甘蓝型油菜MSL不育系花药发育的细胞学研究
2017-11-13罗灿灿沈家葆黄小芳胡胜武
罗灿灿,沈家葆,黄小芳,郭 媛,胡胜武
(1.旱区作物逆境生物学国家重点实验室,陕西杨凌 712100;2.西北农林科技大学 农学院,陕西杨凌 712100)
甘蓝型油菜MSL不育系花药发育的细胞学研究
罗灿灿1,2,沈家葆2,黄小芳2,郭 媛2,胡胜武1,2
(1.旱区作物逆境生物学国家重点实验室,陕西杨凌 712100;2.西北农林科技大学 农学院,陕西杨凌 712100)
为了揭示MSL (male sterility lembke) 的小孢子败育时期及其特点,从而促进中国及世界油菜杂种优势利用的步伐。选用MSL衍生系的不育材料和可育材料为试验材料,采用常规压片和石蜡切片技术,观察其花药发育过程。通过对MSL不育材料的细胞学观察,发现MSL可发育到四分体时期,四分体不同程度皱缩,绒毡层径向伸长,并含有一个特别大的液泡,液泡将细胞核和细胞质挤向细胞的一边,胞质浓缩。随着发育的进行,绒毡层进一步伸长,向内挤压四分体,腔室体积变小。紧接着液泡化并且伸长了的绒毡层和浓缩的四分体溶解在一起,花粉囊横切面的形状发生改变,由圆形变成长条形,最后整个花药室萎缩。结果表明MSL的败育时期为四分体时期,由于绒毡层的异常而导致败育。
甘蓝型油菜;细胞核雄性不育;MSL;花药败育;细胞学观察
油菜作为重要的油料作物,杂种优势显著,油菜杂种优势利用的主要授粉控制系统包括细胞核雄性不育、细胞质雄性不育、化学杀雄、自交不亲和,其中细胞核雄性不育,是油菜杂种优势育种中一个重要的授粉控制系统[1]。随着大家对核不育的重视,核不育在高粱[2]、大豆[3]、油菜[4]、小麦[5]和棉花[6]上取得成功应用。
目前,油菜中已报道的核不育类型有显性核不育[7]、双隐性核不育[8-10]和3对基因控制的隐性核不育[11-12]。油菜MSL (male sterility lembke) 系统是德国NPZ (Norddeutsche Pflanzenzucht Hans-Georg Lembke) 育种公司1984年发现的一种天然突变体,是目前欧洲杂交油菜生产的主要授粉控制系统[13-14]。经典遗传学和分子遗传学对MSL进行遗传分析和验证,认为MSL由1对隐性不育基因和1对隐性上位抑制基因互作控制[15],到目前为止,有关MSL花药败育的细胞学特征还未见报道。
前人对油菜核不育材料花药败育的细胞学观察发现,大多花药败育发生于花粉母细胞至单核花粉时期。比如隐性核不育材料S45A和117A在四分体至单核小孢子阶段发生败育,表现为小孢子不能形成花粉壁并且绒毡层细胞径向肥大和液泡化[16]。隐性核不育材料9012A[11]及其衍生材料1740A[17]花药败育于四分体时期,9012A败育是由于绒毡层细胞未能成功转化为分泌型细胞,无法分泌胼胝质酶降解胼胝质,导致四分体不能从胼胝质中释放出来[16,18];1740A败育是由于胼胝质不能解体和绒毡层细胞的径向肥大所致[17]。显性核不育株Rsl046A[19]、Shaan-GMS的衍生材料0A30A[20]、温敏核不育系TE5A[21]和核不育系宜3A[22]花药败育原因都是减数分裂异常导致无法形成二分体及四分体。温敏核不育系H50S[23]和373S[24],光温敏核不育系Huiyou50S[25]均为单核花粉败育型。
本研究以MSL的衍生系5A135AB为试验材料,采用常规压片和石蜡切片,观察其花药发育过程,明确MSL花药败育时期和特点,为揭示该材料的雄性不育机理及深入利用奠定基础。本试验结果在油菜杂种优势利用方面具有重要的理论与实践意义。
1 材料与方法
1.1 材 料
供试材料为两型系5A135AB,其中不育株为5A135A,可育株为5A135B。5A135AB是从杂交种Marthow自交后代中分离而来,该杂交种是基于德国NPZ育种公司的油菜MSL雄性不育系配置而成。Marthow为捷克作物研究所Miroslav Klima博士惠赠。供试材料于2015年9月中旬播种于西北农林科技大学北校区农学院标本园,田间管理同一般大田常规管理。
1.2 方 法
1.2.1 常规压片 2016年4月初,取初花期不育株5A135A和可育株5A135B主花序以及上部分枝的花蕾,立刻放入卡诺固定液[V(无水乙醇)∶V(冰乙酸)=3∶1]中固定1 h,100%(体积分数,下同)、95%、85%梯度酒精洗涤各2 h,然后转入75%的酒精中,于4 ℃冰箱保存备用。使用时先取花蕾6枚雄蕊中的1枚雄蕊,放在1 mol/L的盐酸中解离20 min,然后经1%醋酸洋红染色压片,在Olympus BX51 光学显微镜下镜检花药发育时期并照相[20]。
1.2.2 石蜡切片 将保存在75%酒精中的已确定发育时期的材料经85%、95%、100%、100%梯度酒精脱水各2 h,经酒精和二甲苯体积比分别为1∶1、0∶1、0∶1透明各2 h,60 ℃浸蜡3次,每次2 h,56~58 ℃进行石蜡包埋,将修好的花蕾包埋块横切,切片厚度为6~8 μm,40 ℃的蒸馏水粘片,放入60 ℃的烘箱2 h烘干,3次二甲苯各25 min脱蜡,100%、95%、85%、70%、50%、30%梯度酒精复水各5 min,蒸馏水水洗5 min,0.1%甲苯胺蓝水溶液染色5 s,阿拉伯树胶封片,Olympus BX51 光学显微镜观察、照相[26]。
2 结果与分析
2.1 5A135A花药败育的压片观察
根据常规压片结果,甘蓝型油菜对照可育株5A135B花药发育的主要细胞学时期包括减数分裂期、四分体时期、小孢子时期、花粉后期、成熟花粉期。减数分裂期花粉母细胞在胼胝质的包裹下进行减数分裂(图1-A),减数分裂结束后形成四分体(图1-B),胼胝质降解,小孢子从四分体中释放出来,合成花粉壁,细胞核被液泡挤向一边,小孢子三边加厚(图1-C),随着发育的进行,小孢子体积增大(图1-D),花粉进一步发育,形成2个生殖核,花粉粒发育成熟(图1-E)。不育材料5A135A减数分裂时期发育正常,花粉母细胞在胼胝质内经历2次减数分裂(图1-F)、形成正常的四分体(图1-G),但是在四分体发育后期,包裹在胼胝质内的四分体被挤压变形(图1-H),四分体不断皱缩(图1-I),最后四分体倾向于解体(图1-J)。不育材料花药发育只能发育到四分体时期,被胼胝质包裹的四分体不能被成功释放,挤压变形,不断皱缩直至解体。所以,5A135A败育时期发生在四分体时期。
2.2 5A135A花药败育的石蜡切片观察
从石蜡切片结果来看,可育株5A135B花药发育主要经历花粉母细胞时期、减数分裂期、四分体时期、单核期、双核期和成熟期。花粉母细胞早期粘连在一团(图2-A),随后以多边形分离,开始减数分裂(图2-B),形成四分体(图2-C),接着四分体周围的胼胝质解体,释放出小孢子,随着小孢子外壁的孢粉素和脂类物质的积累,单核小孢子三边加厚(图2-G);单核小孢子第一次有丝分裂,形成双核花粉粒,此时绒毡层发生解体(图2-H);双核花粉粒再进行一次有丝分裂,形成成熟花粉粒,绒毡层几乎完全降解(图2-I)。
不育材料5A135A在花粉母细胞时期发育正常,花粉母细胞形状规则、排列紧密,绒毡层细胞整齐地排列在花粉母细胞的外圈(图2-D);减数分裂时期绒毡层细胞体积变大,内含大液泡,减数分裂体由胼胝质包裹进行减数分裂(图2-E);和可育材料相比,不育材料在四分体时期出现明显差异,绒毡层径向伸长向内挤压四分体,绒毡层细胞内含有一个特别大的液泡,液泡将细胞核和细胞质挤向细胞的一边,胞质浓缩(图2-F)。随着发育的进行,未发现形成小孢子,并且绒毡层进一步伸长,向内挤压四分体,腔室体积变小(图2-J);花粉囊的横切面开始变形,变成长条形(图2-K),最后,绒毡层和皱缩了的四分体全部解体,整个花粉囊萎缩(图2-L)。根据石蜡切片结果,判断5A135A不育材料在四分体以后小孢子无法释放出来,同时绒毡层异常膨大,向内挤压四分体,花药室变形,最终导致败育。
3 讨 论
油菜MSL系统是德国NPZ育种公司发明的一种油菜不育系统,是目前欧洲油菜杂种生产的主要授粉控制系统[13-14]。本研究通过对MSL衍生系5A135A花药败育的压片及石蜡切片观察结果显示,其败育时期为四分体时期。主要特征是,四分体不同程度皱缩,绒毡层径向伸长,并含有一个特别大的液泡,液泡将细胞核和细胞质挤向细胞的一边,胞质浓缩。随着发育的进行,绒毡层进一步伸长,向内挤压四分体,腔室体积变小。紧接着液泡化并且伸长了的绒毡层和浓缩的四分体溶解在一起,花粉囊的形状发生改变,由圆形变成长条形,最后整个花药室萎缩。本研究结果显示,MSL败育时期和隐性核不育材料9012A[16,18]及其衍生材料1740A[17]相同,均为四分体时期。败育特征也很相似,绒毡层细胞大液泡化并且径向伸长,向内挤压四分体。有研究发现在白菜[27]、辣椒[28]、马铃薯[29]、红麻[30]花药败育特点中也存在类似的现象。在花药发育的过程中,输送花药小孢子发育所需的营养物质是绒毡层主要的功能之一。已有研究表明,异常发育的绒毡层会引起花药败育[31]。分为2种形式,分别是绒毡层细胞的程序化死亡进程异常[32]和绒毡层细胞自身的发育异常[33]。本研究的MSL败育原因属于后者,是绒毡层细胞在四分体时期异常径向伸长,无法正常提供营养物质,反而向内挤压小孢子,侵占小孢子生长所必需的空间,最终使得花药败育。在油菜中,已发现一些基因与绒毡层细胞的发育相关,如曾芳琴[34]报道的 BnCYP704BJ基因和夏秀云[35]研究的 BnATA20基因, BnCYP704BJ基因如果突变,会以影响绒毡层分泌合成脂类物质的方式引起败育, BnATA20基因的突变体以绒毡层异常膨大的形式败育。因此,随着生物科技的发展,已经可以人为地通过基因工程方式获得雄性不育系。
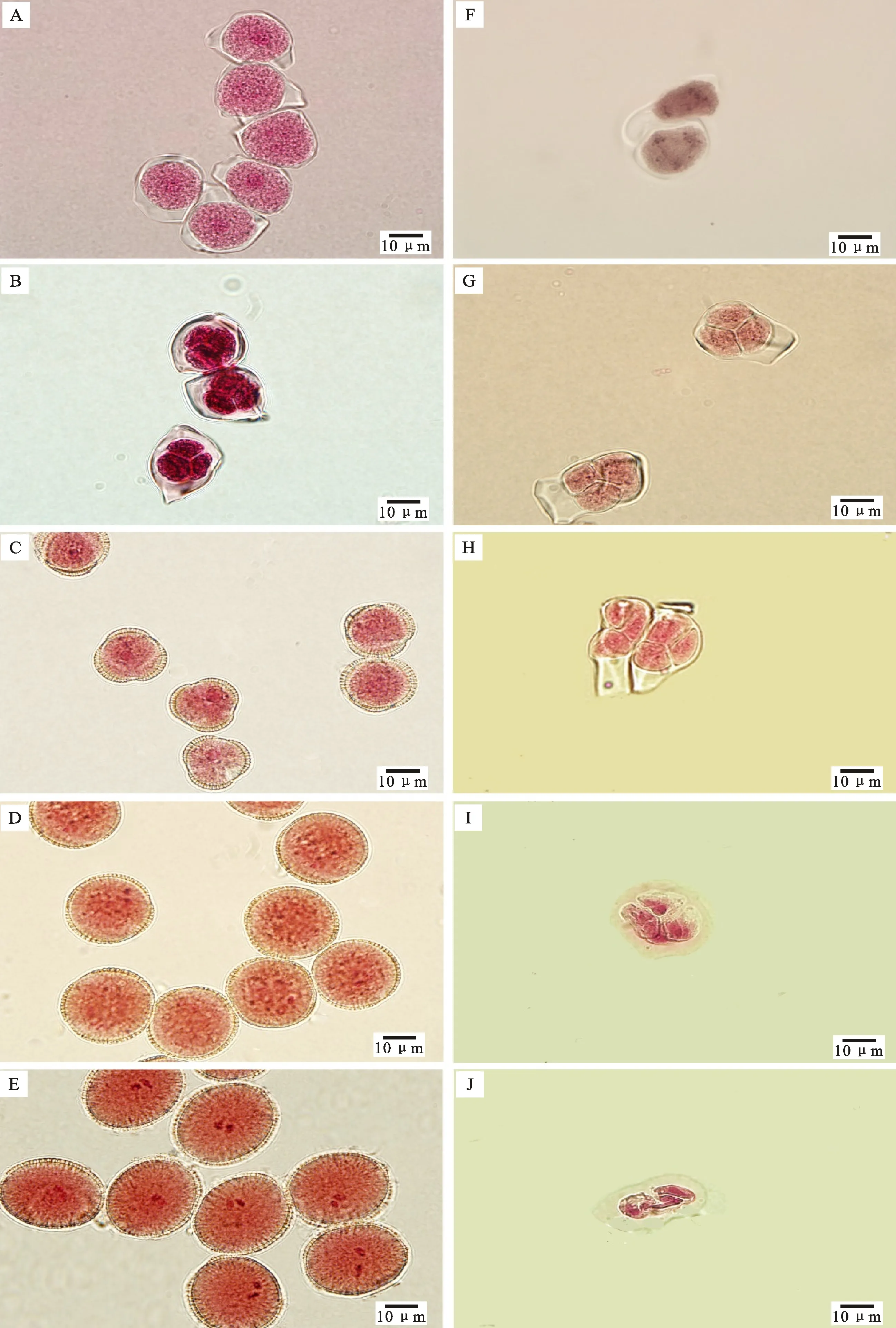
A.可育、减数分裂期 Fertile,meiosis stage;B.可育、四分体时期 Fertile,tetrad stage;C.可育、小孢子时期 Fertile,microspore stage;D.可育、花粉后期 Fertile,later pollen stage;E.可育、成熟花粉期 Fertile,mature pollen stage;F.不育、减数分裂期 Sterile,meiosis stage;G.不育、四分体时期 Sterile,tetrad stage;H,I,J.不育、败育的四分体形态 Sterile,deformed tetrad morphology;标尺为10 μm Scale bars=10 μm
图1甘蓝型油菜可育株5A135B和不育株5A135A花药发育的主要细胞学时期
Fig.1Majorcytologicaleventsduringantherdevelopmentinfertileplants5A135Bandsterileplants5A135AinB.napus

A.可育、花粉母细胞时期 Fertile,pollen mother cell stage;B.可育、减数分裂期 Fertile,meiosis stage;C.可育、四分体时期 Fertile,tetrad stage;D.不育、花粉母细胞时期 Sterile,pollen mother cell stage;E.不育、减数分裂期 Sterile,meiosis stage;F.不育、四分体时期 Sterile,tetrad stage;G.可育、单核期 Fertile,uninuclear microspore stage;H.可育、双核期 Fertile,binuclear microspore stage;I.可育、成熟期 Fertile,mature pollen stage;J.不育、四分体时期的败育花粉囊 Sterile,degenerated anther sac at tetrad stage;K-L.不育、败育花粉囊 Sterile,degenerated anther sac;T.绒毡层 Tapetum;PMCs.花粉母细胞 Pollen mother cells;MC.减数分裂体 Meiotic cell;Tds.四分体 Tetrads;Msp.小孢子 Microspores;标尺在A、B、D、E、F、J中为10 μm,在C、G、H、I、K、L中为20 μm Scale bars in A,B,D,E,F,J were 10 μm and in C,G,H,I,K,L were 20 μm
图2甘蓝型油菜可育株5A135B和不育株5A135A花药发育主要时期的石蜡切片观察
Fig.2Paraffinsectionofmajoreventsduringantherdevelopmentoffertileplants5A135Bandsterileplants5A135AinB.napus
综上,本研究揭示了MSL的败育时期和败育特征,丰富了油菜核不育材料MSL的理论内容,为日后深入利用该材料奠定了基础,同时对促进中国及世界油菜杂种优势利用的步伐,具有深远的意义。
Reference:
[1] 傅廷栋.杂交油菜的育种与利用[M].武汉:湖北科技出版,2000.
FU T D.Breeding and Utilization of Hybrid Rapeseed[M].Wuhan:Hubei Science and Technology Press,2000 (in Chinese).
[2]DOGGETT H,EBERHART S A.Recurrent selection in sorghum[J].CropScience,1968,8(1):119-121.
[3]PARER J T.The possibility of recurrent selection to increase the oil content in soybeans using genetic male sterility and NMR spectroscopy[J].Bragantia, 1988,48(2):157-172.
[4]MATHIAS R.A new dominant gene for male sterility in rapeseed,BrassicanapusL.[J].JournalofPlantBreeding,1985(94):170-173.
[5]SASAKUMA T,MAAN S S,WILLIAMS N D.EMS (ethyl-methane-sulfonate)-indued male-sterile mutants in euplasmic and alloplasmic common wheat[J].CropScience, 1978,18(5):850-853.
[6]BOWMAN D T,WEAVER J B.Analyses of a dominant male-sterile character in upland cotton.II.Genetic studies[J].CropScience, 1979,19(5):628-630.
[7]李树林,周熙荣,周志疆,等.显性核不育油菜的遗传与利用[J].作物研究,1990,4(3):27-32.
LI SH L,ZHOU X R,ZHOU ZH J,etal.Inheritance of genetic male sterility (GMS) and its utilization in rape (BrassicanapusL.)[J].CropScience,1990,4(3):27-32 (in Chinese with English abstract).
[8]侯国佐,王 华,张瑞茂.甘蓝型油菜细胞核雄性不育材料117A的遗传研究[J].中国油料作物学报,1990(2):7-10.
HOU G Z,WANG H,ZHANG R M.Inheritance of genic male sterile line 117A inBrassicanapusL.[J].ChineseJournalofOilCropSciences, 1990(2):7-10 (in Chinese with English abstract).
[9]李树林,周志疆,周熙荣.甘蓝型油菜隐性核不育系S45AB的遗传[J].上海农业学报,1993,9(4):1-7.
LI SH L,ZHOU ZH J,ZHOU X R.Inheritance of recessive genic male sterile line S45AB of rape (BrassicanapusL.)[J].ActaAgriculturaeShanghai,1993,9(4):1-7 (in Chinese with English abstract).
[10]涂金星,傅廷栋,郑用琏.甘蓝型油菜核不育材料90-2441A的遗传及其等位性分析[J].华中农业大学学报,1997,16(3):255-258.
TU J X,FU T D,ZHENG Y L.Analyses on inheritance and isolocus of the rapeseed GMS 90-2441A (B.napus)[J].JournalofHuazhongAgriculturalUniversity,1997,16(3):255-258 (in Chinese with English abstract).
[11]陈凤祥,胡宝成,李 成,等.甘蓝型油菜细胞核雄性不育性的遗传研究:I.隐性核不育系 9012A 的遗传[J].作物学报,1998,24(4):431-438.
CHEN F X,HU B CH,LI CH,etal.Genetic studies on GMS inBrassicanapusL.I.Inheritance of recessive GMS line 9012A[J].ActaAgronomicaSinica,1998,24(4):431-438 (in Chinese with English abstract).
[12]KE L P,SUN Y Q,LIU P W,etal.Identification of AFLP fragments linked to one recessive genic male sterility (RGMS) in rapeseed (BrassicanapusL.) and conversion to SCAR markers for marker-aided selection[J].Euphytica,2004,138(2):163-168.
[13]FRAUEN M,NOACK J,PAULMANN W,etal.Development and perspective of MSL-hybrids in winter oilseed rape in Europe[C]//Proceedings of 11th international rapeseed conference.Copenhagen Denmark:The University of Copenhapen,2003:6-10.
[14]FRAUEN M,NOACK J,GIRKE A,etal.Ten years experience of development and cultivation of winter oilseed rape hybrids in Europe based on the MSL system[C]//Proceedings of 12th International Rapeseed Conference.Wuhan Huber:Huazhong Agricultural University,2007:39-41.
[15]任梦阳.甘蓝型油菜不育基因 BnMs3/ms3和BnRf/rf的精细定位以及分子标记辅助选育效率评价[D].杭州:浙江师范大学,2012.
REN M Y.Fine mapping of sterility genes BnMs3/ms3 andBnRf/rfinBrassicanapusand efficiency evaluation of marker-assisted breeding[D].Hangzhou:Zhejiang Normal University,2012 (in Chinese with English abstract).
[16]杨光圣,瞿 波,傅廷栋.三个甘蓝型油菜隐性细胞核雄性不育系小孢子发生的细胞学研究[J].华中农业大学学报,1999,18(6):520-523.
YANG G SH,QU B,FU T D.Cytological study of microsporogenesis in three recessive genic male sterile lines ofBrassicanapusL.[J].JournalofHuazhongAgriculturalUniversity,1999,18(6):520-523 (in Chinese with English abstract).
[17]李 慧,胡胜武,李 玮,等.甘蓝型油菜隐性上位互作核不育系的选育及其细胞学研究[J].西北农林科技大学学报(自然科学版),2010,38(1):111-118.
LI H,HU SH W,LI W,etal.Breeding of RGMS lines and investigation on their cytology inBrassicanapusL.[J].JournalofNorthwestA&FUniversity(NaturalScienceEdition),2010,38(1):111-118 (in Chinese with English abstract).
[18]WAN L L,XIA X Y,HONG D F,etal.Abnormal vacuolization of the tapetum during the tetrad stage is associated with male sterility in the recessive genic male sterileBrassicanapusL.line 9012A[J].JournalofPlantBiology,2010,53(2):121-133.
[19]吴建勇.甘蓝型油菜显性细胞核雄性不育差异表达基因及雄配子发育研究[D].武汉:华中农业大学,2006.
WU J Y.Study on differentially expressed genes and male gametes of dominant genic male sterility inBrassicanapusL.[D].Wuhan:Huazhong Agricultural University,2006 (in Chinese with English abstract).
[20]XIAO ZH D,XIN X Y,CHEN H Y,etal.Cytological investigation of anther development in DGMS line Shaan-GMS inBrassicanapusL.[J].CzechJournalofGenetics&PlantBreeding,2013,49(1):16-23.
[21]李可琪,曾新华,袁 荣,等.甘蓝型油菜温敏细胞核雄性不育系TE5A花药发育的细胞学研究[J].中国农业科学,2016,49(12):2408-2417.
LI K Q,ZENG X H,YUAN R,etal.Cytological researches on the anther development of a thermo-sensitive genic male sterile line TE5A inBrassicanapusL.[J].ScientiaAgriculturaSinica,2016,49(12):2408-2417 (in Chinese with English abstract).
[22]杨光圣,瞿 波.甘蓝型油菜显性细胞雄性不育系宜3A花药发育的解剖学研究[J].华中农业大学学报,1999,18(5):405-408.
YANG G SH,QU B.Anatomical studies on the anther’s development of the dominant genic male sterile line YI-3A inBrassicanapusL.[J].JournalofHuazhongAgriculturalUniversity,1999,18(5):405-408 (in Chinese with English abstract).
[23]孙晓敏,胡胜武,于澄宇.油菜生态不育系H50S花药败育的细胞学观察[J].西北农业学报,2009,18(5):153-158.
SUN X M,HU SH W,YU CH Y.Cytological observation of anther development of an ecological male sterile line H50S inBrassicanapusL.[J].ActaAgriculturaeBoreali-occidentalisSinica,2009,18(5):153-158 (in Chinese with English abstract).
[24]李 玮.油菜生态型核不育系373S及叶片黄化突变体研究[D].陕西杨凌:西北农林科技大学,2007.
LI W.Studies on ecotypical nuclear male sterile line 373S and a chlorsis mutant in oilseed rape[D].Yangling Shaanxi:Northwest A&F University,2007 (in Chinese with English abstract).
[25]葛 娟,郭英芬,于澄宇,等.甘蓝型油菜光、温敏雄性不育系Huiyou50S花粉败育的细胞学观察[J].作物学报,2012,38(3):541-548.
GE J,GUO Y F,YU CH Y,etal.Cytological observation of anther development of Photoperiod/Thermo-sensitive male sterile line Huiyou50S inBrassicanapus[J].ActaAgronomicaSinica,2012,38(3):541-548 (in Chinese with English abstract).
[26]HUANG Q X,XIN X Y,GUO Y,etal.Cytological observation of anther structure and genetic investigation of a new type of cytoplasmic male sterile 0A193-CMS inBrassicarapaL.[J].PlantBreeding,2016,135(6):707-713.
[27]单奇伟,陈龙正,徐 海,等.不结球白菜Ogura雄性不育花器官形态及败育细胞学的研究[J].华北农学报,2009,24(S2):25-29.
SHAN Q W,CHEN L ZH,XU H,etal.Morphological studies on flower organ of Ogura cytoplasmic male sterile and its cytological characterization of abortion in Brassica rapeseed[J].ActaAgriculturaeBoreali-Sinica,2009,24(S2):25-29 (in Chinese with English abstract).
[28]邱义兰,刘珠丽,李 红,等.辣椒细胞质雄性不育花药败育及淀粉粒分布的细胞学观察[J].分子细胞生物学报,2008,41(4):283-293.
QIU Y L,LIU ZH L,LI H,etal.Cytological observation of anther abortion and starch distribution of a cytoplasm male sterile pepper (CapsicumannumL.)[J].JournalofMolecularCellBiology,2008,41(4):283-293 (in Chinese with English abstract).
[29]全雪丽,玄春吉,吴京姬,等.马铃薯 (延薯4号) 小孢子发生的细胞学观察[J].种子,2013,32(6):28-30.
QUAN X L,XUAN CH J,WU J J,etal.Cytological observation of microsporogenesis in the potato (Yanshu 4)[J].Seed,2013,32(6):28-30 (in Chinese with English abstract).
[30]周 琼,黎 桦,赵 嘉,等.红麻GMS与CMS小孢子败育过程的细胞学及组织化学比较[J].作物学报,2010,36(8):1414-1424.
ZHOU Q,LI H,ZHAO J,etal.Cytological and cytochemical observation on microspore abortion of GMS and CMS in Kenaf (HibiscuscannabinusL.)[J].ActaAgronomicaSinica,2010,36(8):1414-1424 (in Chinese with English abstract).
[31]HUANG M D,HSING Y I,HUANG A H.Transcriptomes of the anther sporophyte:availability and uses[J].PlantandCellPhysiology,2011,52(9):1459-1466.
[32]VARNIER A L,MAZEYRAT G F,SANG W R S,etal.Programmed cell death progressively models the development of anther sporophytic tissues from the tapetum and is triggered in pollen grains during maturation[J].JournalofStructuralBiology,2005,152(2):118-128.
[33]罗海山,孟德璇,陈晓阳,等.植物绒毡层异常导致花粉败育的机理研究进展[J].作物研究,2013,26(6):725-729.
LUO H SH,MENG D X,CHEN X Y,etal.Progress on the mechanism research of abnormal tapetum leads to pollen abortion in plants[J].CropResearch,2013,26(6):725-729 (in Chinese).
[34]曾芳琴.油菜S45AB隐性核不育分子机理与应用研究[D].武汉:华中农业大学,2010.
ZENG F Q.Molecular mechanism and utilization of recessive genic male sterile line S45AB in rapeseed (BrassicanapusL.)[D].Wuhan:Huazhong Agricultural University,2010 (in Chinese with English abstract).
[35]夏秀云.甘蓝型油菜雄性不育相关基因BnATA20功能验证[D].武汉:华中农业大学,2011.
XIA X Y.Functional verification of male sterile related gene BnATA20 inBrassicanapusL.[D].Wuhan:Huazhong Agricultural University,2011 (in Chinese with English abstract).
CytologicalObservationonAntherDevelopmentofGenicMaleSterileLineMSLinBrassicanapusL.
LUO Cancan1,2,SHEN Jiabao2,HUANG Xiaofang2,GUO Yuan2and HU Shengwu1,2
(1.State Key Laboratory of Crop Stress Biology in Arid Areas,Yangling Shaanxi 712100,China;2.College of Agronomy,Northwest A&F University,Yangling Shaanxi 712100,China)
To characterize MSL (male sterility lembke) anther abortion and promote its utilization in breeding of hybrid rapeseed (BrassicanapusL.) in China,even in the world,cytological observations of the anther development in sterile line which developed on the basis of MSL were employed in the study. The results revealed that anther development of the MSL had been abnormal at tetrad stage,and some tetrads had concentrated. The tapetal cell extended radially and contained a particularly large vacuole. The vacuole squeezed the nucleus and cytoplasm to one side of the cell,which it lead to the cytoplasm concentrated. The tapetum occupied the space of the tetrads. Along with the tapetum continued to swelled,the locule was getting smaller and smaller. Sequentially,the vacuolized,elongated tapetum and the concentrated tetrads dissolved together,and the shape of the anther sac changed from a circle to a long strip shape,and the anther sac atrophied finally. The results showed that anther of MSL aborted at tetrad stage,because it could not form functional tapetal cells.
BrassicanapusL. ; Genetic male sterility; MSL; Anther abortion; Cytological observation
2017-04-04
2017-06-01
Earmarked Fund for China Agricultural Research System(No.CARS-13).
LUO Cancan,female,master student. Research area:genetics and breeding of rapeseed. E-mail: 18956141956@163.com
S565.4
A
1004-1389(2017)10-1470-07
日期:2017-10-18
网络出版地址:http://kns.cnki.net/kcms/detail/61.1220.S.20171018.1733.016.html
2017-04-04
2017-06-01
现代农业产业技术体系建设专项资金(CARS-13)。
罗灿灿,女,硕士研究生,从事油菜遗传育种研究。E-mail: 18956141956@163.com
胡胜武,男,博士,教授,博士生导师,主要从事油菜遗传育种研究。E-mail:swhu83251@nwsuaf.edu.cn
CorrespondingauthorHU Shengwu,male,Ph.D,professor,doctoral supervisor.Research area:genetics and breeding of rapeseed.E-mail:swhu83251@nwsuaf.edu.cn
(责任编辑:成敏Responsibleeditor:CHENGMin)
