One-Pot Synthesis of Hierarchically Nanoporous SSZ-13 for Conversion of Methanol to olefins
2017-11-01LiYupingWangYanyueZhangYiLiuRuiLiXiaofengDouTao
Li Yuping; Wang Yanyue; Zhang Yi; Liu Rui; Li Xiaofeng; Dou Tao,3
(1 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024;2 Research Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024;3 CNPC Key Laboratory of Catalysis, College of Chemical Engineering, China University of Petroleum, Beijing 102249)
One-Pot Synthesis of Hierarchically Nanoporous SSZ-13 for Conversion of Methanol to olefins
Li Yuping1; Wang Yanyue1; Zhang Yi1; Liu Rui1; Li Xiaofeng2; Dou Tao2,3
(1 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024;2 Research Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024;3 CNPC Key Laboratory of Catalysis, College of Chemical Engineering, China University of Petroleum, Beijing 102249)
The main disadvantage of microporous SSZ-13 catalyst used in the methanol to olefins (MTO) process is its rapid deactivation due to its relatively low coke resistance. Meanwhile, the hierarchical zeolites usually exhibit improved catalytic stability thanks to their better mass transfer ability. Herein, the hierarchically nanoporous SSZ-13 zeolites were one-pot synthesized by using N,N,N-trimethyl-1-adamantanammonium hydroxide as a microporous structure directing agent and C18H37N+(CH3)2C6H12N+(CH3)2C6H13(Br-)2(hereinafter abbreviated as C18-6-6Br2) as a mesoporogen. The hierarchically nanoporous SSZ-13 catalyst was characterized by XRD, N2physisorption, SEM, TEM, TG-DTG,27Al and29Si NMR spectroscopy and NH3-TPD techniques. The results showed that the hierarchical SSZ-13 zeolite synthesized in the presence of the C18-6-6Br2surfactant exhibits aggregates of primary nanocrystals and contains the well-developed mesopores and excellent acidity. Compared to its conventional counterpart, the hierarchical SSZ-13 zeolite has longer catalytic lifetime and higher selectivity for ethylene and propylene in the MTO reaction, which can be attributed to the synergistic effect of their good acidity and improved diffusion properties resulted from the hierarchical pore structure.
hierarchical zeolites; SSZ-13; template synthesis; mesopore; methanol-to-olefins
1 Introduction
Methanol can be expediently produced via syngas from the abundant nonpetroleum sources such as coal, natural gas, and biomass[1-2]. The conversion of methanol to olefins (MTO) has become an increasingly important alternative to naphtha cracking units for providing light olefins[3-4].In the earliest MTO studies, zeolites with 8-membered ring (8-MR) channels attracted much attention because they exhibited high selectivity for ethylene and propene in the MTO reaction[5-6]. Among the 8-MR zeolites,silicoaluminophosphate zeotype SAPO-34 with a CHA topology is a well-known excellent catalyst for MTO reaction[7-8]. The aluminosilicate analog of SAPO-34 is SSZ-13, the synthesis of which was first reported by Zones in 1985[9]. However, there are few studies describing the detailed catalytic MTO reactions over this zeolite, which has been attracting attention because of its relatively low coke resistance[10]. Accordingly, improving the coking resistance of H-SSZ-13 can make the catalyst more suitable for industrial applications.In recent years, the hierarchical zeolites have been synthesized by constructing mesopores in zeolites in order to improve the diffusion properties and catalytic stability of the zeolite phase[11-12]. There are several strategies for introducing mesoporosity including the soft templating methods, the hard templating methods and the post synthesis modification by steaming, and the acid leaching or alkaline treatment[13-14]. One approach that has been used to improve the catalytic performance of H-SSZ-13 is the desilication by alkaline treatment[10]. However, even though the mesoporosity is introduced into the material,the desilicated H-SSZ-13 still shows a shorter lifetime than the parent H-SSZ-13 zeolite in the MTO reaction.
Hensen, et al.[15-17]synthesized the mesoporous SSZ-13 by using N,N,N-trimethyl-1-adamantanammonium hydroxide (TMADaOH) as a structure-directing agentof the zeolite and a diquaternary ammonium-type surfactant ([C22H45N+(CH3)2C4H8N+(CH3)2C4H9]Br2) as a mesopore-generating soft template. This resulted in the introduction of mesopores into the H-SSZ-13 crystals,which could provide better accessibility to the micropores and a substantial decrease in coke formation during the MTO reaction. Related diquaternary ammonium surfactants have earlier been used to synthesize the ZSM-5 zeolite nanosheets[18]. Hensen, et al.[19]also prepared the hierarchical SSZ-13 using another amphiphilic surfactant containing a mono-quaternary ammonium head group(C16H33-[N+methyl piperidine]) as a soft template to replace some of the TMADaOH in the SSZ-13 synthesis gel. This produced highly mesoporous SSZ-13, which also showed a remarkably improved catalytic performance in the MTO reaction as compared to the bulk SSZ-13. In addition, the synthesis of hierarchical CHA zeolites using organosilane surfactants (CH3O)3Si-C3H6-N+(CH3)2CnH2n+1(n=12, 16 or 18) as a mesopore-generating agent was also reported by Hensen[15]and Ryoo’s groups[20]. However, there are hardly any other reports that are related to the hierarchical SSZ-13 materials until now.
In this work, we expand on soft templating studies by investigating the influence of another diquaternary ammonium-type surfactant of C18-6-6Br2as the mesoporogen in the synthesis of hierarchical SSZ-13 with the purpose of further verifying its template effect.The surfactant was composed of a long-chain alkyl group(C18) and two quaternary ammonium groups spaced by a C6alkyl linkage, and its different alkyl chain and linker length might produce some influence on the mesopore size and mesopore volume of the hierarchical SSZ-13 zeolite. The structural, textural, and acidic properties of the hierarchical SSZ-13 were scrutinized in detail by XRD, electron microscopy, N2physisorption, NMR, and NH3-TPD techniques. The catalytic properties of these hierarchical zeolites for the MTO reaction were compared with a conventional SSZ-13 zeolite.
2 Experimental
2.1 Synthesis of hierarchical SSZ-13 (HS)
The diquaternary ammonium surfactant C18H37-N+(CH3)2-C6H12-N+(CH3)2-C6H13(Br-)2(C18-6-6Br2for brevity) was prepared following a synthesis procedure reported in the literature[18].
In a typical procedure, sodium hydroxide (0.21 g),25% dilute N,N,N-trimethyl-1-adamantanammonium hydroxide solution (TMADaOH, 4.3 g), and deionized water (26 g) were mixed together. Then sodium aluminate(0.3 g) with 41.3% of Al2O3was added to this solution under vigorous stirring. When the solution cleared up,C18-6-6Br2and silica sol (10 mL) with 26 wt% of SiO2were added to the mixture. The precursor mixture was then stirred for another 3 h at 60 °C. The resulting gel with a molar composition of 4Na2O: 1.0Al2O3: 40SiO2:1760H2O: 4TMADaOH: 2C18-6-6Br2was then transferred into a Teflon-lined autoclave and kept at 160 °C for 4 d. The final product was collected by filtration, washed with distilled water and dried overnight at 100 °C. The synthesized material was then calcined in air at 550 °C for 8 h. The protonated form of the sample was obtained via ion exchange with 1 mol/L NH4NO3solution at 90 °C for 3 h, followed by calcination in static air at 550 °C for 5 h. The product is denoted as HS. For comparison,a conventional SSZ-13 zeolite was synthesized using the same gel composition and procedure, except that no C18-6-6Br2was added. This sample is denoted as CS.
2.2 Characterization
The powder X-ray diffraction (XRD) patterns were recorded on a Rigaku D/max-2500 diffractometer with CuKα radiation (36 kV, 30 mA). The scanning electron microscopy (SEM) images were obtained on a TESCAN MIRA 3 LMH scanning electron microscope. The zeolite samples were dispersed on carbon tapes and images were taken with a thin gold film. The transmission electron microscopy (TEM) pictures were taken on a JEOL JEM-2010 electron microscope operating at 200 kV. The N2adsorption-desorption isotherms were recorded at 77 K with a BELSORP-max apparatus. Before measurements,samples were degassed at 473 K for 8 h. The surface area of samples was calculated using the Brunauer-Emmett-Teller (BET) method in a p/p0range of 0.01—0.1. The pore size distribution of samples was calculated from the adsorption branch of the isotherms using the Barret-Joyner-Halenda (BJH) method. The thermogravimetric(TG) and differential thermogravimetric (DTG) analyses were performed on a Rigaku TG Plus 8102 thermal analysis instrument at a heating rate of 10oC/min under an air flow of 30 mL/min. The temperature-programmed desorption of ammonia (NH3-TPD) measurements were carried out on a TL 5000 II analyzer equipped with a thermal conductivity detector. The29Si and27Al MAS NMR spectra of the calcined zeolite samples were recorded on a Bruker Avance III multinuclear solid-state magnetic resonance spectrometer at 99.33 MHz and 156.39 MHz, respectively. The27Al chemical shift was referenced to an aqueous solution of Al(NO3)3and the29Si chemical shift to tetramethylsilane (TMS).
2.3 Catalytic activity measurements
The MTO reactions were carried out in a fixed-bed microreactor at 450 °C under atmospheric pressure. A total of 1 g of catalyst mixed with an equivalent volume of quartz(20—40 mesh) was loaded into the center of a stainless steel reactor (Φ10 mm×300 mm). Prior to the reaction, the catalyst was activated at 450°C in air for 2 h. The weight hourly space velocity (WHSV) was 2 h-1. The analysis of the reaction products was performed using an on-line gas chromatograph (GC-950) equipped with a flame ionization detector and the Agilent PoraPLOT Q capillary columns.
3 Results and Discussion
3.1 Characterization of the hierarchical zeolite SSZ-13
The XRD patterns of the zeolite samples are shown in Figure 1a. The samples exhibit a typical CHA diffraction pattern and no crystalline reflections belonging to other crystalline phases are present. Additionally, it is observed that the HS sample exhibits lower peak intensity and conspicuous line broadening in all reflections than that of the CS sample, which may suggest the presence of smaller crystalline domains in the hierarchical sample in regard to their conventional-sized counterpart (CS).
Figure 1b shows an expanded view of the diffraction patterns from 20° to 23°. Compared to CS, the major peak of HS in this range shifts to higher value. This phenomenon may be attributed to a decreased cell parameter due to the increase of Si/Al ratio[21], which can be further confirmed by subsequent Si-NMR measurements. This would indicate that the addition of C18-6-6Br2causes a slight variation in the framework environment of the hierarchical samples.
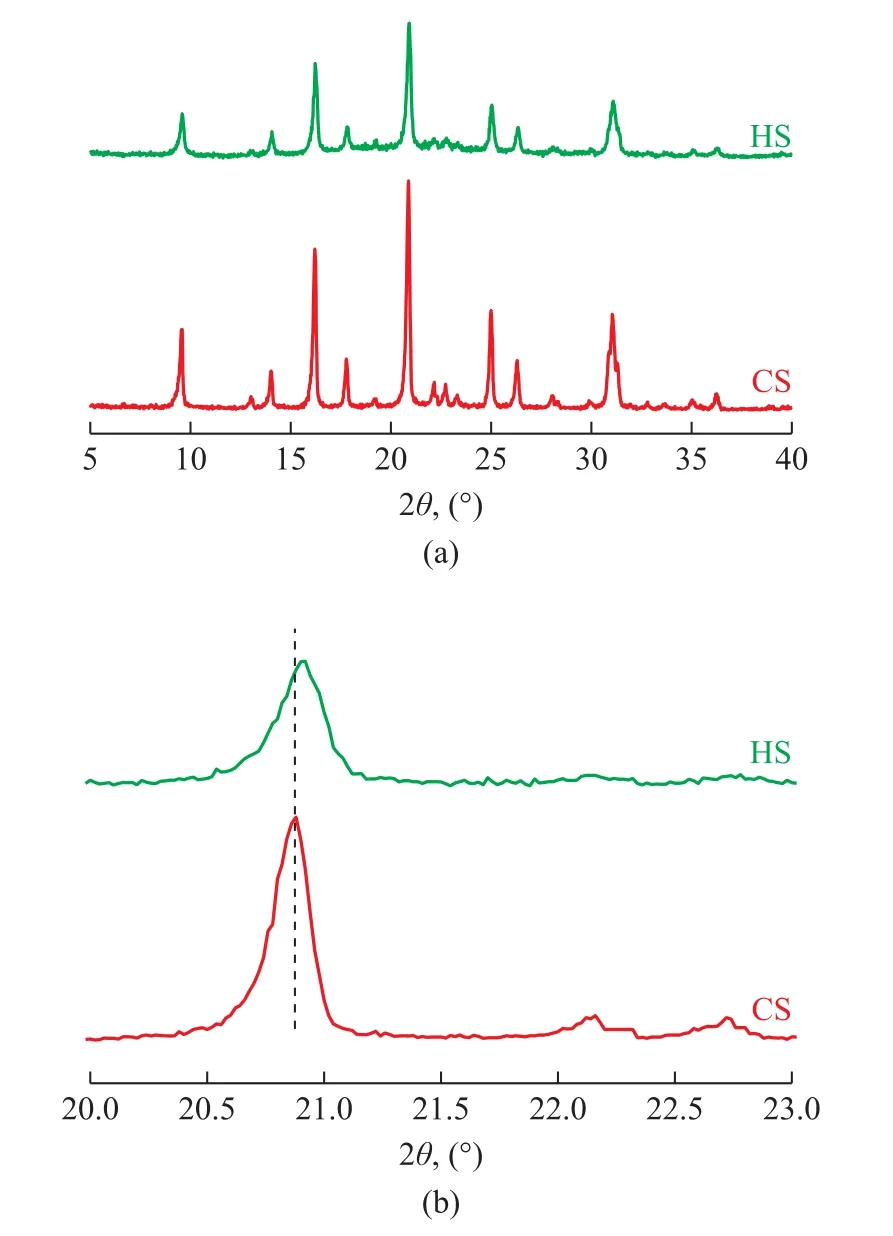
Figure 1 XRD patterns of as-synthesized CS and HS samples (b is the partial enlarged figure of a)
The N2adsorption-desorption isotherms of the samples are shown in Figure 2a. Notably, the CS displays a type-I isotherm without an obvious hysteresis loop, which is usually observed for microporous materials that have relatively low external surface areas. However, the isotherm of the HS sample is a combination of type I and type IV isotherms,which is a characteristic feature of hierarchical materials[22].The high N2uptake by the HS sample at p/p0< 0.2 indicates the existence of micropores. Moreover, the hysteresis loop at higher relative pressures (p/p0> 0.4) also suggests that the mesopores have been introduced into the sample.
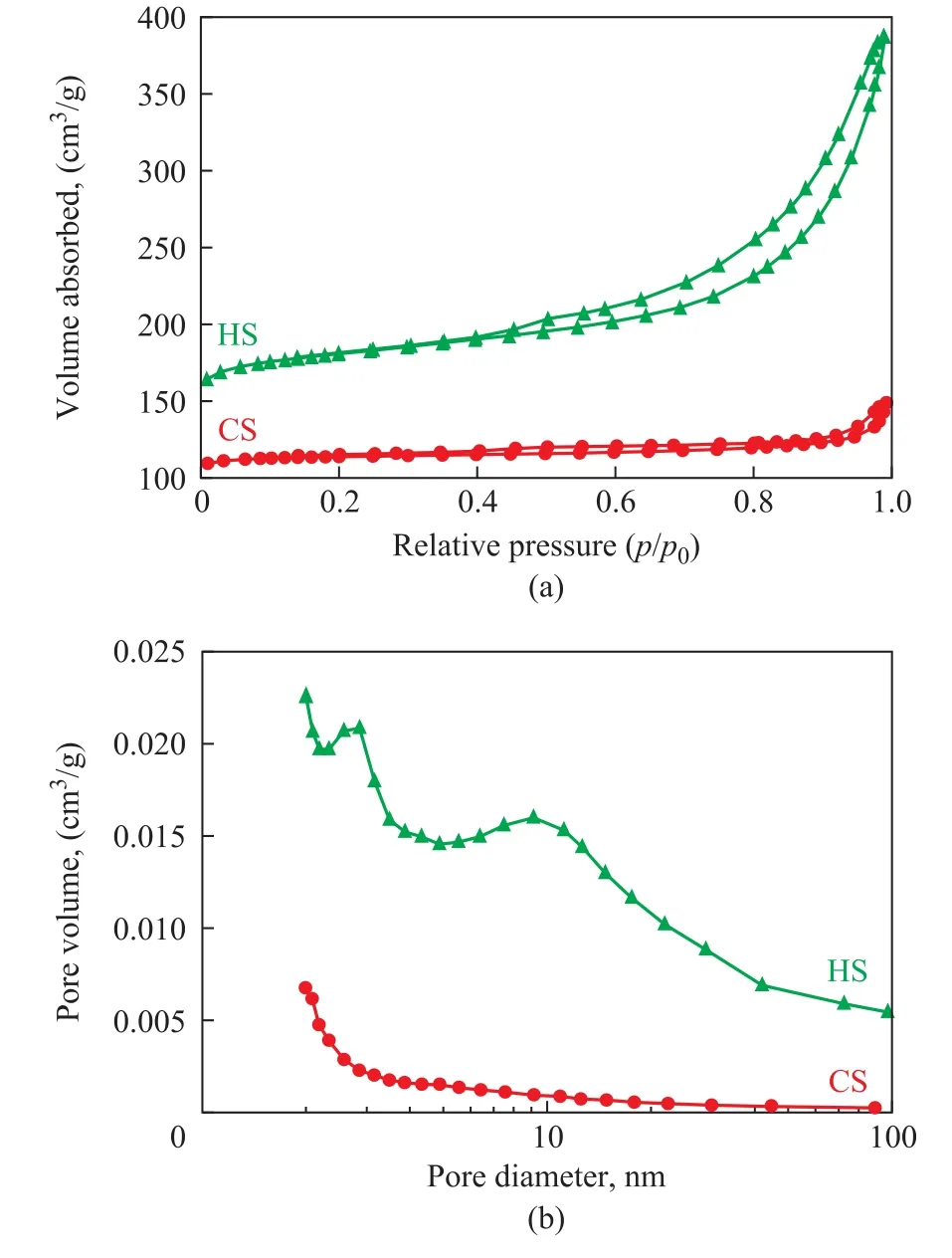
Figure 2 Nitrogen adsorption-desorption isotherms (a)and pore size distributions (b) of CS and HS
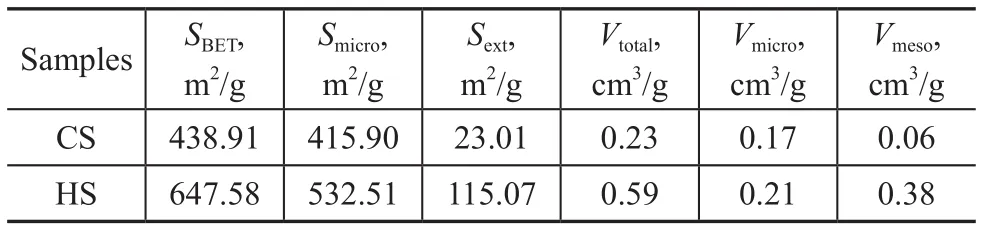
Table 1 Textural parameters of the calcined CS and HS samples
According to the BJH analysis from the adsorption branch,the pore sizes of the HS sample are centered at 2.83 nm and 9.22 nm, indicating that the HS sample does possess hierarchical structure. In contrast to the HS sample, the CS sample only has a negligible amount of mesopores. Table 1 summarizes the textural parameters of the samples. The total BET surface area is higher for the HS (647.58 m2/g) than that for CS (438.91 m2/g). Furthermore, the external surface area (115.07 m2/g) and the mesopore volume (0.38 cm3/g)in HS are much greater than those in CS (23.01 m2/g and 0.06 cm3/g, respectively). These results suggest that adding diquaternary ammonium-type surfactant (C18H37-N+(CH3)2-C6H12-N+(CH3)2-C6H13, labeled as C18-6-6hereinafter) in the synthesis gel can produce sufficient interactions with the zeolite precursors to introduce abundant mesoporosity into the final zeolite sample. Hierarchically structured zeolite SSZ-13 with large external surface area and mesopore volume is believed to be capable of enhancing the mass transport for reactants and products molecules[12].The thermogravimetric analysis (TGA) was used to investigate the effect of the C18-6-6surfactant. The TG-DTG curves of synthesized CS and HS samples are presented in Figure 3. The CS sample had a 2.62% weight loss below 180 °C, which could occur due to the desorption of physically absorbed water. The corresponding weight loss in the HS samples (<180 °C) was only 1.90%. This weight loss might be attributed to the occupation of the spaces of porous material by the C18-6-6surfactant[23].The weight loss of the HS samples in the temperature range from 180 °C to 400 °C supports the aforementioned assumption. The steep weight loss in the temperature range from 180 °C to 290 °C occurred due to the decomposition of the C18-6-6surfactant, and the weight loss in the temperature range from 290 °C to 400 °C was likely associated with the further removal of any organic residue[23]. The weight loss occurring in the temperature range from 180 °C to 400 °C for the HS sample was 16.61%, while the weight loss in this region for CS was only 3.02%. This result indicates that a large amount of C18-6-6surfactant has definitely participated in the assembling of hierarchical structure of SSZ-13. For both the CS and HS samples, the weight loss in the temperature range from 400 °C to 730 °C is resulted from the removal of N,N,N-trimethyl-1-adamantanammonium (TMADa+)hydroxide. The TMADa+hydroxide is removed at a lower temperature in HS (~470 °C) as compared to the case for CS (at 490 °C). This indicates that there is an effortless elimination by combustion and weak interaction with the hierarchical structure.The SEM images of all samples are shown in Figure 4.The CS sample (Figure 4a and 4c), is composed of predominantly nearly cubical particles with dense and smooth external surfaces. The particles are fairly large with a diameter ranging from 13 μm to 17 μm. For the HS sample, there are obvious changes in the morphology and size of the crystals. The HS sample (Figure 4b and 4d) exhibits the well-defined spherical-shape crystals with a decreased diameter of 8 μm and rougher external surface, which are assembled from small primary zeolite nanocrystals that can form more mesoporous voids between the primary particles.These results indicate that C18-6-6plays a key role in the three-dimensional assembly of HS. The hydrophilic diquaternary ammonium groups may attract the zeolite precursors due to electrostatic interactions between the ammonium groups and the inorganic aluminosilicate species, and the zeolite precursors will be directed onto the surfaces of the C18-6-6micelles[23]. During the crystallization process, the C18-6-6micellae are embedded into the zeolite crystals. Simultaneously, the hydrophobic tails linked to the surface of the silica are also integrated into the zeolite framework, which can block the crystal growth. This phenomenon can lead to the formation of cavities among the nano-crystals. After calcination, the cavities are transformed into intercrystalline mesopores[19].The TEM images of the HS sample are shown in Figure 5.
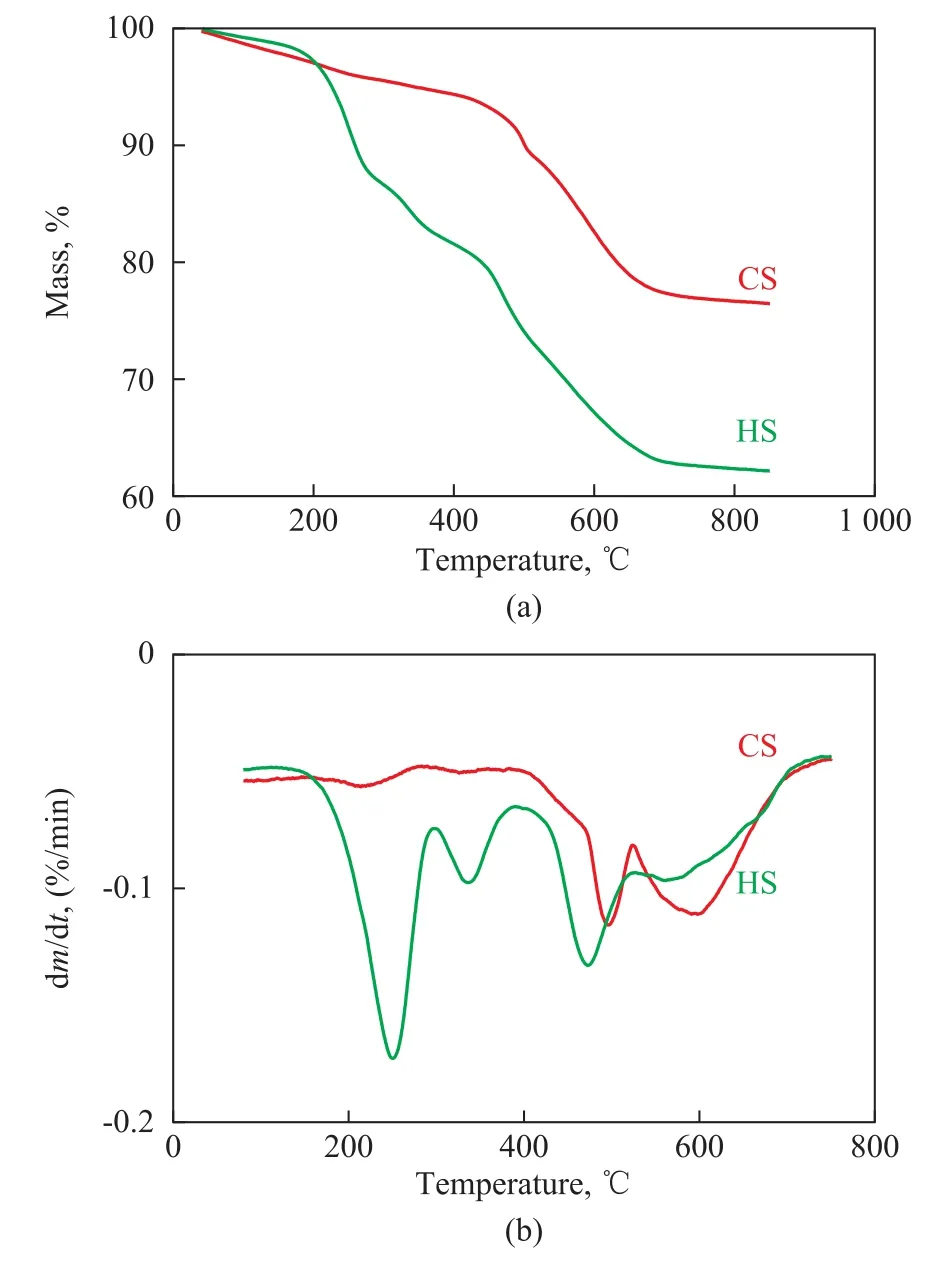
Figure 3 Thermogravimetry (TG, a) and differential thermogravimetry (DTG, b) curves of the synthesized CS and HS samples
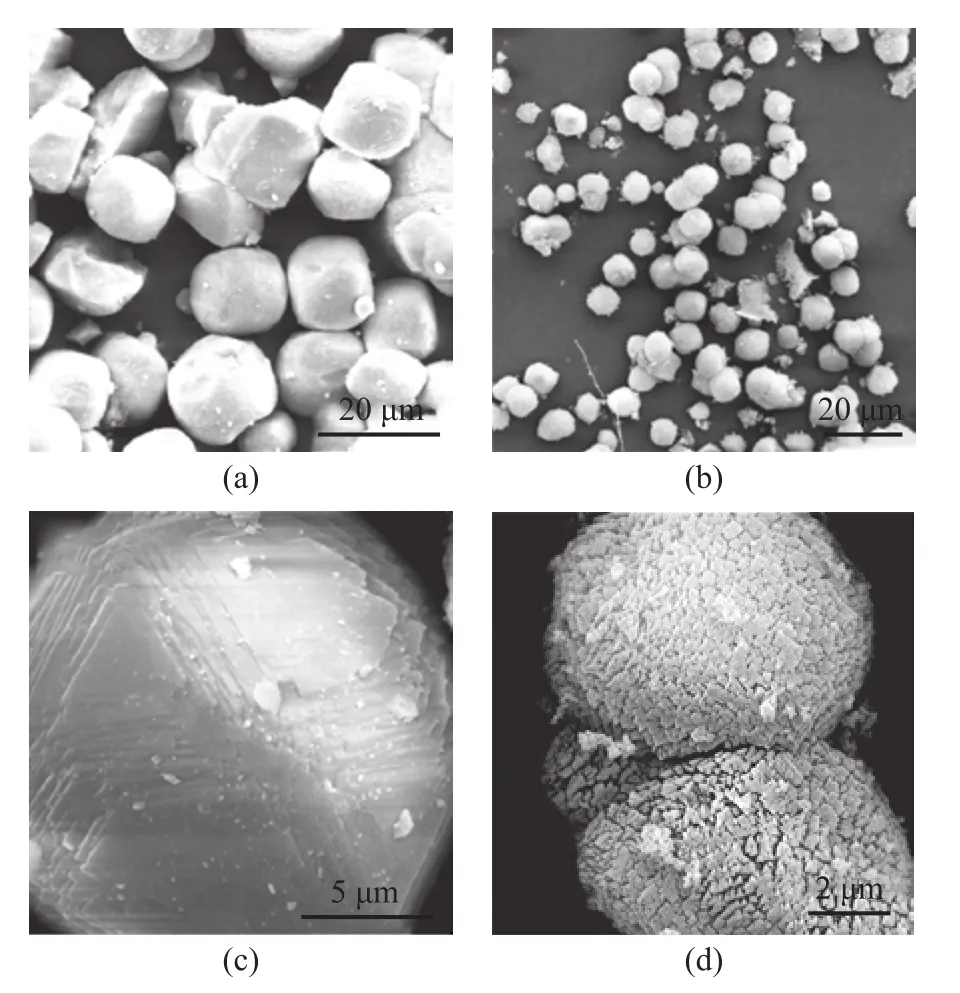
Figure 4 SEM images of CS (a and c) and HS (b and d) samples
The images show that the particles are composed of uniformly arranged nanocrystals, which are in agreement with the SEM results. In addition, the presence of mesoporosity on the surface and edges of the HS crystals is clear (Figure 5a and 5c). The mesopores are less than 10 nm in diameter, which is in accordance with the previous N2sorption isotherm test. The presence of mesopores in the HS crystals could enhance the diffusion of substrates through the material in catalytic reactions.
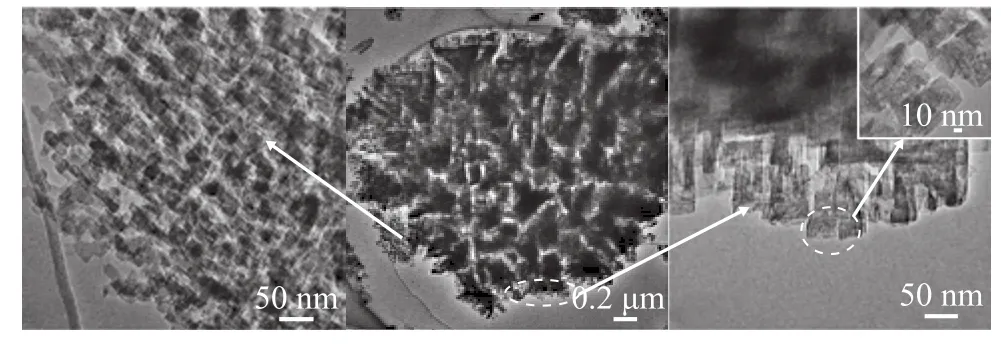
Figure 5 TEM images of the HS sample (a, b and c)
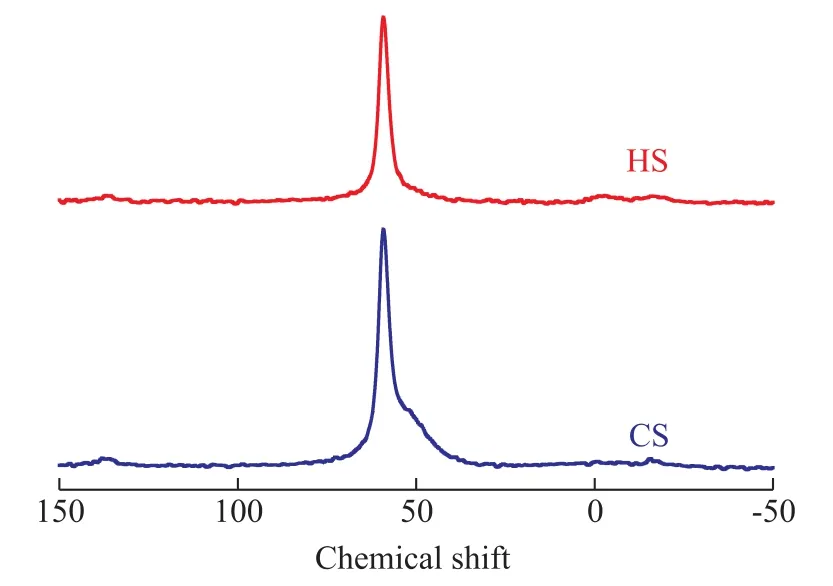
Figure 6 27Al MAS NMR spectra of CS and HS samples
The MAS NMR spectroscopy was used to determine the coordination of the Si and Al atoms, to verify if the Al atoms are associated with the Si atoms at the T-positions,and to calculate the Si/Al ratios of synthesized zeolites.The27Al MAS NMR spectra of CS and HS samples are shown in Figure 6. The pronounced resonance band at 59 indicates that the Al atoms reside predominantly in the CS and HS frameworks[24]. The absence of a peak at 0 in both samples also suggests that there is no non-framework octahedrally coordinated Al, which demonstrates that Al is almost entirely incorporated into the framework of the samples.The29Si MAS NMR spectra and the relative distribution of Si(nA1) units for CS and HS samples are presented in Figure 7. The resonance peaks around -101, -105, and-111 can be assigned to Si (2Si, 2Al), Si (3Si, 1Al) and Si(4Si, 0Al) sites, respectively[24-27]. The peak areas of these resonances were obtained from the deconvolution of the29Si MAS NMR spectra, and the framework Si/Al ratios of the two samples were calculated based on these areas[28].The results showed that the HS sample has higher Si/Al ratio (10.5) than the CS sample (8.7), which is in good agreement with the XRD results. After crystallization, it was observed that the pH value of the mother liquor for HS (9.98) was lower than that for CS (11.24), indicating that the increased Si/Al ratio for HS might be attributed to the influence on gelling and crystallization process of SSZ-13 zeolites from this diquaternary ammonium surfactant.The changes in the acidic properties of a material can be evaluated using the temperature-programmed desorption of ammonia. The integral of the profiles can give the information about the number of acidic sites,and the shift in the peak temperature is related to the strength of the acidic sites. The NH3-TPD profiles of the CS and HS samples are presented in Figure 8. All the samples have two NH3desorption peaks with the maximum values identified at ca. 220 °C and 490 °C.These peaks can be assigned to the weak and strong acid sites, respectively[29]. As shown in Table 2, the weak acid peak is identified at a slightly lower temperature in the HS sample than the peak of the CS sample. However,the high-temperature desorption peak of the HS sample is con firmed at a higher temperature as compared to the CS sample. The number of acid sites was estimated from the peak areas by assuming one NH3molecule per acid site. Obviously, the HS sample had more total and strong acid sites than the CS sample, which might be attributed to its higher micropore surface areas, abundant external surface areas (Table 1) and the more accessible catalytic active sites. This trend of change in acid sites is similar to our previous studies using C18-6-6Br2as a porogen of hierarchical mordenite[30].
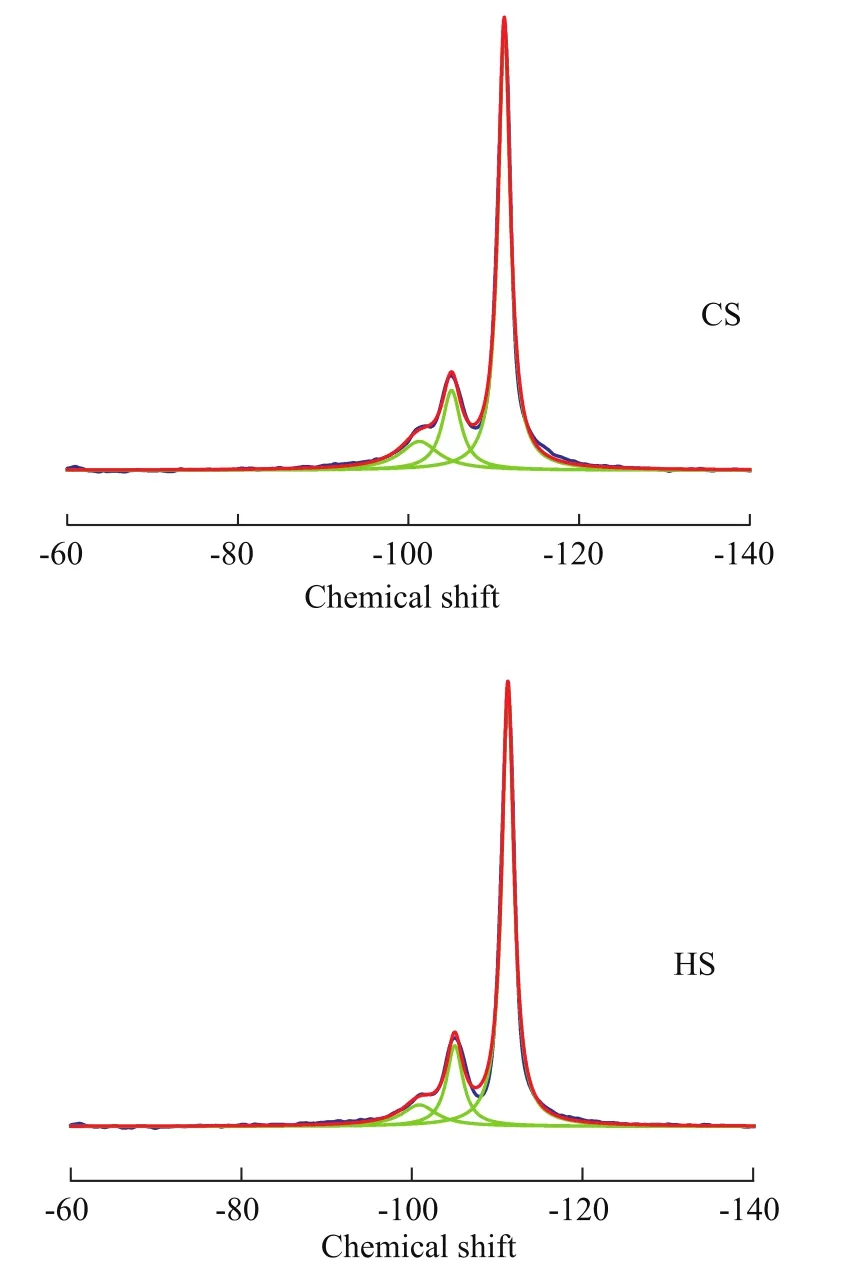
Figure 7 29Si MAS NMR spectra of CS and HS samples
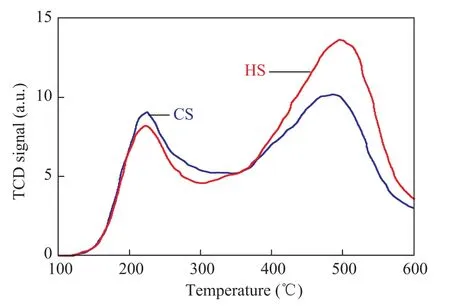
Figure 8 The NH3-TPD pro files of CS and HS samples
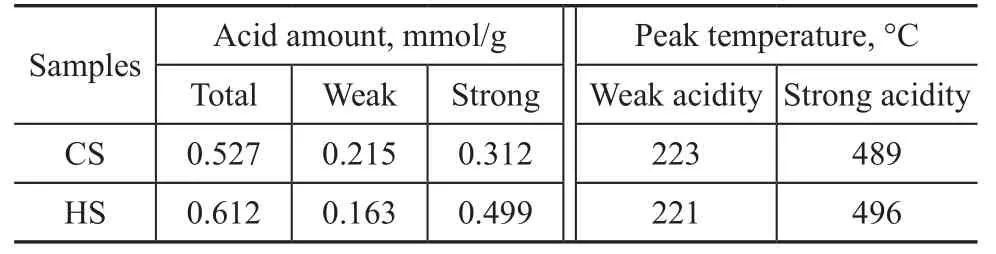
Table 2 Acidity data from NH3-TPD pro files
3.2 Catalytic activity measurements
The catalytic activity of the zeolite samples was then tested for the MTO reaction. Figure 9 shows the methanol conversion as a function of time on stream and Table 3 gives the corresponding product distribution (with the values being determined when methanol conversion began to decrease from 100%). Both the CS and HS catalysts could almost completely convert the methanol feed initially and then the catalytic activity gradually decreased with the time on stream. Catalyst lifetime is defined as the time when the methanol conversion decreased to 80%. It is noted that the lifetime of HS that reached 86 min was more than two times longer than that of CS (39 min). The selectivity (82.71%) for ethylene and propylene achieved by the HS samples was by 18.73%more than that achieved by the CS sample (63.98%).
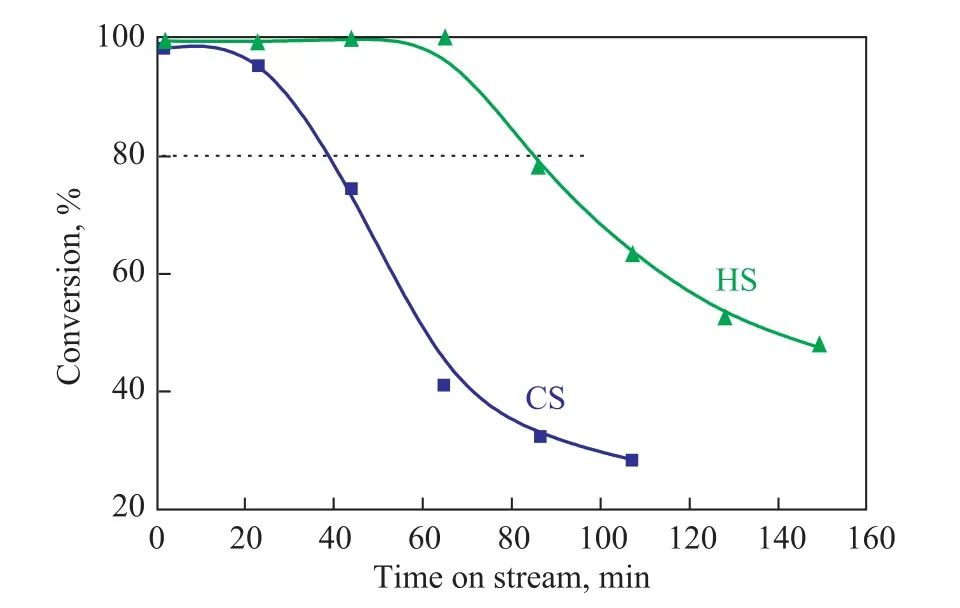
Figure 9 Conversion of methanol over the samples in the MTO reaction
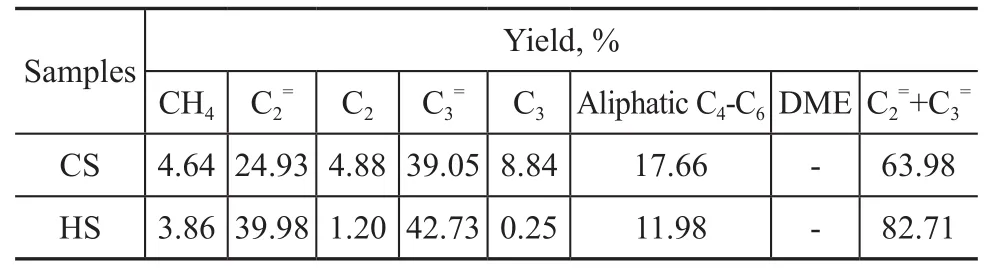
Table 3 Product distribution over the SSZ-13 zeolites during the MTO reaction
In view of the difference of the acidity and textural properties between the hierarchical and conventional SSZ-13, the lifetime enhancement achieved by the hierarchical SSZ-13 sample might be mainly attributed to the synergistic effect of their hierarchical pore structure and good acidity because it is usually thought that the decreased acidic strength and concentration of zeolites could retard the coke formation and thus prolong the catalytic lifetime[31]. The mesoporous voids between the primary particles in the hierarchical SSZ-13 zeolite could significantly facilitate the mass transfer and increase the accessibility to the catalytically active sites in the microporous channels, which might help to prolong the catalytic lifetime and improve the product selectivity.
It is well known that the coke formation is often responsible for the deactivation of catalytic sites[32-33].TGA was also done on the spent HS and CS catalysts after the reaction (over 150 min and 108 min, respectively)(Figure 10), and there was less coke found on the HS catalyst (15.4%) than on the CS catalyst (17.8%). The slightly lower coke deposition after a longer reaction time for the HS catalyst is probably attributed to its hierarchical structure. This structure can facilitate the diffusion of the coke precursors from the narrow micropores to the outside surface of the catalyst during the MTO reaction, which reduces the chance of coke formation. In contrast, in the CS catalyst, the reaction products have to diffuse through a much longer path to escape from the microporous channels. The products would be more likely to become trapped and could eventually form coke[34-36]. As for the higher selectivity for olefins, the hierarchical porosity can significantly improve the catalytic selectivity due to the enhanced diffusion efficiency[37-38]. Wang P. F., et al. have also found out that SAPO-34 with small crystallite size and large external surface area exhibited a better olefins selectivity except for the case of longer lifetime in MTO reaction[33]. In addition, the higher Si/Al ratio in the HS sample may also lead to better olefins selectivity[39].
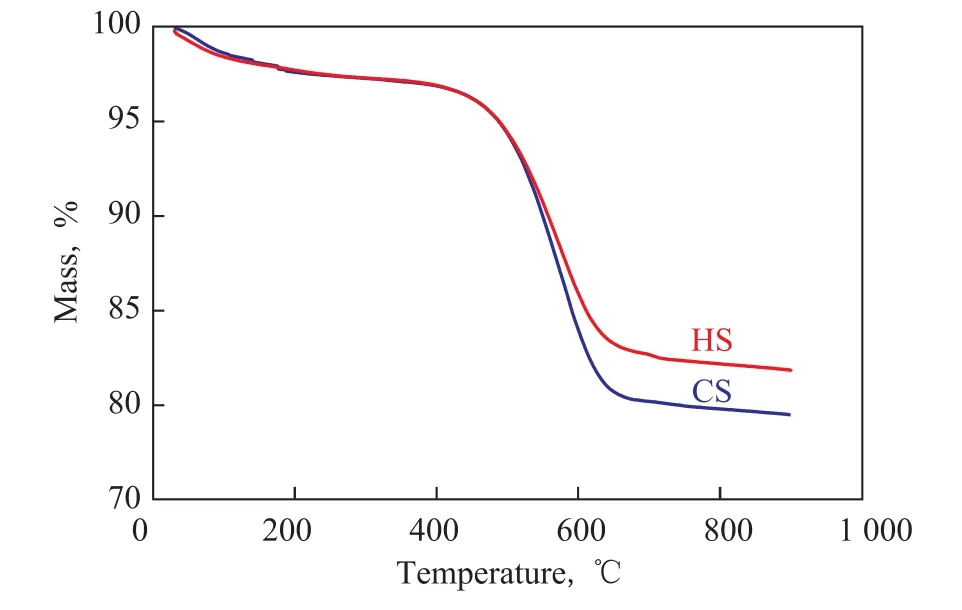
Figure 10 TGA curves of the coked CS and HS catalysts after the reaction
4 Conclusions
The hierarchical SSZ-13 zeolite was synthesized using TMADaOH and C18-6-6Br2diquaternary ammoniumtype surfactant as the microporous and the mesoporous templates, respectively. The hierarchical SSZ-13 zeolite displays the aggregates of nanocrystals with coarse surface,which leads to more intercrystalline voids between the nanocrystals. Compared to the conventional counterpart,the hierarchical SSZ-13 sample has much larger external surface area and mesopore volume in the porous networks.The HS catalyst exhibited superior MTO performance with a catalytic lifetime of about two times longer than that of CS and improved the selectivity for light olefins(C2H4+ C3H6) by 18.74% more than that of CS. This phenomenon can be attributed to the synergistic effect of their hierarchical pore structure and excellent acidity. The developed mesoporosity can increase the accessibility to the catalytically active sites in the microporous channels and minimize the diffusion path length of the coke precursors out of the micropores. Judging clearly from its improved catalytic stability and selectivity for light olefins, HS is a better catalyst for MTO reactions than the conventional microporous SSZ-13 zeolite.
Acknowledgments: The authors gratefully acknowledge the National Natural Science Foundation of China (No. 51371123),the Natural Science Foundation of Shanxi Province (No.201701D121024), and the Research Project Supported by Shanxi Scholarship Council of China (No. 2017-042) for providing financial support for this study.
[1] Wang Q, Wang L, Wang H, Li Z X, et al. Synthesis,characterization and catalytic performance of SAPO-34 molecular sieves for methanol-to-olefin (MTO) reaction[J].Asia-Paci fic Journal of Chemical Engineering, 2011, 6 (4):596-605
[2] Xiang Y Y, Zhou J S, Lin BW, et al. Exergetic evaluation of renewable light olefins production from biomass via synthetic methanol[J]. Applied Energy, 2015, 157: 499-507
[3] Park J W, Kim S J, Seo M, et al. Product selectivity and catalytic deactivation of MOR zeolites with different acid site densities in methanol-to-olefin (MTO) reactions[J].Applied Catalysis A: General, 2008, 349(1): 76-85
[4] Martin N, Li Z B, Martinez-Triguero J, et al.Nanocrystalline SSZ-39 zeolite as an efficient catalyst for the methanol-to-olefin (MTO) process[J]. Chemical Communications, 2016, 52(36): 6072-6075
[5] Hereijgers B P C, Bleken F, Nilsen M H, et al. Product shape selectivity dominates the Methanol-to-Olefins(MTO) reaction over H-SAPO-34 catalysts[J]. Journal of Catalysis, 2009, 264(1): 77-87
[6] Bleken F, Bjørgen M, Palumbo L, et al. The effect of acid strength on the conversion of methanol to olefins over acidic microporous catalysts with the CHA topology[J].Topics in Catalysis, 2009, 52(3): 218-228
[7] Li J, Wei Y X, Liu G Y, et al. Comparative study of MTO conversion over SAPO-34, H-ZSM-5 and H-ZSM-22:Correlating catalytic performance and reaction mechanism to zeolite topology[J]. Catalysis Today, 2011, 171: 221-228
[8] Tian P, Wei Y X, Ye M, et al. Methanol to olefins (MTO):From fundamentals to commercialization[J]. ACS Cataysis,2015, 5(3): 1922-1938
[9] Zones S I. Zeolite SSZ-13 and its method of preparation:The United States, US 4544538[P]. 1985
[10] Sommer L, Mores D, Svelle S, et al. Mesopore formation in zeolite H-SSZ-13 by desilication with NaOH[J].Microporous and Mesoporous Materials, 2010, 132: 384-394
[11] Zhou J, Hua Z L, Liu Z C, et al. Direct synthetic strategy of mesoporous ZSM-5 zeolites by using conventional block copolymer templates and the improved catalytic properties[J]. ACS Catalysis, 2011, 1(4): 287-291
[12] Chen L H, Li X Y, Rooke J C, et al. Hierarchically structured zeolites: Synthesis, mass transport properties and applications[J]. Journal of Materials Chemistry, 2012,22(34): 17381-17403
[13] Verboekend D, Pérez-Ramírez J. Desilication mechanism revisited: highly mesoporous all-silica zeolites enabled through pore-directing agents[J]. Chemistry - A European Journal, 2011, 17(4): 1137-1147
[14] Na K, Choi M, Ryoo R. Recent advances in the synthesis of hierarchically nanoporous zeolites[J]. Microporous and Mesoporous Materials, 2013, 166: 3-19
[15] Wu L L, Degirmenci V, Magusin P C, et al. Dual template synthesis of a highly mesoporous SSZ-13 zeolite with improved stability in the methanol-to-olefins reaction[J].Chemical Communications, 2012, 48: 9492-9494
[16] Wu L L, Degirmenci V, Magusin P C M M, et al.Mesoporous SSZ-13 zeolite prepared by a dual-template method with improved performance in the methanol-toolefins reaction[J]. Journal of Catalysis, 2013, 298: 27-40
[17] Zhu X C, Hofmann J P, Mezari B, et al. Trimodal porous hierarchical SSZ-13 zeolite with improved catalytic performance in the methanol-to-olefins reaction[J]. ACS Catalysis, 2016, 6(4): 2163-2177
[18] Choi M, Na K, Kim J, et al. Stable single-unit-cell nanosheets of zeolite Mfi as active and long-lived catalysts[J]. Nature, 2009, 461 (7261): 246-249
[19] Zhu X C, Rohling R, Filonenko G, et al. Synthesis of hierarchical zeolites using an inexpensive mono-quaternary ammonium surfactant as mesoporogen[J]. Chemical Communications, 2014, 50(93):14658-14661
[20] Kim J, Jo C, Lee S, et al. Bulk crystal seeding in the generation of mesopores by organosilane surfactants in zeolite synthesis[J]. Journal of Materials Chemistry A,2014, 2(30): 11905-11912
[21] Delprato F, Delmotte L, Guth J L, et al. Synthesis of new silica-rich cubic and hexagonal faujasites using crownether-based supramolecules as templates[J]. Zeolites, 1990,10(6): 546-552
[22] Yuan E H, Tang Z C, Mo Z L, et al. A new method to construct hierarchical ZSM-5 zeolites with excellent catalytic activity[J]. J Porous Mater , 2014, 21(6): 957-965
[23] Liu B Y, Tan Y Z, Ren Y Q, et al. Fabrication of a hierarchically structured beta zeolite by a dual-porogenic surfactant[J]. Journal of Materials Chemistry, 2012, 22 (35):18631-18638
[24] Eilertsen E A, Arstad B, Svelle S, et al. Single parameter synthesis of high silica CHA zeolites from fluoride media[J]. Microporous and Mesoporous Materials, 2012,153: 94-99
[25] Wang J C, Peng Z L, Chen Y, et al. In-situ hydrothermal synthesis of Cu-SSZ-13/cordierite for the catalytic removal of NOx from diesel vehicles by NH3[J]. Chemical Engineering Journal, 2015, 263: 9-19
[26] Xu L, Du A P, Wei Y X, et al. Synthesis of SAPO-34 with only Si(4Al) species: Effect of Si contents on Si incorporation mechanism and Si coordination environment of SAPO-34[J]. Microporous and Mesoporous Materials,2008, 115(3): 332-337
[27] Yun J H, Lobo R F. Effects of temperature pretreatment on propane cracking over H-SSZ-13 zeolites[J]. Catalysis Science & Technology, 2015, 5(93): 264-273
[28] Fyfe C A, Feng Y, Grondey H, et al. One- and twodimensional high-resolution solid-state NMR studies of zeolite lattice structures[J]. Chemical Reviews, 1991,91(7): 1525-1543
[29] Kim D J, Wang J, Crocker M. Adsorption and desorption of propene on a commercial Cu-SSZ-13 SCR catalyst[J].Catalysis Today, 2014, 231: 83-89
[30] Li Y P, Sun C J, Fan W B, et al. One-pot synthesis of hierarchical mordenite and its performance in the benzylation of benzene with benzyl alcohol[J]. Journal of Materials Science, 2015, 50(14): 5059-5067
[31] Sun Q M, Ma Y H, Wang N, et al. High performance nanosheet-like silicoaluminophosphate molecular sieves:synthesis, 3D EDT structural analysis and MTO catalytic studies[J]. Journal of Materials Chemistry A, 2014, 2(42):17828-17839
[32] Freiding J, Kraushaar-Czarnetzki B. Novel extruded fixedbed MTO catalysts with high olefin selectivity and high resistance against coke deactivation[J]. Applied Catalysis A: General, 2011, 391: 254-260
[33] Wang P F, Yang D X, Hu J, et al. Synthesis of SAPO-34 with small and tunable crystallite size by two-step hydrothermal crystallization and its catalytic performance for MTO reaction[J]. Catalysis Today, 2013, 212: 62.e1-62.e8
[34] Rownaghi A A, Rezaei F, Hedlund J. Uniform mesoporous ZSM-5 single crystals catalyst with high resistance to coke formation for methanol deoxygenation[J]. Microporous and Mesoporous Materials, 2012, 151: 26-33
[35] Milina M, Mitchell S, Crivelli P, et al. Mesopore quality determines the lifetime of hierarchically structured zeolite catalysts[J]. Nature Communications, 2014, 5: 3922
[36] Sun Q M, Wang N, Xi D Y, et al. Organosilane surfactantdirected synthesis of hierarchical porous SAPO-34 catalysts with excellent MTO performance[J]. Chemical Communications, 2014, 50(4): 6502-6505
[37] Li B, Hu Z J, Kong B, et al. Hierarchically tetramodalporous zeolite ZSM-5 monoliths with template-freederived intracrystalline mesopores[J]. Chemical Science,2014, 5(4): 1565-1573
[38] Kim J, Choi M, Ryoo R. Effect of mesoporosity against the deactivation of Mfi zeolite catalyst during the methanolto-hydrocarbon conversion process[J]. Journal of Catalysis,2010, 269: 219-228
[39] Yuen L T, Zones S I, Harris T V, et al. Product selectivity in methanol to hydrocarbon conversion for isostructural compositions of Afiand CHA molecular sieves[J].Microporous and Mesoporous Materials, 1994, 2: 105-117
date: 2017-04-01; Accepted date: 2017-08-26.
Dr. Li Yuping, Telephone: +86-18636860353, E-mail: yupingli123@163.com.
杂志排行
中国炼油与石油化工的其它文章
- Thermal Decomposition Behavior of Terephthalate in Inert Gas
- Molecular Simulation of Competitive Adsorption on Fe(110) Between Gasoline Detergent and Deposit: I. Physical Adsorption
- Characterization and Apparent Kinetics of Polymerization of 1-Decene Catalyzed by Boron Trifluoride/Alcohol System
- Preparation of Cu-, Zn-, Co-Zeolites and Application for Adsorptive Desulfurization of Saudi Arabian Medium Crude
- Study on Con fined Impinging Jet Mixer and Mechanism offlash Nanoprecipitation
- Antimicrobial Degradation Performance of Novel Polyacrylamide Derivatives by Microbial Consortia for Enhanced Oil Recovery
