Ghrelin对AngⅡ 诱导的心肌细胞凋亡的影响及机制
2017-11-28杨春艳冯朝晖
杨春艳,冯朝晖,杨 萍,任 平
(1.吉林大学中日联谊医院 心内科,吉林 长春130033;2.吉林大学第一医院 胸外科)
Ghrelin对AngⅡ 诱导的心肌细胞凋亡的影响及机制
杨春艳1,冯朝晖1,杨 萍1,任 平2*
(1.吉林大学中日联谊医院 心内科,吉林 长春130033;2.吉林大学第一医院 胸外科)
目的阐明Ghrelin对血管紧张素Ⅱ( AngⅡ)诱导的心肌细胞凋亡的影响及其可能机制。方法体外培养H9C2心肌细胞,分为空白对照组、Ang Ⅱ组、Ang Ⅱ+Ghrelin组及单纯Ghrelin组,采用MTT法检测细胞存活率,TUNEL染色观察心肌细胞凋亡情况,并应用RT-PCR法检测Bcl-2、Bax、Caspase-3、1型及2型Ang Ⅱ 受体(AT1R,AT2R)mRNA表达。结果与空白对照组相比,Ang Ⅱ组心肌细胞存活率明显下降;细胞凋亡数目明显增加;Caspase-3及促凋亡分子Bax表达明显增加,而抗凋亡分子Bcl-2表达明显减少;AT1R 及AT2R表达均明显增加,AT1R增加尤为显著。与Ang Ⅱ组相比,Ghrelin+Ang Ⅱ组心肌细胞存活率明显增加;细胞凋亡数目明显减少;Caspase-3及促凋亡分子Bax表达明显减少,而抗凋亡分子Bcl-2表达明显增加;AT1R表达明显减少,而AT2R表达无明显变化。结论AT1R及AT2R均参与Ang Ⅱ诱导心肌细胞凋亡进程,Ghrelin可抑制Ang Ⅱ诱导的心肌细胞凋亡,其机制可能与Ghrelin下调通过下调AT1R表达有关。
Ghrelin;血管紧张素Ⅱ;心肌细胞凋亡;血管紧张素Ⅱ受体
(ChinJLabDiagn,2017,21:1982)
心肌细胞凋亡可导致心肌细胞数目的减少进而影响心肌收缩性,并可引起心肌细胞代偿性肥大及纤维化,促进心血管系统疾病的进展。因此,抑制或改善心肌细胞凋亡是是临床关心的一个重要课题,探寻可抑制心肌细胞凋亡的有效药物,并明确其抑制心肌细胞的凋亡分子机制具有重要意义。促生长激素释放多肽(Ghrelin)是生长激素促分泌素(GHS)受体的内源性配体,具有多种生物学活性,包括刺激生长激素(GH)分泌、调节代谢、促进摄食与肥胖等[1]。目前,有研究发现Ghrelin及其受体可在心脏表达[2],并与心脏上的结合位点具有高度亲合力[3-5],提示心脏为Ghrelin作用的靶器官。Ghrelin还具有增加心肌收缩力、舒张血管、保护内皮细胞、改善心肌能量代谢以及预防保护心梗后心衰的形成等多种心血管保护作用[6-9]。然而,Ghrelin发挥心血管保护作用的细胞及分子机制尚有待进一步研究。H9c2心肌细胞是从胚胎大鼠心室肌中分离克隆的一个细胞系[10],以往的研究表明,它是体外研究心肌细胞凋亡模型的理想细胞[11,12]。在本研究中,我们培养H9c2细胞,探讨Ghrelin对血管紧张素Ⅱ(Ang Ⅱ)诱导的H9c2细胞凋亡的影响及其可能机制,为临床应用Ghrelin治疗心血管疾病提供实验依据。
1 材料与方法
1.1主要试剂酰基化Ghrelin购自于中肽生物科技有限公司,Ang Ⅱ 与MTT购自于美国Sigma-Aldrich公司 (St.Louis,MO,USA),TUNEL检测试剂盒购自罗氏公司(South San Francisco,California,USA)。
1.2H9c2细胞培养及分组H9c2 细胞用含10%血清的高糖DMEM培养液培养,于培养瓶,37℃,5%CO2培养箱孵育,传至2-3代后用于实验。实验分为以下四组:空白对照(Con)组(培养液对照),Ang Ⅱ组(培养液+ 10-7mol/L Ang Ⅱ ),Ang Ⅱ+ghrelin组(培养液+10-7mol/L Ang Ⅱ +10-7 mol/L Ghrelin ),Ghrelin组(培养液+ 10-7mol/L Ghrelin)。
1.3MTT法检测H9c2心肌细胞活性将浓度为5-6×105/ml的H9c2心肌细胞悬液接种于96孔板,待细胞长至70%-80%时给予Ang Ⅱ及Ghrelin干预,并于加药刺激24 h后每孔加入20 μl MTT (5 mg/ml),于37℃细胞培养箱继续孵育4 h。去除细胞上清,每孔加入150 μl 二甲基亚砜(DMSO),避光摇晃10 min,于490 nm 处测细胞吸光度,并计算细胞存活百分率。
1.4原位末端标记检测法(TUNEL)染色检测H9c2心肌细胞凋亡将生长于玻片上的四组心肌细胞应用TUNEL试剂进行染色,具体步骤按TUNEL说明书进行。染色结束后于暗室光镜下观察,细胞核呈绿色荧光即为TUNEL阳性细胞。
1.5RT-PCR检测Bcl-2、Bax、Caspase-3、AT1R及AT2R的mRNA表达提取总RNA及逆转录反应:Trizols提取各组H9c2细胞的总RNA,分光光度计测定RNA的浓度和纯度,,然后取2 μg RNA进行逆转录,合成cDNA后扩增30个循环。所有引物均由上海生物工程技术有限公司合成,序列见表 1。

表1 RT-PCR引物合成表
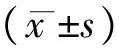
2 结果
2.1Ghrelin及AngⅡ对H9c2心肌细胞凋亡的影响
应用Ang Ⅱ及Ghrelin干预H9c2心肌细胞24小时后,MTT法测心肌细胞存活率发现:与Con组相比,给予Ang Ⅱ刺激后H9c2心肌细胞存活率明显下降(80.5±1.6%),有显著性差异(Plt;0.01); Ghrelin干预组细胞的存活率无明显改变(99.7±4.1%),差异无统计学意义(Pgt;0.05);与Ang Ⅱ组相比,Ghrelin 与Ang Ⅱ共同干预组细胞存活率明显增高(90.4±4.7%),有显著性差异(Plt;0.01),见图1。
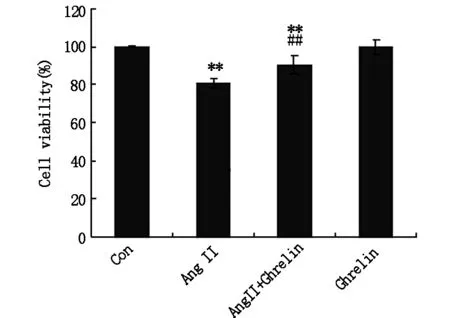
进一步应用TUNEL染色检测H9c2心肌细胞凋亡情况,结果显示:与Con组相比,Ang Ⅱ干预后H9c2心肌细胞凋亡率明显增高,有显著性差异(Plt;0.01);与Ang Ⅱ组相比,Ghrelin与Ang Ⅱ共同干预组H9c2心肌细胞凋亡率明显降低,有显著性差异(Plt;0.01),见图2及图3。
**Plt; 0.01 vs.the control group;##Plt; 0.01 vs.the Ang Ⅱ group.
图1MTT法检测心肌细胞存活率
2.2Ghrelin及AngⅡ对Caspase-3表达的影响
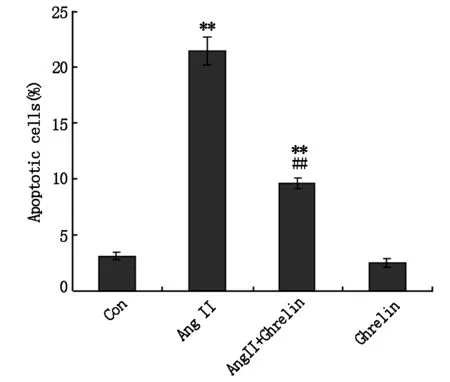
本研究利用Ang Ⅱ诱导H9c2细胞凋亡,在加药刺激后24 h,采用RT-PCR检测Caspase-3表达情况。结果发现Ang Ⅱ 组较空白对照组Caspase-3 mRNA表达水平明显增加 (Plt;0.01),而Ghrelin可以明显下调Ang Ⅱ诱导的Caspase-3表达 (Plt;0.01),见图4。Caspase-3 为与细胞凋亡呈正相关的凋亡途径关键分子,上述结果提示Ang Ⅱ可通过促进凋亡关键分子Caspase-3表达而诱导心肌细胞凋亡,Ghrelin则可通过下调Caspase-3表达而抑制其作用。

图2 TUNEL染色法检测心肌细胞凋亡(×400)
**Plt;0.01 vs.the control group;##Plt; 0.01 vs.the Ang Ⅱ group.
图3TUNEL染色法检测心肌细胞凋亡百分率
图4 RT-PCR检测Caspase-3 mRNA表达
2.3Ghrelin及AngⅡ对Bcl-2及Bax表达的影响
Bcl-2及Bax表达失衡与细胞凋亡密切相关。因此,本研究利用Ang Ⅱ诱导H9c2细胞凋亡,在加药刺激后24 h,采用RT-PCR检测Caspase-3表达情况。结果发现Ang Ⅱ 组较空白对照组Bax mRNA表达明显增加 (Plt;0.01),Bcl-2 mRNA表达明显减少(Plt; 0.01);而Ghrelin可以明显下调Ang Ⅱ诱导的Bax表达增加(Plt;0.01),并上调Ang Ⅱ诱导的Bcl-2表达减少(Plt; 0.01),见图5。上述结果提示:Ang Ⅱ可能通过诱导Bcl-2/Bax表达失衡而导致心肌细胞凋亡,Ghrelin则可抑制Ang Ⅱ诱导的Bcl-2/Bax表达失衡发挥心血管保护作用。
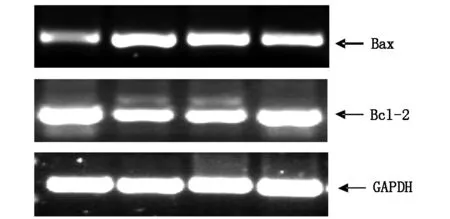
图5 RT-PCR检测Bcl-2及Bax mRNA表达
2.4Ghrelin及AngⅡ对AT1R及AT2R表达的影响
为明确AT1R及AT2R在Ang Ⅱ诱导H9c2细胞凋亡中的作用,实验采用RT-PCR检测AT1R及AT2R mRNA表达水平,研究结果显示,Ang Ⅱ刺激培养的H9c2细胞24 h后,AT1R及AT2R mRNA较空白对照组表达均增加(BothPlt;0.01),Ghrelin可明显降低Ang Ⅱ诱导的AT1R表达增加 (Plt; 0.01),而对AT2R表达无明显影响 (Pgt;0.05),见图6。
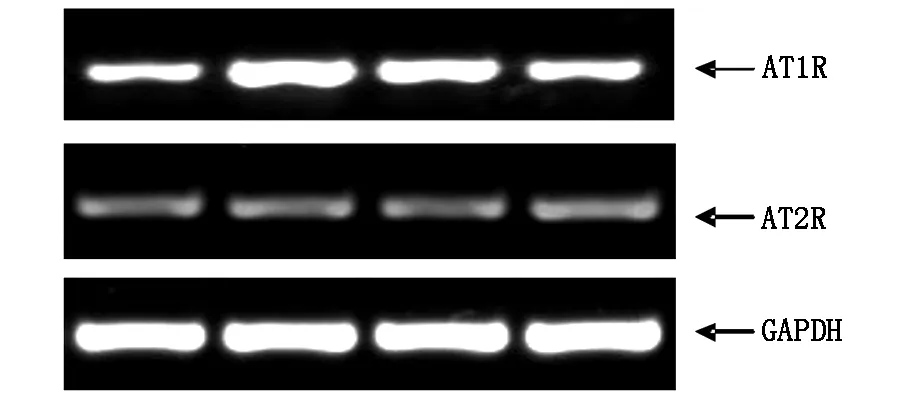
图6 RT-PCR检测AT1R及AT2R mRNA表达
3 讨论
以往的研究表明Ghrelin除刺激生长激素分泌、调节摄食及促进新陈代谢外,尚具有许多心血管保护作用[6-9]。然而,Ghrelin保护心血管作用的相关分子机制尚未完全明确。肾素-血管紧张素-醛固酮系统(RAAS)是维持心血管内环境稳定的重要机制之一,Ang Ⅱ是RAAS的核心分子,在许多心血管疾病进程中发挥重要作用[13-15]。以往的研究表明,Ghrelin可以保护Ang Ⅱ诱导的内皮细胞损伤[16],Ghrelin可以抑制Ang Ⅱ诱导的人主动脉内皮细胞移行[17]。因此,我们推测:在心血管系统,Ghrelin可能通过抑制Ang Ⅱ诱导的心肌损伤而发挥心血管保护作用。越来越多的研究表明,Ang Ⅱ可诱导心肌细胞凋亡[18],为此,我们用Ang Ⅱ诱导H9c2细胞凋亡,并用Ghrelin加以干预,探讨Ghrelin对Ang Ⅱ诱导的H9c2细胞凋亡的影响。结果发现Ang Ⅱ可诱导H9c2 细胞凋亡,而Ghrelin可抑制Ang Ⅱ诱导的H9c2细胞凋亡。
Caspase-3 为凋亡途径的关键分子,其表达与细胞凋亡正相关[19,20]。为进一步明确Ghrelin对心肌细胞凋亡的影响,本研究进一步采用RT-PCR法检测Caspase-3表达情况,结果发现Ang Ⅱ 组Caspase-3 mRNA表达水平明显增加,而ghrelin可以明显下调Ang Ⅱ诱导的Caspase-3表达。进一步证实Ang Ⅱ可通过诱导凋亡途径的关键分子Caspase-3活化而导致心肌细胞凋亡,Ghrelin则可抑制其作用。
Bcl-2及Bax表达失衡与细胞凋亡密切相关[21-22],当Bcl-2表达相对较高而bax表达较低时,细胞活力增强;反之,则可促进细胞凋亡。为探讨Ghrlein抑制Ang Ⅱ诱导的H9c2细胞凋亡的分子机制,我们还应用PCR法检测了Bcl-2 与Bax的mRNA表达水平,结果发现在Ang Ⅱ诱导的H9c2细胞凋亡过程中Bax的表达增加而Bcl-2表达减少,导致Bcl-2及Bax表达失衡,Bcl-2/Bax比值降低,而ghrelin则对其具有抑制作用。上述结果提示,ghrelin可能通过减轻Ang Ⅱ诱导的Bax/Bcl-2比值增加而纠正其表达失衡,从而抑制Ang Ⅱ诱导的H9c2细胞凋亡。
Ang Ⅱ是影响心血管系统的重要内分泌因子之一,主要通过1型及2型受体(AT1R,AT2R)发挥生物学作用[23],其中Ang Ⅱ与AT1R的结合率约50%-70%[24]。Ang Ⅱ通过AT1R及AT2R发挥生物学作用,然而Ang Ⅱ是经由AT1R还是AT2R介导心肌细胞凋亡,仍存在很大争议[25-27]。为明确AT1R及AT2R在Ang Ⅱ诱导H9c2心肌细胞凋亡中的作用,研究应用RT-PCR法检测AT1R及AT2R表达,结果发现Ang Ⅱ刺激后,AT1R及AT2R表达均上调,而Ghrelin可下调AT1R表达,但对AT2R表达无明显影响。上述结果提示:AT1R及AT2R均参与Ang Ⅱ诱导心肌细胞凋亡过程,通过Ghrelin下调AT1R表达而抑制Ang Ⅱ诱导的心肌细胞凋亡,进一步提示Ang Ⅱ通过AT1R介导促凋亡分子Bax及Caspase-3表达,抑制抗凋亡分子Bcl-2表达,进而诱导心肌细胞凋亡。此外,Ang Ⅱ干预后AT2R表达亦增加,可明确Ghrelin不通过AT2R抑制Ang Ⅱ诱导的心肌细胞凋亡,但AT2R是拮抗AT1R的作用还是与AT1R共同介导Ang Ⅱ诱导的心肌细胞凋亡尚不能明确,仍有待于进一步研究。结合2008年Yanfei等[28]研究发现的新生乳鼠AT2R过表达可诱导心肌细胞凋亡,推测本研究发现的AT2R上调可能也对Ang Ⅱ诱导的心肌细胞凋亡起促进作用。
综上所述,我们目前的体外研究不仅明确了ghrelin 可抑制Ang Ⅱ诱导的H9c2细胞凋亡,更重要的是,明确了ghrelin 抑制Ang Ⅱ诱导的H9c2细胞凋亡的可能机制,即:Ghrelin可通过下调H9c2细胞上的AT1受体,影响凋亡相关基因bax、Bcl-2及凋亡关键分子caspase - 3的表达,进而抑制Ang Ⅱ诱导的H9c2细胞凋亡。
[1]Kojima M,Hosoda H,Date Y,et al.Ghrelin is a growth-hormone-releasing acylated peptide from stomach[J].Nature,1999,402:656.
[2]Gnanapavan S,Kola B,Bustin SA,et al.The tissue distribution of the mRNA of ghrelin and subtypes of its receptor,GHS-R,in humans[J].J Clin Endocrinol Metab,2002,87:2988.
[3]Papotti M,Ghè P,Cassoni P,et al.Growth hormone secretagogue binding sites in peripheral human tissues[J].J Clin Endocrinol Metab,2000,85:3803.
[4]Bodart V,Febbraio M,Demers A,et al.CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart[J].Circ Res,2002,90:844.
[5]Katugampola SD,Pallikaros Z,Davenport AP.[125I-His(9)]-ghrelin,a novel radioligand for localizing GHS orphan receptors in human and rat tissue:up-regulation of receptors with athersclerosis[J].Br J Pharmacol,2001,134:143.
[6]Nagaya N,Uematsu M,Kojima M,et al.Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure[J].Circulation,2001,104:1430.
[7]Mohammad Reza Aliparasti,Mohammad Reza Alipour,Shohreh Almasi,et al.Effect of Ghrelin on Aldolase Gene Expression in the Heart of Chronic Hypoxic Rat[J].Int J Endocrinol Metab,2012,10(3):553.
[8]Gianfranco Mitacchione,Jeffrey C.Powers,Gino Grifoni,et al.The Gut Hormone Ghrelin Partially Reverses Energy Substrate Metabolic Alterations in the Failing Heart[J].Circ Heart Fail,2014 ,7(4):643.
[9]Tobias Daniel Trippel,Volker Holzendorf,Martin Halle,et al.Ghrelin and hormonal markers under exercise training in patients with heart failure with preserved ejection fraction:results from the Ex-DHF pilot study[J].ESC Heart Fail,2017,4(1):56.
[10]Kimes BW,Brandt BL.Properties of a clonal muscle cell line from rat heart [J].Exp Cell Res,1976,98(2):367.
[11]Ekhterae D,Lin Z,Lundberg MS,et al.ARC inhibits cytochrome c release from mitochondria and protects against hypoxia-induced apoptosis in heart-derived H9c2 cells[J].Circ Res,1999,85(12):e70.
[12]Ray-Jade Chen,His-Chin Wu,Mu-Hsin Chang,et al.Leu27IGF2 plays an opposite role to IGF1 to induce H9c2 cardiomyoblast cell apoptosis via Gq signaling[J].J Mol Endocrino.,2009,43:221 .
[13]Justin F.X.Ainscough,Mark J.Drinkhill,Alicia Sedo,et al.Angiotensin Ⅱ type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction[J].Cardiovasc Res,2009,81:592.
[14]NP Mel'nikova,SS Timoshin,EY Jivotova,et al.Angiotensin-Ⅱ activates apoptosis,proliferation and protein synthesis in the left heart ventricle of newborn albino rats[J].Int J Cardiol,2006,112(2):219.
[15]Keisuke Shinohara,Takuya Kishi,Yoshitaka Hirooka,et al.Circulating angiotensin Ⅱ deteriorates left ventricular function with sympathoexcitation via brain angiotensin Ⅱ receptor[J].Physiol Rep.2015,3(8):e12514.
[16]Deng B,Fang L,Chen X,et al.Effect of ghrelin on angiotensin Ⅱ induced human umbilicus vein endothelial cell oxidative stress and endothelial cell injury[J].Zhong Nan Da Xue Xue Bao Yi Xue Ban,2010,35(10):1037.
[17]F Rossi, C Bertone,S Petricca,et al.Ghrelin inhibits angiotensin Ⅱ-induced migration of human aortic endothelial cells[J].Atherosclerosis,2007,192(2):291.
[18]Jin-Jiang Pang,Rong-Kun Xu,Xiang-Bin Xu,et al.Hexarelin protects rat cardiomyocytes from angiotensin Ⅱ-induced apoptosis in vitro[J].Am J Physiol Heart Circ Physiol,2004,286:H1063.
[19]Jun Chen,Tetsuya Nagayama,Kunlin Jin,et al.Induction of Caspase-3-Like Protease May Mediate Delayed Neuronal Death in the Hippocampus after Transient Cerebral Ischemia[J].J Neurosci,1998,18:4914 .
[20]Peter R Hurst,Jocelyn M Mora,Mark A.Fenwick.Caspase-3,TUNEL and ultrastructural studies of small follicles in adult human ovarian biopsies[J].Hum Reprod,2006,21:1974.
[21]Mirta S Albamonte,Miguel A Willis,María I Albamonte,et al.The developing human ovary:immunohistochemical analysis of germ-cell-specific VASA protein,BCL-2/BAX expression balance and apoptosis[J].Hum Reprod,2008,23:1895.
[22]C Zhou,X Li,W Du,et al.Antitumor effects of ginkgolic acid in human cancer cell occur via cell cycle arrest and decrease the Bcl-2/Bax ratio to induce apoptosis[J].Chemotherapy,2010,56(5):393.
[23]Mukoyama M,Nakajima M,Horiuchi M,et al.Expression cloning of type 2 angiotensin Ⅱ receptor reveals a unique class of seven-transmembrane receptors[J].J Biol Chem,1993,268:24539.
[24]Sechi LA,Griffin CA,Grady EF,et al.Characterization of angiotensin Ⅱ receptor subtypes in rat heart[J].Circ Res,1992,71:1482.
[25]Suzuki J,Matsubara H,Urakami M,et al.Rat angiotensin Ⅱ (type 1A) receptor mRNA regulation and subtype expression in myocardial growth and hypertrophy[J].Circ Res,1993,73:439.
[26]Lopez JJ,Lorell BH,Ingelfinger JR,et al.Distribution and function of cardiac angiotensin AT1- and AT2-receptor subtypes in hypertrophied rat hearts[J].Am J Physiol,1994,267:H844.
[27]Nio Y,Matsubara H,Murasawa S,et al.Regulation of gene transcription of angiotensin Ⅱ receptor subtypes in myocardial infarction[J].J Clin Invest,1995,95:46.
[28]Yanfei Qi,Hongwei Li,Adam Mecca,et al.Overexpression of Angiotensin Ⅱ type 2 receptor (AT2R) in neonatal cardiomyocytes induces apoptosis[J].FASEB J,2008,22:1238.
EffectandMechanismofGhrelinonH9c2CardiomyocytesapoptosisinducedbyAngⅡ
YANGChun-yan,FENGZhao-hui,YANGPing,etal.
(Departmentofcardiology,China-JapanUnionHospital,JilinUniversity,Changchun130033,China)
ObjectiveTo clarify the effect and possible mechanism of ghrelin on H9c2 cardiomyocytes apoptosis induced by Angiotensin Ⅱ (Ang Ⅱ).MethodsH9c2 cells were cultured and divided into blank control group,group of Ang Ⅱ and Ang Ⅱ + Ghrelin and Ghrelin group.MTT was used to detect cell survival,TUNEL staining was used to observe the apoptosis of H9c2 cells,and the RT-PCR was used to detect the mRNA expression of bcl-2,Bax,Caspase- 3,type 1 and type 2 Ang Ⅱ receptors (AT1R,AT2R).ResultsCompared with the control group,the survival rate of H9c2 cells decreased significantly in Ang Ⅱ group.The number of apoptosis was significantly increased.The expression of Caspase-3 and Bax was significantly increased,while the expression of bcl-2 was significantly decreased.AT1R and AT2R were significantly increased,especially AT1R.However,ghrelin could significantly increase the survival rate of myocardial cells,reduce the number of apoptosis,down-regulate the expression of Caspase-3,Bax and AT1R,and up-regulate the expression of bcl-2.Interestingly,there was no significant change in AT2R expression.ConclusionAT1R and AT2R were both involved in the induction of myocardial apoptosis in Ang Ⅱ.Ghrelin inhibited the apoptosis of myocardial cells induced by Ang Ⅱ,and its mechanism could be related to the down-regulation of AT1R.
heart failure;ghrelin;angiotensin Ⅱ;myocardial apoptosis
国家自然科学基金(81570360,81400298)
*通讯作者
1007-4287(2017)11-1982-06
R541.6+1
A
杨春艳(1980- ),女,博士,主治医师,主要从事心力衰竭发病机制及治疗研究。
2017-02-13)
