ADV. The course of treatment was 60 weeks for all groups. The patients were followed up for 24 weeks after drug withdrawal. The three groups were compared in terms of clinical outcome [HBeAg disappearance rate and seroconversion rate, HBsAg clearance rate, HBV DNA clearance rate, and alanine aminotransferase (ALT) normalization rate]. An analysis of variance orttest was used for comparison of continuous data between groups, and the chi-square test was used for comparison of categorical data between groups.ResultsAfter 60 weeks of treatment, there were significant differences in HBeAg disappearance rate and seroconversion rate between the three groups (85.2% vs 81.5% vs 69.2%,χ2=6.253,P<0.05), and groups A and B had significantly higher rates than group C (allP<0.012 5); there was a significant difference in HBV DNA clearance rate between the three groups (81.5% vs 55.6% vs 80.8%,χ2=7.409,P<0.05), and groups A and C had a significantly higher rate than group B (bothP<0.012 5); there was a significant difference in ALT normalization rate between the three groups (81.5% vs 80.8% vs 57.7%,χ2=7.425,P<0.05), and group A had a significantly higher rate than group C (P<0.012 5). After 24 weeks of drug withdrawal, there were significant differences in HBeAg disappearance rate and seroconversion rate between the three groups (81.5% vs 81.5% vs 65.4%,χ2=6.723,P<0.05), and groups A and B had significantly higher rates than group C (allP<0.012 5); there was a significant difference in ALT normalization rate between the three groups (81.5% vs 74.1% vs 53.8%,χ2=9.690,P<0.05), and group A had a significantly higher rate than group C (P<0.012 5). Most adverse reactions occurred within 24 weeks of treatment and mainly manifested as influenza-like symptoms such as low-grade fever, headache, and sore muscle, and most of the patients were relieved spontaneously without intervention. Some patients experienced bone marrow suppression manifesting as reductions in leukocytes, neutrophils, and platelets and were relieved after the treatment with granulocyte colony-stimulating factor.ConclusionADV given at first to reduce HBV DNA and followed by the addition of PEG-IFNα-2a can achieve a similar effect as ADV given concurrently with PEG-IFNα-2a and has certain significance in shortening the duration of PEG-IFNα-2a treatment and reducing the dose of PEG-IFNα-2a.
hepatitis B, chronic; interferon alfa-2a; adefovir dipivoxil
R512.62; R452
A
1001-5256(2017)12-2311-05
10.3969/j.issn.1001-5256.2017.12.011
2017-07-14;修回日期:2017-09-18。 作者简介:武晓丽(1970-),女,副主任医师,主要从事肝炎、肝硬化诊治方面的研究。
引证本文:WU XL. Influence of combination mode of PEG-IFNα-2a and adefovir dipivoxil on outcome of patients with HBeAg-positive chronic hepatitis[J]. J Clin Hepatol, 2017, 33(12): 2311-2315. (in Chinese)
武晓丽. PEG-IFNα-2a与阿德福韦酯的联用方式对HBeAg阳性慢性乙型肝炎疗效的影响[J]. 临床肝胆病杂志, 2017, 33(12): 2311-2315.
(本文编辑:林 姣)
