新型TiO2-铜卟啉催化剂的合成及其光催化甲基橙降解研究
2017-11-04陈韶蕊
陈韶蕊
(河北科技大学理学院,河北石家庄 050018)
1008-1542(2017)05-0453-07
10.7535/hbkd.2017yx05007
新型TiO2-铜卟啉催化剂的合成及其光催化甲基橙降解研究
陈韶蕊
(河北科技大学理学院,河北石家庄 050018)
为了研究不同长度侧链催化剂对光催化降解甲基橙溶液的影响,更好地解决水污染治理问题,以1-萘酚为原料,经两步反应得到中间体4-(3-萘氧基-烷氧基)-苯甲醛,利用Lindsey法合成4个结构新颖的卟啉及铜卟啉衍生物,中间体和目标产物的结构经MS,NMR和元素分析进行确认,制备了TiO2-铜卟啉催化剂,并进行了光催化甲基橙降解实验。结果表明:当铜卟啉衍生物苯环上连有侧链时,其光催化活性优于四苯基铜卟啉催化剂,但侧链的长度对活性的影响不明显。该研究为今后合成高效催化剂提供了思路。
有机光化学;萘酚;卟啉;合成;甲基橙;光催化
近年来,随着世界工业化进程的加快和人口的增长,生产、生活过程中排放的各种污水对环境造成了严重破坏,污染水体的治理已成为环保领域里的一个重要课题。作为高级氧化技术之一,光催化降解具有反应条件温和、设备简单、易于操作、二次污染小、运行成本低、有望用太阳光作为反应光源等优点,是一种非常有发展前景的水污染治理技术,近年来受到了广泛关注[1-3]。
卟啉类化合物是一种高度共轭的大环化合物,卟啉及其金属卟啉类化合物因具有高效的可见光吸收特性及特殊的光电、催化性质,广泛应用于光催化动力学疗法[4-5]、太阳能的光电转化[6-8]、选择性催化氧化[9]、光催化环氧化等方面[10-11]。TiO2作为光催化剂,具有无毒、稳定、便宜和资源丰富等多种性质,在光催化降解环境污染物方面引起了广泛关注[12-13]。但由于TiO2在可见光区无吸收,限制了其对太阳能的吸收及光催化降解有机物。而利用TiO2-金属卟啉这种“有机-无机”复合光敏催化材料,却能够直接利用可见光,敏化分子氧,产生单线态氧,快速消除水体中的生物难降解有机污染物,从而使水质得到净化[14-18]。
近年来人们对TiO2-金属卟啉光催化降解有机物进行了大量研究,但有关尾式卟啉侧链长度对光催化活性的影响未见报道。笔者采用Lindsey法设计了不同长度侧链的尾式铜卟啉衍生物,其结构经NMR,MS等进行确认,制备了TiO2-铜卟啉催化剂,利用扫描电子显微镜 (SEM)、漫反射紫外可见光谱(DRS)及X射线衍射(XRD)对催化剂进行表征,研究了不同长度侧链的催化剂对光催化降解甲基橙溶液的影响。目标化合物的合成路线见图1。

图1 目标化合物的合成路线Fig.1 Synthesis route of target compounds
1 实验部分
1.1 主要仪器和试剂
X4型数字显微熔点测定仪,温度计未较正,AV500型核磁共振仪 (美国Bruker公司提供),质谱采用英国VG公司的VGZAB-HS型质谱仪进行测定,氙灯光化学反应仪 (北京纽比特科技有限公司提供),X射线衍射 (XRD)采用Rigaku D/MAX 2500仪器测定,漫反射紫外可见光谱(DSR)采用Shimadzu UV2550仪器测定。
5,10,15,20-四苯基铜卟啉(CuTPP),自制;所用试剂均为市售分析纯。
1.2 中间体1的合成
中间体1的合成参照文献[19]。
1.2.1 3-萘氧基-1-溴丙烷(中间体1a)
淡黄色液体,产率为91.6%。1H NMR(500 MHz,CDCl3) δ(×10-6),2.42 (m,2H,—CH2),3.89(t,J= 6.3 Hz,2H—CH2Br),4.33(t,J=5.9 Hz,2H,—OCH2),6.86(m,1H,Naph),7.41(m,1H,Naph),7.48(m,1H,Naph),7.53(m,2H,Naph),7.85(m,1H,Naph),8.28(m,1H,Naph)。MS:m/z215.1 ([M+H]+) amu。
1.2.2 4-萘氧基-1-溴丁烷(中间体1b)[20]
熔点为42~44 ℃,产率为89.4%。1H NMR(500 MHz,CDCl3)δ(×10-6),2.11 (m, 2H,—CH2), 2.18 (m, 2H, —CH2), 3.56(t,J=6.5 Hz, 2H,—CH2Br), 4.19(t,J=5.8 Hz, 2H,—OCH2), 6.81(m, 1H, Naph), 7.36(m, 1H, Naph), 7.44(m, 1H, Naph), 7.48(m, 2H, Naph), 7.81(m, 1H, Naph), 8.27(m, 1H, Naph)。MS:m/z229.4 ([M+H]+) amu。
1.2.3 5-萘氧基-1-溴戊烷(中间体1c)[19]
淡黄色液体,产率为90.5%。1H NMR(500 MHz, CDCl3) δ(×10-6),1.78(m, 2H, —CH2), 2.00~2.06 (m, 4H, —CH2), 3.46 (t, 2H,—CH2Br), 4.20(t, 2H,—OCH2), 6.81(m, 1H, Naph), 7.32(m, 1H, Naph), 7.44(m, 1H, Naph), 7.53(m, 2H, Naph), 7.83(m, 1H, Naph), 8.28(m, 1H, Naph)。MS:m/z243.2 ([M+H]+) amu。
1.2.4 6-萘氧基-1-溴己烷(中间体1d)
熔点为76~78 ℃,产率为87.8%。1H NMR(500 MHz, CDCl3) δ(×10-6),1.56(m, 2H,—CH2), 1.78~1.91 (m, 6H,—CH2), 3.42(t, 2H, —CH2Br), 4.11(t, 2H,—OCH2), 6.85(m, 1H, Naph), 7.34(m, 1H, Naph), 7.45(m, 1H, Naph), 7.56(m, 2H, Naph), 7.85(m, 1H, Naph), 8.27(m, 1H, Naph)。MS:m/z257.3 ([M+H]+) amu。
1.3 中间体2的合成
中间体2的合成参照文献[19]。
1.3.1 4-(3-萘氧基-丙氧基)-苯甲醛(中间体2a)
熔点为46~48 ℃,产率为62.3%。1H NMR(500 MHz, CDCl3) δ(×10-6),2.26 (m, 2H,—CH2), 4.18(m,J= 6.0 Hz, 2H,—CH2O), 4.26(m,J=6.0 Hz, 2H,—OCH2), 6.81(m, 1H, Naph), 7.00(m, 2H, Ph), 7.35(m, 1H, Naph),7.45(m, 2H, Ph), 7.47(m, 1H, Naph), 7.81(m, 1H, Naph), 8.24(m, 1H, Naph), 9.88(s, 1H,—CHO)。MS:m/z257.3 ([M+H]+) amu。
1.3.2 4-(4-萘氧基-丁氧基)-苯甲醛(中间体2b)
熔点为72~74 ℃,产率为67.1%。1H NMR(500 MHz, CDCl3) δ(×10-6),2.14 (m, 4H,—CH2), 4.18(m,J=6.0 Hz, 2H,—CH2O), 4.23(m,J=6.0 Hz, 2H,—OCH2), 6.81(m, 1H, Naph), 7.00(m, 2H, Ph), 7.25(m, 1H, Naph), 7.45(m, 2H, Naph), 7.48(m, 1H, Naph), 7.81(m, 3H, Naph+Ph), 8.24(m, 1H, Naph), 9.88(s, 1H, —CHO)。MS:m/z271.3 ([M+H]+) amu。
1.3.3 4-(5-萘氧基-戊氧基)-苯甲醛(中间体2c)
熔点为56~58 ℃,产率为60.1%。1H NMR(500 MHz, CDCl3) δ(×10-6),1.78 (m, 2H,—CH2), 1.92(m, 2H, —CH2), 2.01(m, 2H,—CH2), 4.08(t,J=6.0 Hz, 2H,—OCH2), 4.17(t,J=6.0 Hz, 2H,—OCH2), 6.79(m, 1H, Naph), 6.99(m, 2H, Ph), 7.35(m, 1H, Naph),7.43~7.49(m, 3H, Naph), 7.78(m, 1H, Naph), 7.80(m, 2H, Ph), 8.26(m, 1H, Naph), 9.87(s, 1H,—CHO)。MS:m/z285.1([M+H]+) amu。
1.3.4 4-(6-萘氧基-己氧基)-苯甲醛(中间体2d)
熔点为86~88 ℃,产率为61.1%。1H NMR(500 MHz, CDCl3) δ(×10-6),1.57~1.70 (m, 4H,—CH2), 1.92 (m, 2H,—CH2), 1.98 (m, 2H,—CH2), 4.05(t,J=6.0 Hz, 2H,—OCH2), 4.16(t,J=6.0 Hz, 2H,—OCH2), 6.78(m, 1H, Naph), 6.98(m, 2H, Ph), 7.36(m, 1H, Naph),7.37~7.48(m, 3H, Naph), 7.78(m, 1H, Naph), 7.81(m, 2H, Ph), 8.26(m, 1H, Naph), 9.87(s, 1H,—CHO)。MS:m/z299.4 ([M+H]+) amu。
1.4 目标化合物的合成
1.4.1 化合物3的合成
在氮气保护下,将5.0 mmol的中间体2,1.59 g(15 mmol)的苯甲醛,1.4 mL (20.0 mmol)的吡咯及600 mL的氯仿在室温下搅拌15 min,滴加0.03 mL的三氟化硼乙醚和10 mL氯仿的溶液,于室温下反应48 h,再加入0.85 g (3.75 mmol)的DDQ继续反应30 h,蒸除溶剂,经柱色谱分离得到化合物3[21]。
1)5,10,15-三苯基-20-(4-(3-萘氧基丙氧基)-苯基)卟啉(化合物3a)
熔点大于300 ℃,产率为7.6%。Anal. Calcd. for C57H42N4O2(%):C, 84.00; H, 5.19; N, 6.87。Found C, 83.82; H, 5.27; N, 6.96. 1H NMR(500 MHz, CDCl3) δ(×10-6),-2.76(s, 2H,—NH), 2.43(m, 2H,—CH2), 4.32(m, 2H,—CH2O), 4.40(m, 2H,—CH2O), 7.27(m, 2H, Ph), 7.53(m,2H, Ph), 7.73(m, 12H, Ph), 8.11(m, 2H, Ph), 8.22(m, 7H, Naph), 8.87 (m, 9H, pyrrole+Ph)。MS:m/z815.4 ([M+H]+) amu。UV-vis (CH2Cl2): λmax(nm), 418(Soret band), 515, 550, 591, 646 (Q bands)。
2)5,10,15-三苯基-20-(4-(4-萘氧基丁氧基)-苯基)卟啉(化合物3b)
熔点大于300 ℃,产率为6.9%。Anal. Calcd. for C58H44N4O2(%): C, 84.03; H, 5.35; N, 6.76。Found C, 84.23; H, 5.39; N, 6.87。1H NMR(500 MHz, CDCl3) δ(×10-6),-2.76(s, 2H, -NH), 2.28(m, 4H, —CH2), 4.35(m, 4H,—CH2O), 6.88(m, 2H, Ph), 7.23~7.81(m, 15H, Ph), 8.36(m, 2H, Ph), 8.38(m, 7H, Naph), 8.89(m, 8H, pyrrole)。MS: m/z 829.3([M+H]+) amu。UV-vis (CH2Cl2): λmax(nm), 418(Soret band), 515, 551, 591,645 (Q bands)。
3)5,10,15-三苯基-20-(4-(5-萘氧基戊氧基)-苯基)卟啉(化合物3c)
熔点大于300 ℃,产率为6.7%。Anal. Calcd. for C59H46N4O2(%): C, 84.06; H, 5.50; N, 6.65。Found C, 84.32; H, 5.67; N, 6.78。1H NMR(500 MHz, CDCl3) δ(×10-6),-2.74(s, 2H,—NH), 1.84(m, 2H,—CH2), 2.02 (m, 4H,—CH2), 4.17(m, 4H,—CH2O), 7.20(m, 2H, Ph), 7.40(m, 2H, Ph), 7.76(m, 13H, Ph), 8.07(m, 2H, Ph), 8.21(m, 7H, Naph), 8.88 (m, 8H, pyrrole)。MS:m/z843.5 ([M+H]+) amu。UV-vis (CH2Cl2): λmax(nm), 418(Soret band), 516, 551, 591, 646 (Q bands)。
4)5,10,15-三苯基-20-(4-(6-萘氧基己氧基)-苯基)卟啉(化合物3d)
熔点大于300 ℃,产率为7.0%; Anal. Calcd. for C60H48N4O2(%): C, 84.08; H, 5.65; N, 6.54。Found C, 84.25; H, 5.89; N, 6.66。1H NMR(500 MHz, CDCl3) δ(×10-6),-2.74(s, 2H,—NH), 1.69(m,4H,—CH2), 1.97 (m,4H, —CH2), 4.15(m, 4H,—CH2O), 7.19(m, 2H, Ph), 7.37(m, 2H, Ph), 7.45(m, 2H, Ph), 7.73(m, 11H, Ph), 8.20(m, 2H, Ph), 8.33(m, 7H, Naph), 8.89(m, 8H, pyrrole)。MS:m/z857.1 ([M+H]+) amu。UV-vis (CH2Cl2): λmax(nm), 418(Soret band), 516, 551, 591, 646 (Q bands)。
1.4.2 化合物4的合成
将化合物3 (0.10 mmol)、醋酸铜(0.026 g, 0.18 mmol)及25 mL二氯甲烷于室温下反应1~2 h,TLC检测反应完全,经过滤、蒸除溶剂得到产品,柱色谱分离后得到化合物4[22]。
1)5,10,15-三苯基-20-(4-(3-萘氧基丙氧基)-苯基)铜卟啉(化合物4a)
熔点大于300 ℃,产率为93.6%。UV-vis (CH2Cl2) : λmax(nm), 416(Soret band), 539 (Q band)。Anal. Calcd. for C57H40CuN4O2(%):C, 78.11; H, 4.60; N, 6.39。Found C, 78.25; H, 4.80; N, 6.36。
2)5,10,15-三苯基-20-(4-(4-萘氧基丁氧基)-苯基)铜卟啉(化合物4b)
熔点大于300 ℃,产率为94.9%。UV-vis (CH2Cl2): λmax(nm), 416(Soret band), 539 (Q band)。Anal. Calcd. for C58H42CuN4O2(%): C,78.23; H, 4.75; N, 6.29。Found C, 78.45; H, 4.89; N, 6.46。
3)5,10,15-三苯基-20-(4-(5-萘氧基戊氧基)-苯基)铜卟啉(化合物4c)
熔点大于300 ℃,产率为92.7%。UV-vis (CH2Cl2) : λmax(nm), 416(Soret band), 539 (Q band)。Anal. Calcd. for C59H44CuN4O2(%): C, 78.34; H, 4.90; N, 6.19。Found C,78.52; H, 4.89; N, 6.06。
4)5,10,15-三苯基-20-(4-(6-萘氧基己氧基)-苯基)铜卟啉(化合物4d)
熔点大于300 ℃,产率为90.3%。UV-vis (CH2Cl2) : λmax(nm), 416(Soret band), 539 (Q band)。Anal. Calcd. for C60H46CuN4O2(%):C, 78.45; H, 5.05; N, 6.10。Found C, 78.25; H, 5.29; N, 6.06。
1.5 Cu-卟啉-TiO2光敏剂的制备
将6 μmol的Cu-卟啉 (化合物4) 溶于25 mL的二氯甲烷中,加入0.2 g的TiO2,于室温搅拌8 h, 蒸除溶剂并在烘箱中干燥,得到光敏剂4a-TiO2, 4b-TiO2, 4c-TiO2和4d-TiO2[23]。
1.6 光催化实验
称取0.1 g的光敏剂,加入到100 mL质量浓度为10 mg/ mL的甲基橙溶液中,避光搅拌30 min达到吸咐平衡。在300 W,24 V氙灯光照下搅拌30 min。取样3 mL,将样品离心10 min,取上清液在464 nm下测定其吸光度值[24]。补加蒸馏水,使甲基橙溶液恢复至原来刻度,重复上述步骤,总体照射时间为150 min。
2 结果与讨论
2.1 光敏剂4a, 4b, 4c, 4d-TiO2的漫反射紫外可见光谱分析
化合物4a, 4b, 4c, 4d-TiO2的漫反射紫外可见光谱见图2。由图2可知,TiO2在400 nm以上没有吸收,而4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2在420 nm (Soret band) 和541 nm (Q band)均有特征吸收,此吸收峰与铜卟啉(化合物4)在二氯甲烷中的吸收峰一致,且发生了红移(416~420 nm)。由此数据可知,铜卟啉负载到TiO2表面,并且拓宽了TiO2吸收范围。
2.2 光敏剂4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2的X射线衍射分析
4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2的X射线衍射分析见图3。

图2 4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2的漫反射紫外可见光谱Fig.2 UV-vis diffuse reflectance spectra of the bare TiO2, 4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2

图3 4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2的X射线衍射Fig.3 XRD pattern of the bare TiO2 and 4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2
由图3可知,4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2在25.4°, 37.9°, 48.1°, 54.1°, 62.8°和68.4° 出现了特征衍射峰,其衍射峰位置与TiO2一致,说明铜卟啉负载在TiO2表面但并不影响其晶体结构。
2.3 4b-TiO2和TiO2的扫描电子显微镜分析
图4是纯TiO2和4b-TiO2的SEM图。由图4可知,两种物质具有相同的表面状态,这说明铜卟啉负载在TiO2表面,但并不影响其晶体结构。

图4 TiO2和4b-TiO2的扫描电子显微镜Fig.4 SEM pattern of the bare TiO2 and 4b-TiO2 catalysts
2.4 光催化实验结果分析
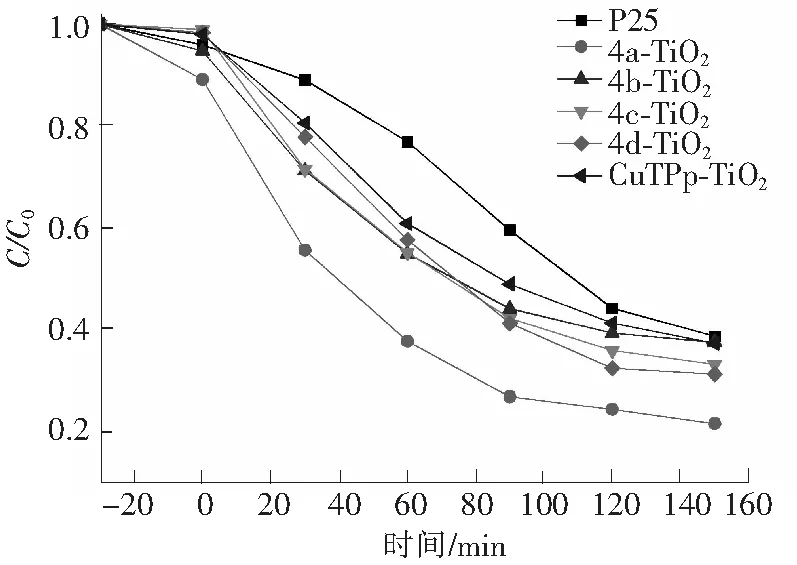
图5 TiO2, CuTPp, 4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2光催化降解甲基橙Fig.5 Photocatalytic degradation of methyl orange by bear TiO2, CuTPp and 4a-TiO2, 4b-TiO2,4c-TiO2, 4b-TiO2 catalysts
以不同时间样品的吸光度与甲基橙原液吸光度的比值为纵坐标,时间为横坐标作图,得到图5。其中CuTPp是四苯基铜卟啉(自制)。由图5可知,CuTPp, 4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2与TiO2的催化活性顺序为4a-TiO2>4d-TiO2>4c-TiO2>4b-TiO2>CuTPp-TiO2>TiO2。
由图5可知:CuTPP, 4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2与TiO2相比均表现出更强的光催化活性,尤其是4a-TiO2的光催化活性最好,且4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2的催化活性均优于CuTPp-TiO2。利用制备的催化剂光催化降解甲基橙,在光照射下卟啉和TiO2都可以被激发。卟琳激发后成为激发态的卟琳分子,激发态的卟琳分子将电子传递至TiO2的导带上,自身成为卟琳正离子,导带上的电子与水中的溶解氧反应生成·O-,生成的·O-通过一系列反应产生氧化性极强的·OH,·OH能够催化氧化溶液中的甲基橙分子,最终生成无毒的小分子降解产物。根据此过程可推测,当苯环上连有取代基时可增强CuPp-TiO2的相互作用,因而增强其催化活性,但CuPp和TiO2之间的相互作用主要是共价键和物理作用,因此取代基的长度对催化活性的影响并不明显[25]。

图6 4a-TiO2, 4b-TiO2, 4c-TiO2, 4d-TiO2催化剂的稳定性Fig.6 Stability of 4a-TiO2, 4b-TiO2,4c-TiO2, 4b-TiO2 catalysts
2.5 催化剂稳定性分析
催化剂的稳定性在实际应用中具有重要的作用。为了进一步研究催化剂的稳定性,当一次降解完成后,将所使用的催化剂回收,于120 ℃烘干重复使用,研究结果如图6所示。
由图6可知:在第1次光催化降解完成后,4a-TiO2, 4b-TiO2, 4c-TiO2和4d-TiO2的效率分别为75.8%,60.4%,66.4%和68.3%;重复使用4次后,效率分别为68.8%,53.7%,58.5%和59.8%。由此可知催化剂具有较好的稳定性。
3 结 论
本研究采用Lindsey法,合成了4个结构新颖的卟啉及铜卟啉衍生物,中间体和目标产物的结构经MS,NMR和元素分析进行确认,制备了TiO2-铜卟啉催化剂光催化降解甲基橙溶液。研究发现:同TiO2-CuTPP催化剂相比,当苯环上连有取代基时其光催化活性增强,但链的长度对活性的影响不明显。此研究为今后进一步研究合成高效催化剂提供了思路和借鉴。
/
[1] 罗利军,王娟,潘学军,等. 二氧化钛选择性光催化降解有机污染物研究进展[J].化学通报,2013, 76(4): 332-337.
LUO Lijun, WANG Juan, PAN Xuejun, et al. Progress in selective degradation of organic contaminants by titanium dioxide[J]. Chemistry Bulletin, 2013, 76(4): 332-337.
[2] 汤小胜,李汝杏,汤平,等.纳米二氧化钛光催化技术降解废水中苯酚的研究[J].湖北理工学院学报,2017, 33(1): 26-30.
TANG Xiaosheng, LI Ruxing, TANG Ping, et al. Research on degradation of phenol in wastewater by nanometer titanium dioxide photocatalysis technology[J]. Journal of Hubei Polytechnic University, 2017, 33(1): 26-30.
[3] 吴勇民,李甫,黄咸雨.含酚废水处理新技术及其发展前景[J].环境科学与管理,2007, 32(3): 150-154.
WU Yongmin, LI Fu, HUANG Xianyu. New techniques for ttreatment of phenol containing wastewater and prospect[J]. Environmental Science and Management, 2007, 32(3): 150-154.
[4] LIAO P Y, WANG X R, ZHANG X H, et al. Synthesis of 2-morpholinetetraphenylporphyrins and their photodynamic activities[J]. Bioorganic Chemistry, 2017, 71: 299-304.
[5] SLOMP A M, BARREIRA S M W, CARRENHO L Z B, et al. Photodynamic effect of meso-(aryl)porphyrins and meso-(1-methyl-4-pyridinium)porphyrins on HaCaT keratinocytes[J]. Bioorganic & Medicinal Chemistry Letters, 2017, 27(2): 156-161.
[6] KEAWIN T, TARSANG R, SIRITHIP K, et al. Anchoring number-performance relationship of zinc-porphyrin sensitizers for dye-sensitized solar cells: A combined experimental and theoretical study[J]. Dyes and Pigments, 2017, 136: 697-706.
[7] GUO C, PENG Q, LIU Q, et al. Selective oxidation of ethylbenzene with air catalyzed by simple [mu]-oxo dimeric metalloporphyrins under mild conditions in the absence of additives[J]. Journal of Molecular Catalysis A: Chemical, 2003, 192 (1/2): 295-302.
[8] CHENG H L, HUANG Z S, WANG L Y, et al. Synthesis and photovoltaic performance of the porphyrin based sensitizers with 2H-[1,2,3]triazolo[4,5-c]pyridine and benzotriazole as auxiliary acceptors[J]. Dyes and Pigments, 2017, 137: 143-151.
[9] KARIMI B, GHOREISHI-NEZHAD M, CLARK J H. Selective oxidation of sulfides to sulfoxides using 30% hydrogen peroxide catalyzed with a recoverable silica-based tungstate interphase catalyst[J]. Organic Letters, 2005, 7(4): 625-628.
[10] MELE G, DEL SOLE R, VASAPOLLO G, et al. Photocatalytic degradation of 4-nitrophenol in aqueous suspension by using polycrystalline TiO2impregnated with functionalized Cu(Ⅱ)-porphyrin or Cu(Ⅱ)-phthalocyanine[J]. Journal of Catalysis, 2003, 217 (2): 334-342.
[11] CASTRO K A D F, LIMA F H C, SIMǒES M M Q, et al. Synthesis, characterization and catalytic activity under homogeneous conditions of ethylene glycol substituted porphyrin manganese(Ⅲ) complexes[J]. Inorganica Chimica Acta, 2017, 455: 575-583.
[12] YU D, BAI J, LIANG H, et al. AgI-modified TiO2supported by PAN nanofibers: A heterostructured composite with enhanced visible-light catalytic activity in degrading MO[J]. Dyes and Pigments, 2016, 133: 51-59.
[13] MA Y, WANG X, JIA Y, et al. Titanium dioxide-based nanomaterials for photocatalytic fuel generations[J]. Chemical Reviews,2014, 114(19): 9987-10003.
[14] WANG C, YANG G M, LI J,et al. Novel meso-substituted porphyrins: Synthesis, characterization and photocatalytic activity of their TiO2-based composites[J]. Dyes and Pigments, 2009, 80(3): 321-328.
[15] WANG C, LI J, MELE G, et al. The photocatalytic activity of novel, substituted porphyrin/TiO2-based composites[J]. Dyes and Pigments, 2010, 84(2): 183-189.
[16] 刘莹, 杨毅华, 刘守信. 提高 TiO2光催化处理废水效率的研究进展[J]. 河北科技大学学报, 2014, 35 (1): 58-63.
LIU Ying, YANG Yihua, LIU Shouxin. Ways to enhance the efficiency of TiO2photocatalytic in wastewater treatment and the research progress[J]. Journal of Hebei University of Science and Technology, 2014, 35(1): 58-63.
[17] ZHAO X, LIU X, YU M M, et al. The highly efficient and stable Cu, Co, Zn-porphyrineTiO2photocatalysts with heterojunction by using fashioned one-step method[J]. Dyes and Pigments, 2017, 136: 648-656.
[18] LYU X F, QIAN H, MELEC G, et al. Impact of different TiO2samples and porphyrin substituents on the photocatalytic performance of TiO2/copper porphyrin composites[J]. Catalysis Today, 2017, 281: 45-52.
[19] KANG I J, WANG LW, HSU S J, et al. Design and synthesis of indole, 2,3-dihydro-indole, and 3,4-dihydro-2H-quinoline -1-carbothioic acid amide derivatives as novel HCV inhibitors[J]. Bioorganic and Medicinal Chemistry Letters,2009, 19(15): 4134-4138.
[20] BODENDIEK S B, MAHIEUX C, HANSEL W, et al. 4-Phenoxybutoxy-substituted heterocycles:A structure-activity relationship study of blockers of the lymphocyte potassium channel Kv1.3[J]. European Journal of Medicinal Chemistry, 2009, 44(5): 1838-1852.
[21] KANG M S, OH J B, ROH S G, et al. Novel extended π-conjugated dendritic Zn(II)-porphyrin derivatives for dye-sensitized solar cell based on solid polymeric electrolyte: Synthesis and characterization[J]. Bulletin of the Korean Chemical Society, 2007, 28(1): 33-40.
[22] SANTOS L J, CARVALHODA-SILVA D, REBOUC J S, et al. Synthesis of new porphyrin/fullerene supramolecular assemblies: A spectroscopic and electrochemical investigation of their coordination equilibrium in solution[J]. Tetrahedron, 2011, 67(1): 228-235.
[23] SUN W J, LI J, YAO G P, et al. Surface-modification of TiO2-with new metalloporphyrins and their photocatalytic activity in the degradation of 4-notrophenol[J]. Applied Surface Science, 2011, 258(2): 940-945.
[24] MELE G, GARCIA-LOPEZ E, PALMISANO L, et al. Photocatalytic degradation of 4-nitrophenol in aqueous suspension by using polycrystalline TiO2-impregnated with lanthanide double-decker phthalocyanine complexes[J]. The Journal of Physical Chemistry C, 2007, 111 (17):6581-6588.
[25] 吕向菲.不同外围取代基卟琳和金属卟琳的合成及其修饰TiO2的研究 [D]. 西安:西北大学,2011.
LYU Xiangfei, Study on the Synthesis of Different Substituents Porphyrin and Metal Porphyrin and Modification of TiO2[D]. Xi’an: Northwest University, 2011.
Study on the synthesis of novel TiO2-copper porphyrin catalyst and photocatalytic degradation of methyl orange
CHEN Shaorui
(School of Science, Hebei University of Science and Technology, Shijiazhuang,Hebei 050018, China)
In order to study the effect of different length side chain catalysts on photocatalytic degradation of methyl orange solution, solving the poroblem of water pollution control, four novel porphyrins and their corresponding copper complexes are synthesized from the starting material 1-naphthol, and their structures are characterized by MS, NMR and elemental analysis. Novel TiO2-porphyrins hybrid systems are prepared and its photocatalytic activity is investigated by photodegradation of methyl orange in aqueous solution under visible light. The results indicate that when there are side chains on the benzene ring of copper-porphyrin derivatives, the photocatalytic activity of substituted TiO2-copper porphyrins is better than TiO2-copper tetraphenyl porphyrin, but the effect of the side chains' length on the activity is not obvious. This study provides an idea for the synthesis of highly efficient catalysts in the future.
organic photochemistry;naphthol;porphyrins;synthesis;methyl orange;photocatalysis
O69
A
2017-06-12;
2017-08-16;责任编辑:张士莹
河北省自然科学基金(B2012208036);河北科技大学五大平台项目(1182120)
陈韶蕊(1971—),女,河北赵县人,副教授,博士,主要从事有机合成方面的研究。
E-mail:sjz_wgq@126.com
陈韶蕊.新型TiO2-铜卟啉催化剂的合成及其光催化甲基橙降解研究[J].河北科技大学学报,2017,38(5):453-459.
CHEN Shaorui.Study on the synthesis of novel TiO2-copper porphyrin catalyst and photocatalytic degradation of methyl orange [J].Journal of Hebei University of Science and Technology,2017,38(5):453-459.
