Characterization and Apparent Kinetics of Polymerization of 1-Decene Catalyzed by Boron Trifluoride/Alcohol System
2017-11-01WangSihanWangLiboLiuTongMaLiliHuoHongliangWangJian
Wang Sihan; Wang Libo; Liu Tong; Ma Lili; Huo Hongliang; Wang Jian
(1. Daqing Petrochemical Research Center of PetroChina, Daqing 163714;2. Northeast Petroleum University, Daqing 163318)
Characterization and Apparent Kinetics of Polymerization of 1-Decene Catalyzed by Boron Trifluoride/Alcohol System
Wang Sihan1,2; Wang Libo1; Liu Tong1; Ma Lili1; Huo Hongliang1; Wang Jian2
(1. Daqing Petrochemical Research Center of PetroChina, Daqing 163714;2. Northeast Petroleum University, Daqing 163318)
Poly-α-olefin (PAO) synthetic oil is the base oil of high-quality lubricants, and has a huge market potential.We illustrate PAO synthesis by catalytic polymerization of 1-decene with a boron trifluoride (BF3)/alcohol system. Gas chromatography-mass spectrometry, proton nuclear magnetic resonance and13C nuclear magnetic resonance analysis confirmed dimer, trimer and tetramer structures of PAO. Each component contained branched chains with a 1,2 insertion of a head-to-tail link; a 2,1 insertion of a tail-to-tail link and a methyl-containing linked structure. At a low conversion rate, the reaction rate was related directly with the reaction temperature and the catalyst/1-decene concentration. An apparent kinetic equation for PAO formation was determined during 1-decene polymerization.
poly-α-olefin; 1-decene; boron tri fluoride; product structure; apparent kinetics
1 Introduction
Poly-α-olefin (PAO) synthetic oils are base oils of highquality lubricants with a high viscosity index, a low volatility, a low liquidity, a strong shearing resistance,and a high-temperature oxidation resistance[1-2], which are always in short supply. Low-viscosity PAO (with a viscosity in the range of 1—10 mm2/s at 100 °C)has gained the highest market share and accounts for more than 80% of the total demand. The Ziegler-Natta catalyst system, metallocenes and cationic catalysts are the main catalytic systems for PAO synthesis[3-5].Metallocene catalyzed 1-decene polymerization yields mainly tetrameric and pentameric PAO with a high kinematic viscosity, which is only suitable for highviscosity PAO formation[6-7]. Although the Ziegler-Natta system catalyzed 1-decene polymerization produces PAO of a low kinematic viscosity, it has a wider product distribution[8-10].
Boron trifluoride (BF3) cationic catalytic systems in which alcohol acts as an initiator are most suitable for the low-viscosity PAO formation with a low pour point and a high viscosity index[11-12].
The degree of polymerization, the branching degree and the atomic packing modes determine the PAO properties.Jiang, et al.[13]characterized 1-decene polymerization with a metallocene catalyst rac-Et(1-Ind)2ZrCl2/C6H5NH(CH3)2B(C6H5)4Al(i-Bu)3by proton nuclear magnetic resonance (1H-NMR) spectrometry and13C nuclear magnetic resonance (13C-NMR) spectrometry,and showed that the polymerization could yield a regular fragment structure. Huang[14]and Sheng, et al.[15]investigated PAO products formed in different catalytic systems by gas chromatography-mass spectrometry (GCMS) and13C-NMR spectrometry, and demonstrated that PAO products consisted mainly of trimers and tetramers,and many linked isomers existed in the molecular structure.
Compared with the structural study, a kinetic investigation of PAO is more complex and limited. Jiang,et al.[13]studied the kinetics of 1-decene polymerization and established a kinetic model by using a monomer concentration and relative molecular weight of the polymer, and combined these data with a material balance.The reaction rate, number-average molecular weight andweight-average molecular weight were predicted by this model. By using an aluminum chloride (AlCl3)/ethanol catalytic system for 1-decene oligomerization, Wu, et al.[16]studied the effect of temperature on the reaction rate. The apparent activation energy was calculated and a simplified dynamic equation for 1-decene oligomerization was proposed.
By using a BF3/alcohol catalytic system, we reported the PAO synthesis via 1-decene polymerization, its product characterization and apparent kinetics. The kinetic equation for PAO synthesis from 1-decene polymerization was established by exploring the relationship between the density and the reaction conversion rate. The kinetic equation is of significant importance to verify the PAO structure and to control the polymerization reaction.
2 Experimental
2.1 Materials and methods
BF3was 99% pure obtained from the Qike Chemical Co., Ltd (Zibo, Shandong, China). 1-Decene and alcohol were 99.5% pure obtained from the Aladdin Reagent Co.NaOH was 99% pure obtained from the Boke Chemical Co., Ltd (Cangzhou, Hebei, China). The reagents were used without further purification.
The kinematic viscosity was determined using a CAV 2200 automatic kinematic viscosity analyzer (Cannon Instrument Company, Mexico, USA). Pour point was determined on a DSY-006A petroleum products pourpoint/cloud-point tester (North Dalian Analytical Instrument Co., Ltd., Dalian, China). NMR spectra were recorded with a VNMRS-400 MHz spectrometer (Varian Company, CA, USA) operating at 400 MHz and 25 °C in CDCl3with tetramethyl silane (TMS) serving as an internal reference. The GC-MS analyses were performed on a 6890 GC-5973 MSD gas chromatograph-mass spectrometer with an Agilent 19091S-001 column (Agilent Technologies, Santa Clara, CA, USA).
2.2 General procedure for 1-decene oligomerization
1-Decene oligomerization was carried out in a 1-L stainless-steel reactor equipped with mechanical stirring. The reactor was heated under vacuum at 160 °C for 2 h and then was cooled down to room temperature. The reactor was purged with nitrogen for 10 min. The desired amount of 1-decene and the alcohol initiator was added to the reactor at a 100:1 molar ratio under stirring. At a specified reaction temperature, the BF3catalyst was introduced in the reactor, and the system pressure was maintained at 0.4 MPa. The reaction temperature was controlled by a cooling system. Sampling and analysis were conducted at regular intervals. After the completion of reaction,the reaction material was released and the residual catalyst was recycled. The product was transferred to a 500-mL round-bottomed flask and washed to neutral pH value with a 5% NaOH aqueous solution. The final PAO product was obtained by vacuum distillation to remove the monomer.
3 Results and Discussion
3.1 Structural and property relationships of PAO product
3.1.1 Product properties
The kinematic viscosity, viscosity index and pour point of the PAO products were determined, and the property indicators of the PAO were compared with the same type of product from a foreign company. The results in Table 1 show that the kinematic viscosity of PAO was 4.3 cSt(in the low-viscosity range) and 2318 cSt at 100 °C and-40 °C, respectively. The high-viscosity index reached 132 and the pour point was -60 °C. Its viscositytemperature properties and the low-temperature performance were better than the reference product.

Table 1 Comparison of main performance indicators of PAO products
3.1.2 GC-MS analysis
The polymerization products were characterized by GCMS, with the results shown in Figure 1. Five peaks at different retention times are visible in the GC spectrum.Peaks at 0—5 min, 5—10 min, 10—17 min, 17—22 min,and 22—25 min are attributed to monomer, dimer, trimer,tetramer and pentamer, respectively, and their mass fractions were 4.60%, 10.55%, 62.40%, 17.54% and 4.91%,respectively. The GC-MS results showed that the products were mainly composed of dimmer, trimer and tetramer with a small amount of monomer and high polymer.

Figure 1 GC-MS spectrum of PAO product
3.1.3 1H-NMR characterization
Purified monomer, dimer, trimer, tetramer and pentamer were obtained from a true boiling point cutting of the PAO product. The1H-NMR spectra (Figure 2) show signals for -CH3groups in the product fractions and PAO. The measured values of -CH3(Table 2) were greater than the theoretical values reported by Shubkin[17].The increased amount of branches was attributed to the rearrangement of active species in BF3·HA that initiated 1-decene polymerization.
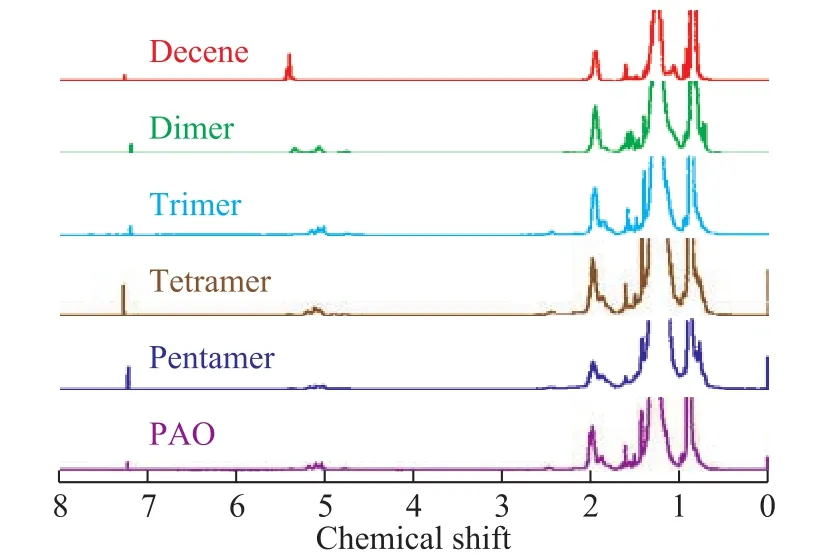
Figure 2 1H-NMR spectra of fractions

Table 2 Number of methyl groups in each fraction
3.1.4 13C-NMR characterization
The trimer content was the major PAO product formed during 1-decene oligomerization. After hydrogenation, the trimer was characterized by13C-NMR spectrometry. Results showed that trimer existed as three kinds of linked isomers, covering a 1,2 insertion of a head-to-tail link, a 2,1 insertion of a tail-to-tail link, and a methyl-linked structure (a,b, c), as shown in Figures 3 and 4. The analysis for the chemical shift of the carbon atoms was consistent with the calculated value[18]. The trimer skeleton of the head-to-tail link (a) has two kinds of carbon atoms(10 and 18), and their chemical shifts were 30.96 and 39.45, respectively. The chemical shift of the tertiary carbon atom (17) in the tail-to-tail link isomer (b)was 39.15. The methyl-linked isomer (c) contained a tertiary carbon atom (17) and a quaternary carbon atom (13), with a chemical shift of 34.12. These results show that the molecular structure of the trimer contained five methyl groups, which were consistent with the results of characterization made by1H-NMR spectrometry.

Figure 3 13C-NMR spectrum of trimer

Figure 4 Trimer structures
3.2 Relationship between density and conversion rate
The density of the reaction system varied with the monomer conversion rate. The monomer content and reaction-system density were determined at different reaction times, and the monomer conversion rate was calculated.

Figure 5 Relationship between density and conversion
The system density increased with an increasing monomer conversion as shown in Figure 5. By using the least-squares method for the experimental data, a linear equation was obtained as shown below:

in which ρ is the density and X is the conversion rate.
Equation (1) shows that the system density is related directly with the conversion rate. The apparent kinetic equation was established under low-conversion-rate conditions because a high-viscosity system has a blocking effect on the diffusion process[19].
3.3 Fitting of apparent kinetics equation
The reaction rate depends mainly on the 1-decene concentration, the active-center concentration and the reaction temperature. The fitting of kinetic equations is based mainly on the following three equations:

where R is the reaction rate, mol/(L·s); M is the 1-decene consumption, mol/L; t is the reaction time, s; k is the reaction rate constant; Ccais the catalyst concentration,mol/L; Cmis the 1-decene concentration, mol/L; a is the order of the catalyst concentration; b is the order of the 1-decene concentration; A is the pre-exponential factor;Ea is the activation energy, J/mol; R is the gas constant,and T is the reaction temperature, K.
Here, the R is calculated by the concentration of 1-decene consumed in unit time. And we assumed that the entire catalyst was activated into the active center because the concentration of activated catalyst can hardly be determined.
3.3.1 Effect of 1-decene concentration on kinetics
To elucidate the relationship between the 1-decene concentration and the polymerization performance in our system, the concentration of 1-decene was changed from 3.70 mol/L to 4.40 mol/L at a constant temperature of 30 °C and a catalyst concentration of 0.02 mol/L as shown in Table 3.
Equation (5) is obtained by taking the logarithm of Equation (3).

At a constant reaction temperature and a constant catalyst concentration, Equation (5) is reduced to Equation (6).


Table 3 Effect of 1-decene concentration on the reaction rate
By fitting the experimental data in Equation 6, a linear relationship is obtained between logR and logCm, which shows that the reaction rate decreases with a decreasing monomer concentration and vice versa (Figure 6). The slope of Equation (6) is 2, which implies that R exhibited a second-order dependence on the 1-decene concentration.

Figure 6 Relationship between logR and logCm
3.3.2 Effect of catalyst concentration on kinetics
To investigate the influence of catalyst concentration on 1-decene polymerization for PAO synthesis, the catalyst concentration was changed from 0.01 mol/L to 0.03 mol/L at a constant temperature of 30 °C and a 1-decene concentration of 3.7 mol/L, as shown in Table 4.

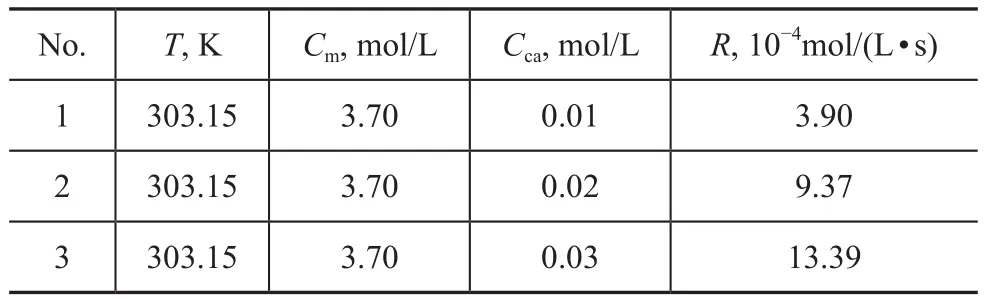
Table 4 Effect of catalyst concentration on reaction rate
For a constant reaction temperature and a 1-decene concentration, Equation (5) reduces to Equation (7).By fitting the experimental data in Equation (7), a linear relationship is obtained between logR and logCca(Figure 7), which shows that the average reaction rate decreases with a decreasing catalyst concentration and vice versa.The slope of Equation (7) is 1.1, which implies a firstorder dependence of R on the catalyst concentration.

Figure 7 Relationship between logR and logCca
3.3.3 Effect of reaction temperature on kinetics
Reaction temperature plays an important role in polymerization, and can affect the rate of chain initiation,chain propagation and chain termination. Temperature affects the catalytic behavior and product properties simultaneously.
Equation (8) is obtained by taking the logarithm of Equation (4).

To investigate the temperature influence on the performance of the 1-decene polymerization for PAO synthesis, the results in Table 5 were obtained by changing the reaction temperature from 30 °C to 50 °C,while the concentration of 1-decene (3.7 mol/L) and catalyst (0.02 mol/L) were kept constant.
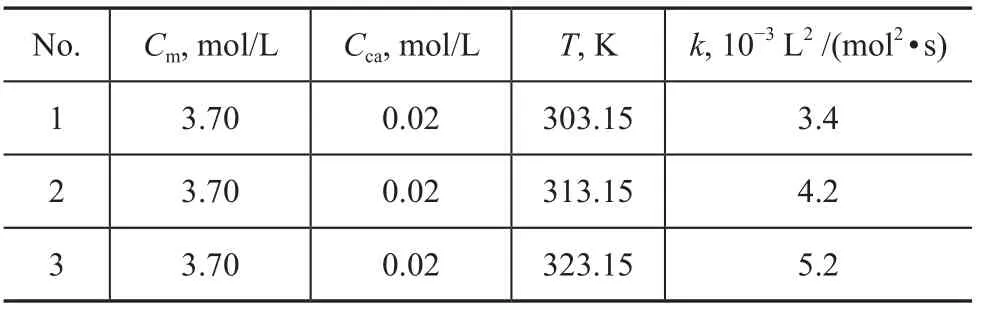
Table 5 Effect of temperature on reaction rate constant
The effect of reaction temperature on the rate constant(lnk verses -T-1) is shown in Figure 8. The graph shows a linear relationship between the rate constant and the reaction temperature. By using the least-square method,values of the slope (2 110.68) and intercept (1.27) were calculated from the graph. By fitting experimental data to Equation 8, useful parameters (Ea= 17.548 kJ, A = 3.56,k = 3.56e-17548/RT) were obtained.
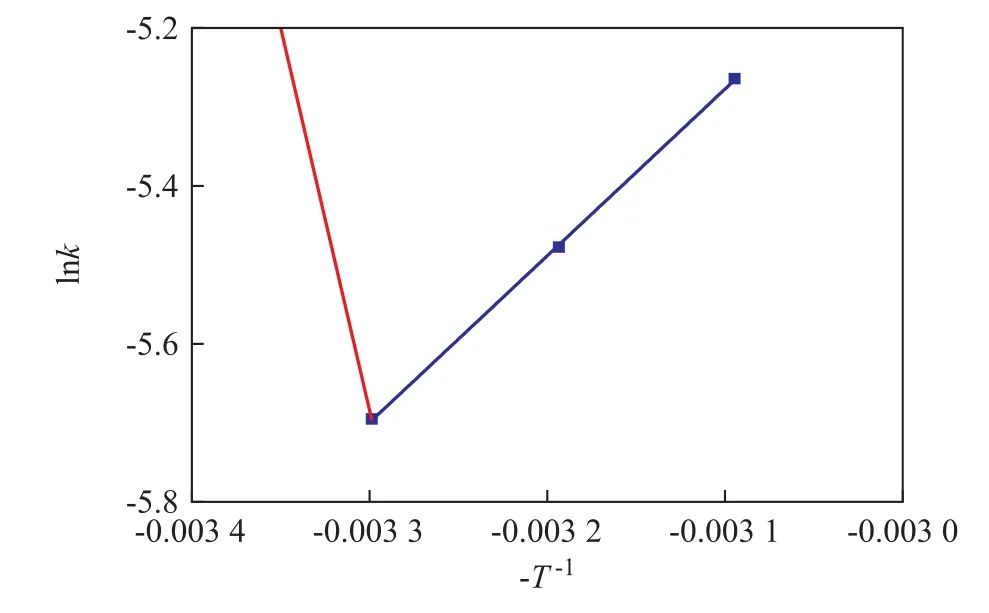
Figure 8 Relationship between lnk and -T-1
3.3.4 Validation of apparent kinetics equation
Equation (9) is the final form of the apparent kinetic equation for our system.

The average reaction rate (R) was determined experimentally under different reaction conditions and compared with the theoretical value that was calculated from the apparent kinetic equation. The experimental results showed less than a 10% deviation from the theoretical value, as shown in Table 6. The average reaction-rate deviation indicates that the experimentally determined catalytic activity also deviates less than 10% from theoretical activity. This result agrees with the dynamic model that was established by Jiang[20], and demonstrates that the obtained kinetic equation is good in practice for BF3/alcohol catalyzed synthesis of PAO during 1-decene polymerization.

Table 6 Veri fication of rate- fitting equation
4 Conclusions
PAO was synthesized through 1-decene polymerization using a BF3/alcohol catalytic system and characterized by GC-MS,1H-NMR and13C-NMR. Analysis showed that the PAO was composed mainly of dimer, trimer and tetramer, which carried numerous branched chains. The13C-NMR showed that the PAO existed in three kinds of linked structures, covering a 1,2 insertion of a head-totail link; a 2,1 insertion of a tail-to-tail link, and a linked structure containing methyl groups. The results show that the system density was related to the reaction conversion rate and increased with an increasing conversion rate.An apparent kinetic equation, R = 3.56e-17548/RT· Cca· Cm2
was established for PAO synthesis via the BF3/alcohol catalytic polymerization of 1-decene. The experimental reaction rate deviated by less than 10% from the theoretical calculated value, which could provides a high reference value for the lubricating oil field.
Acknowledgement: This work was supported by the Natural Science Foundation of China (21576048) and the PetroChina Innovation Foundation (2014D-5006-0503).
Reference
[1] Liu W M, Xu J, Feng D P, et al. The research status and prospect of synthetic lubricating oils[J]. Tribology, 2013,33(1): 91-101
[2] Zhang J T, Hou X Y, Li K W, et al. Status quo of application and processing technology of PAO (group IV)base stocks[J]. Journal of Xi’an Shiyou University (Natural Science Edition), 2007, 22(5): 52-56 (in Chinese)
[3] Zhang Y, Duan Q H, Liu Y N, et al. Progress on research of catalysts for 1-olefins oligomerization to poly (alpha)olefin[J]. Specialty Petrochemicals, 2011, 28(1): 82-86 (in Chinese)
[4] Kramer A I, Surana P, Nandpurkar P J, et al. Compiler:High viscosity poly-alpha olefins based on 1-hexene,1-dodecene and 1-tetradecene: US 2007/0225533[P].2007-09-27
[5] Yang N, Nandapurkar P J. Low viscosity poly-alpha-olefin based on 1-decene and 1-dodecene. US 7592497[P]. 2009-09-22
[6] Lü C S, Zhao J F. Synthesis and characterization of oligomers from 1-decene catalyzed by metallocene catalyst with constrained geometry[J]. Chemical Industry and Engineering Progress, 2009, 28(8): 1371-1399 (in Chinese)
[7] Wu M M, Hagemeister M P, Yang N. Process to produce polyalphaolefins. US 8513478[P]. 2013-08-20
[8] Duan M J. Study on Synthesis and Properties of Polyalpha-olefin (PAO)[D]. Jinan: Shandong University, 2012
[9] Huang Q G, Chen L G, Fu Z F, et al.1-Decene oligomerization catalyzed by Ziegler-Natta catalyst[J]. Petrochemical Technology, 2004, 33(10): 928-931 (in Chinese)
[10] Yang L. Synthesis of Poly-Alpha-olefin Used for Synthetic Lube Base Oil[D]. East China University, 2012.
[11] Zhang Z F, Ding H S, Quan C G, et al. Synthesis and properties of poly-alpha-olefin with 1-decene[J].Journal of Liaoning University of Petroleum and Chemical Technology, 2005, 25(3):24-27 (in Chinese)
[12] Cupples B L, Heilman W J, Kresge N A. Method of making alpha-olefin oligomers: US, US4045508A[P],1977-08-30
[13] Jiang H B, Wu S G. Synthesis and characterization of polymers from 1-decene catalyzed by bridged metallocene[J]. Chemical Industry and Engineering Progress, 2015, 34(4): 1088-1121 (in Chinese)
[14] Huang Q G, Chen L G, Sheng Y P, et al. Synthesis and characterization of oligomer from 1-decene catalyzed by AlCl3/TiCl4/SiO2/Et2AlCl[J]. Journal of Applied Polymer Science, 2006, 101(3): 584-590
[15] Sheng Y P, Huang Q G, Chen W, et al. Synthesis and characterization of oligomer from 1-decene catalyzed by metallocene complex/MAO[J]. Journal of Chemical Industry and Engineering China, 2007, 58(3): 759-764
[16] Wu J M, Xu S, Mi P K, et al. Reaction kinetics of 1-decene oligomerization catalyzed by AlCl3-ethanol complex[J].Petroleum Processing and Petrochemicals, 2015, 46(10):12-16 (in Chinese)
[17] Shubkin R L, Baylerian M S, Maler A R. olefin oligomer synthetic lubricants: structure and mechanism of formation[J]. Industrial & Engineering Chemistry Product Research & Development, 1980, 19(1): 15-19
[18] Grant H K, Paul G E. Carbon 13 magnetic resonance(II): Chemical shift data for the alkanes[J]. Journal of the American Chemical Society, 1964, 86(15): 2984-2990
[19] Tan T E, Dou M, Zhou M H. Principles of Chemical Engineering[M]. Beijing: Chemical Industry Press, 2006
[20] Jiang H B, Fan Z M. Kinetics of homogeneous polymerization of 1-decene over metallocene/organic boride catalytic system[J]. CIESC Journal, 2016, 67(7):2815-2823 (in Chinese)
Manufacture of Olefins through Catalytic Oxidative Coupling of Methane at Low Temperature
The East China Normal University (ECNU) has made important progress in production of olefins via low-temperature oxidative coupling of methane (OCM), with its relevant achievements published in the Journal “Scientific Progress”.
The research staffs after being inspired by the study on“manufacture of methanol through low-temperature electrochemical oxidation of methane” have inferred that effectively reducing the temperature for activation of oxygen molecules could be the “key” that would open the entrance to the low-temperature OCM reaction. Thereby they have put forward a new idea to “ignite the lowtemperature OCM reaction through activation of oxygen molecules by low-temperature chemical cycles”. They have selected a cocatalyst TiO2which can combine with MnxOyto form a compound at low temperature in order to modify the relevant catalytic material that can significantly decrease the reaction temperature from 800—900 °C to 650 °C, resulting in more than 20% increment of methane conversion and over 60% of product selectivity.
Meanwhile, this low-temperature chemical cycle can achieve a synergistic catalytic performance with sodium tungstate to realize the highly selective adjustment of the target product. The research team has also proposed the criterion for determining the threshold value relating to the catalyst lattice oxygen conversion rate, which means that under whatever reaction condition when the catalyst lattice oxygen conversion rate exceeds the threshold value this catalyst is considered to possess good catalytic activity. Furthermore,the said catalyst has good stability while retaining its activity after having smoothly operated for 500 hours at 720 °C.In the meantime, the methane conversion and ethylene selectivity remain at 26% and over 76%, respectively.
date: 2017-03-02; Accepted date: 2017-05-02.
Ma Lili, Telephone: +86-15045926375;E-mail: nepumalili@163.com.
杂志排行
中国炼油与石油化工的其它文章
- Thermal Decomposition Behavior of Terephthalate in Inert Gas
- Molecular Simulation of Competitive Adsorption on Fe(110) Between Gasoline Detergent and Deposit: I. Physical Adsorption
- Preparation of Cu-, Zn-, Co-Zeolites and Application for Adsorptive Desulfurization of Saudi Arabian Medium Crude
- Study on Con fined Impinging Jet Mixer and Mechanism offlash Nanoprecipitation
- Antimicrobial Degradation Performance of Novel Polyacrylamide Derivatives by Microbial Consortia for Enhanced Oil Recovery
- Hydrophobic and Magnetic Reduced Graphene Oxide Nanocomposite for Emulsified Oil Removal
