Study on Con fined Impinging Jet Mixer and Mechanism offlash Nanoprecipitation
2017-11-01CaoShuyanXuXuWangXiaoxiao
Cao Shuyan; Xu Xu; Wang Xiaoxiao
(School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002)
Study on Con fined Impinging Jet Mixer and Mechanism offlash Nanoprecipitation
Cao Shuyan; Xu Xu; Wang Xiaoxiao
(School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002)
Nanoparticles have been given considerable attention and applied in many fields because of their properties that are superior to and more distinct than those of conventional materials. In practice, a stable and reproducible manufacturing process is highly desirable. This review presents the flash nanoprecipitation, a new technique that can rapidly produce nanoparticles. Moreover, the mixing process, the mechanism of particle formation, and the mixer design are discussed.Furthermore, the factors controlling the size stability of the produced nanoparticles are summarised in this review.
flash nanoprecipitation; mechanism; particle stability
1 Introduction
Nanoparticles (NPs) possess one-dimensional or multidimensional nanoscale and show different properties at the nanometre scale, including the physical, chemical,distinct optical, electrical, magnetic, and pharmacological properties. These properties make NPs widely used in many applications, such as coatings[1], pigments[2],medicines[3-5]and catalysis[6].
Two main methods can be used to prepare NPs. One is called the top-down, which usually involves superfine grinding of a material to the nanometre range. The other method is called the bottom-up, which involves controlling the nanoprecipitation and self-assembly into nanoscale. However, the process which called the bottom-up is time consuming. Therefore, the flash nanoprecipitation (FNP), which is used to produce NPs,is introduced in this review. The FNP possesses the following advantages, namely: 1) the particle size can be small with an even size distribution; 2) the preparation is rapid and can save time; 3) the device is simple and easy to operate; and 4) the method is environmentally friendly.This review aims to present the advanced methods for preparing NPs through FNP. The particle size and size distribution that have been influenced by nucleation and the supersaturation kinetics are discussed, and the parameters that influence the phase of NP form are discussed.
2 Mechanism
2.1 Nucleation and growth
The classical nucleation theory is widely used in explaining the nucleation and crystallisation. Under a certain supersaturation degree, the nuclei can form primarily in the concentration of the solute molecules that can grow through aggregation. Moreover, the nuclei assume a spherical shape. The following derivation summarises the process[7-8].
The Arrhenius relationship can be used to describe the nucleation rate using the following equation:

where B refers to the nucleation rate, T is the absolute temperature, K1is a constant, k is the Boltzmann constant and ΔG is the Gibbs free energy of the nucleation. As a spherical particle, the Gibbs free energy contributes to the radius r:[8]
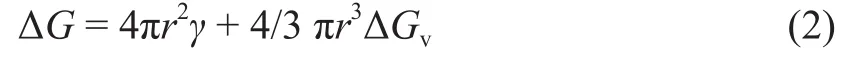
where γ is the surface tension and ΔGvis the Gibbs free energy in the formation of bulk phase. The ΔGvchanges when the critical nucleus radius rcvaries:

The particle size is inversely proportional to the maximum bulk free energy.
The nucleation rate is linked to the supersaturation of the solution. Supersaturation is a state wherein the solute concentration exceeds the solubility under a certain pressure and temperature. Nevertheless, no precipitation occurs. Supersaturation (S) is de fined as follows:

where C is the solubility of the solute at a certain particle radius, and C∞is the equilibrium solubility. Mixing the organic and aqueous phases is necessary under homogeneous supersaturation. Fewer nuclei are observed at low supersaturation than in high supersaturation environments. The following relationship can be obtained using the above equations:In the classical nucleation theory, the precipitation is closely related with the solute concentration (Figure 1)[9]:
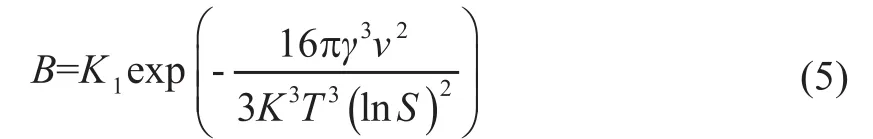
Figure 1 Schematic representation of the concentration relationships in controlled particle formation according to the model representation of LaMer[9]
When the concentration continues to increase beyond the critical nucleation concentration, the nuclei will begin to form and grow. High supersaturation allows the growth of a large amount of nuclei; therefore, supersaturation is important for FNP.
2.2 Spinodal decomposition
In the classical nucleation theory, nuclei that form and grow are widely used. However, the spinodal and binodal decomposition exists in the phase program, and the metastable region should be discussed.
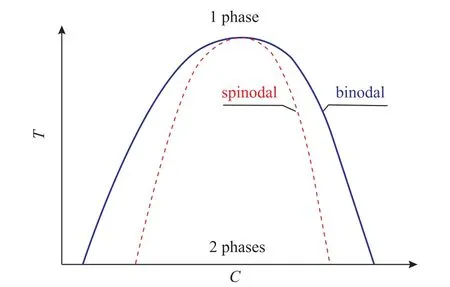
Figure 2 Phase diagram of a two component system with miscibility gap[10]
This type of phase transformation is called spinodal decomposition. In the phase program, no apparent phase boundary is observed, and a miscibility gap exists.Moreover, the supersaturation phase spreads towards the direction of the increasing concentration gradient. The boundary of the unstable region is called the bimodal, and the spinodal region is inside (Figure 2)[10]. The spinodal and binodal curves reach a critical point where the spinodal decomposition may occur. High supersaturation takes place in the FNP process; therefore, the borderline to spinodal decomposition can be crossed, and the phase separation occurs spontaneously without any actual nuclei.
Spinodal decomposition can be contrasted with the nucleation and growth. Critical nucleation energy (ΔG)is essential in the former process which is driven by the thermodynamic force. The process can be remarkably slow because the energy barrier is difficult to reach.However, the spinodal decomposition is controlled by a dynamic force and may be too fast to be observed.
2.3 Thermodynamic self-assembly
In FNP, an amphiphilic diblock copolymer and an organic active drug are dissolved in an organic solvent and mixed rapidly using an antisolvent. Johnson[11]discussed the mechanism of self-assembly in the block copolymer NPs.Several key components, including the mixing time and the copolymer selection, have been proposed. The mixing time must be rapid and less than the induction time;moreover, the aggregation and induction time should match each other. The narrowest particle size distribution can be achieved when the aggregation and induction time are equal. Furthermore, different copolymers can significantly influence the size distribution. Correctly selecting copolymers can create functional surfaces to provide steric stabilisation and promote nucleation.
2.4 Kinetically controlled freezing process
Zhu[12]proved that FNP is more likely a kinetic process limited by the time as compared with a thermodynamic process. The NPs in the FNP show no micellar structure but present a non-equilibrium one. Moreover, an evidence can support the non-equilibrium structure, which includes a non-uniform particle size distribution and a size larger than the average equilibrium micellar size.
The self-assembly process mentioned above is controlled by thermodynamic force, and the NPs are kinetically frozen. Zhu[12]stated that the thermodynamic effect could influence the particle formation. Based on the experimental outcome, β-carotene was protected in the PEG corona, and different block copolymers showed different stability against aggregation.
3 Mixer and Mixing Process
3.1 Con fined impinging jets (CIJ)
The first FNP mixer was designed by Prud’homme[13]and was named CIJ. The design aims to produce NPs with a rapid mixing time and narrow size distribution. Numerous investigations on mixing devices have been characterised and proposed. In these methods, CIJ uses two high velocity fluid streams to turbulently mix with each other in the chamber and rapidly reduce the aggregation to reach the precipitation process. The chamber size and the Reynold’s number are important in the mixing process.
The CIJ mixer is faster during mixing than during precipitation[14]. The key to fast mixing includes the high turbulent energy dissipation and a high intensity twofluid stream mixing. High energy dissipation occurs in the chamber because the small size of the chamber can convert the kinetic energy of the jet fluid into a turbulentlike motion. Demyanovich and Bourne[15]provided the relationship between the energy dissipation rate and the particle length scale.
3.2 Multi-inlet vortex mixer (MIVM)
Studies have been done on CIJ, and a new design of MIVM was proposed by Ying Liu and Prud’homme[16].
Further understanding the concept of macro- , mesoand mirco-mixing is also necessary for application in the supersaturation process. These concepts are divided into three length scales, viz.: the vessel, the turbulent eddies and the molecules. FNP belongs to the micromixing process. Micromixing in CIJ can occur in milliseconds,but is limited by the two inlet streams. The equal momenta of the solvent and the antisolvent are necessary,and the final concentration is the average of the two.
MIVM uses four inlet streams to overcome these limitations. The four inlet streams can exhibit different velocities, and each stream individually contributes to the mixing. Computational fluid dynamics simulation was conducted by Liu Ying[16]. MIVM exhibits a better mixing than CIJ at the same flow rate and it can provide a wide distribution of the stream concentration.
3.3 CIJs with dilution (CIJ-D) mixer
Furthermore, Zhu[17]modified CIJs to produce stable and small NPs. Two stream inlets have been preserved and the hand operation has been enabled. The syringe pump has been eliminated and an antisolvent dilution step has been added. Moreover, with dilution, a high level of supersaturation has become achievable, and NPs are not likely to aggregate. The particle size becomes relatively small and the kinetically stabled NPs are formed.Moreover, the CIJ-D mixer exhibits several advantages,including easy usage, low cost and effectiveness for treating a small quantity of materials. Furthermore,this mixer is an alternative among the three devices,especially for laboratory use and testing. Figure 3 shows the dimensions of the CIJ-D mixer chamber.
4 Solvent Elimination
4.1 Flash evaporation
FNP is based on two streams, including the hydrophobic drug dissolved in the organic solvent and the antisolvent,such as water. The solvent should be removed, and acceptable NP suspensions or NPs should remain for other research or biological applications. Traditional drying methods are not suitable because aggregation occurs during drying and makes the restoration impossible.Varun Kumar[18]used flash evaporation to remove the solvent and obtained a low nanosuspension concentration that is below the Food and Drug Administration (FDA)limit.
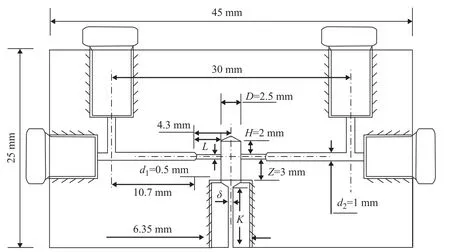
Figure 3 Dimensions of the CIJ-D mixer made of high-density polyethylene[17]
A low pressure is applied in flash evaporation, which is performed under saturated vapour pressure so that the solvent can be removed. The multistep flash evaporation can improve the amount of evaporation. The two stages were performed by Kumar[18], and the removal rate of solvent reached 96%, as calculated by ASPEN software.The NP dispersion showed a stable size distribution against the Ostwald ripening, which can be more useful than the traditional dialysis.
4.2 Spray drying
To date, spray drying is widely used in pharmaceutical industries to convert liquid solutions into powder. Spray drying is formed directly by the atomised droplets. During this process, the medium contained in the solvent, which is in contact with the hot gas, is evaporated to produce dry powder. This technique shows the advantage in FNP for its remarkable reproducibility, consistency and beneficial drug release. Katherine Margulis[19]prepared the curcumin NPs via FNP and used spray drying to obtain the powdered form. Narrow particle size distribution was observed and the particles were stable after solvent removal. However, the spray drying uses high temperature in both the inlet air and vacuum chamber,and this would limit the NP choice. High temperature can cause aggregation that reduces the stability of NPs.Therefore, the strictly controlled conditions and a narrow choice of NP suspensions can be used with this method.
4.3 Freeze drying and spray freeze drying
Freeze drying and spray freeze drying[20-22]are two available methods for removing solvents without extensive aggregation to obtain the dry powder with a relative stability. Freeze drying is a dehydration process that is usually adopted by pharmaceutical and biotechnology companies. During the freeze drying process, water is frozen and NPs are removed by reducing the surrounding pressure to sublimate directly from solid to gas. However, without proper stabilisers, NPs tend to fuse under stresses as the phase separation progresses.
With the rapid developments in biotechnology, the spray freeze drying has become important in the pharmaceutical industry. The NP in the form of aerosols can sublime from the lower layer of the cryogenic liquid and then the cold vapour phase would enter the gas phase after being discharged through a nozzle. Small droplets from the vapour phase begin to freeze when their contact with the cryogenic liquid layer would completely lead to the formation of frozen droplets. At low temperature and pressure, solvents are removed by sublimation, leaving dried particles. Spray freeze drying is a combination and complementary method of spray drying and freeze drying.
5 Particle Stability
5.1 Ostwald ripening
The Ostwald ripening, or particle coarsening, is a significant factor for particle formation. This ripening refers to the process in which small particles tend to shrink and also precipitate on the surface of large particles. Moreover, large particles tend to grow, thereby increasing the size distribution. The process is controlled by thermodynamic forces, which rely on the theory that small particles possess a higher surface tension than large particles. The Ostwald ripening of particles can be effectively controlled because the particle size distribution is considerably well-prepared through FNP.
5.2 Aggregation of NPs
Irreversible NP aggregation is caused by the van der Waals interactions between particles. NP aggregation occurs immediately after particle formation when no stabilizer is present; this stabilizer, which can be a lowmolecular-weight surfactant or an amphiphilic polymer(e. g. PEG-b-PLGA block copolymer), is absorbed at the NP surface. Nevertheless, the stabilizer is usually not required when the NP contains surface charges or hydrophilic moieties[23]. Moreover, the particles collide with one another and the van der Waals force can cause the particles to come in contact to form a flocculation, if the repulsive force between the particles is small. Zhu, et al.[24]found that β-carotene nanoparticles stabilized by the inherent surface charge could flocculate in a 1% saline solution.
5.3 Sedimentation or floating of particles
Particle movement depends on the density of the particles and the base liquid. When the density of the particle is greater than that of the base liquid, the particles will precipitate; otherwise, the particles will float.Furthermore, the particle movement depends on the particle size. Large particles can easily precipitate. The particles prepared through FNP are remarkably small, and the Brownian movement is highly active. Therefore, the sedimentation and floating of particles can be effectively controlled.
6 Conclusions
In this review, the particle size and its distribution influenced by nucleation and supersaturation kinetics are discussed. The stability of NPs is also investigated.Given the control over the particle size and flexibility in incorporating multiple activities, the particles prepared through rapid precipitation are exploited for several applications. As the understanding of the mechanism of this process broadens, the scope offNP application will continue to increase.
Acknowledgements: The work was financially supported by the National Natural Science Foundation of China (No. 21544005),the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 15KJB430034), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Reference
[1] Taylor J W, Winnik M A. Functional latex and thermoset latex films[J]. JCT Res., 2004, 1(3): 163-190
[2] Schuman T, Karlsson A, Larsson J, et al. Characteristics of pigment- filled polymer coatings on paperboard[J]. Prog Org Coat, 2005, 54(4): 360-371
[3] Gharpure K M, Wu S Y, Li C, et al. Nanotechnology:Future of Oncotherapy[J]. Clin Cancer Res, 2015, 21(14):3121-3130
[4] Dickherber A, Morris S A, Grodzinski P. NCI investment in nanotechnology: achievements and challenges for the future[J]. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology, 2015, 7(3): 251-265
[5] Yang J, Han S, Zheng H, et al. Preparation and application of micro/nanoparticles based on natural polysaccharides[J].Carbohydr Polym, 2015, 123: 53-66
[6] Klapper M, Clark C G, Muellen K, Jr. Application-directed syntheses of surface-functionalized organic and inorganic nanoparticles[J]. Polym Int, 2008, 57(2): 181-202
[7] Brick M C, Palmer H J, Whitesides T H. Formation of colloidal dispersions of organic materials in aqueous media by solvent shifting[J]. Langmuir, 2003, 19(16): 6367-6380
[8] D'Addio S M, Prud'homme R K. Controlling drug nanoparticle formation by rapid precipitation[J]. Adv Drug Delivery Rev, 2011, 63(6): 417-426
[9] Sugimoto T, Shiba F, Sekiguchi T, et al. Spontaneous nucleation of monodisperse silver halide particles from homogeneous gelatin solution I: Silver chloride[J].Colloids and Surfaces A-Physicochemical and Engineering Aspects, 2000, 164(2/3): 183-203
[10] Horn D, Rieger J. Organic nanoparticles in the aqueous phase - Theory, experiment, and use[J]. Angewandte Chemie - International Edition, 2001, 40(23): 4331-4361
[11] Johnson B K, Prud’Homme R K. Mechanism for rapid self-assembly of block copolymer nanoparticles[J]. Phys Rev Lett, 2003, 91(11): 118302
[12] Zhu Z. Effects of amphiphilic diblock copolymer on drug nanoparticle formation and stability[J]. Biomaterials, 2013,34(38): 10238-10248
[13] Johnson B K, Prud'homme R K. Chemical processing and micromixing in con fined impinging jets[J]. AIChE J, 2003,49(9): 2264-2282
[14] Mahajan A J, Kirwan D J. Micromixing effects in a twoimpinging-jets precipitator[J]. AIChE J, 1996, 42(7):1801-1814
[15] Demyanovich R J, Bourne J R. Rapid micromixing by the impingement of thin liquid sheets. 1. A photographic study of the flow pattern[J]. Ind Eng Chem Res, 1989, 28(6):825-830
[16] Liu Y, Cheng C, Prud'homme R K, et al. Mixing in a multiinlet vortex mixer (MIVM) for flash nano-precipitation[J].Chem Eng Sci, 2008, 63(11): 2829-2842
[17] Han J, Zhu Z, Qian H, et al. A simple confined impingement jets mixer for flash nanoprecipitation[J]. J Pharm Sci, 2012, 101(10): 4018-4023
[18] Kumar V, Prud'homme R K. Nanoparticle stability:Processing pathways for solvent removal[J]. Chem Eng Sci, 2009, 64(6): 1358-1361
[19] Margulis K, Magdassi S, Lee H S, et al. Formation of curcumin nanoparticles by flash nanoprecipitation from emulsions[J]. J Colloid Interface Sci, 2014, 434: 65-70
[20] Cavalli R, Caputo O, Carlotti M E, et al. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles[J]. International Journal of Pharmaceutics(Amsterdam), 1997, 148(1): 47-54
[21] D'Addio S M, Chan J G Y, Kwok P C L, et al. Aerosol delivery of nanoparticles in uniform mannitol carriers formulated by ultrasonic spray freeze drying[J]. Pharm Res, 2013, 30(11): 2891-2901
[22] Wang Z L, Finlay W H, Peppler M S, et al. Powder formation by atmospheric spray-freeze-drying[J]. Powder Technol, 2006, 170(1): 45-52
[23] Lepeltier E, Bourgaux C, Couvreur P. Nanoprecipitation and the "Ouzo effect": Application to drug delivery devices[J]. Adv Drug Delivery Rev, 2014, 71: 86-97
[24] Zhu Z, Margulis-Goshen K, Magdassi S, et al.Polyelectrolyte stabilized drug nanoparticles via flash nanoprecipitation: A model study with beta-carotene[J]. J.Pharm Sci, 2010, 99(10): 4295-4306
Successful Bench Test of Novel Catalyst for Shape-Selective Disproportionation of Toluene at Liaoyang Petrochemical Company
The PetroChina’s Liaoyang Petrochemical Company(LPC) has successfully carried out the bench-scale tests of shape-selective catalyst for toluene disproportionation.The catalyst evaluation tests have revealed that this catalyst can achieve a toluene conversion of more than 30%, with the xylene selectivity exceeding 90%to be on a par with the similar catalysts used in China to fill a domestic gap in the field of shape-selective disproportionation of toluene. This study adopts the catalyst modification technique by means of the liquid phase deposition of silicon to precisely adorn the catalyst pores and control the surface acidity in an attempt to maximize the toluene conversion and the para-xylene selectivity.
Currently LPC is working on the optimization of process regime for manufacturing the catalyst to provide basic data necessary for the forthcoming model scaleup tests and commercial scaleup tests.
date: 2017-05-02; Accepted date: 2017-07-04.
Xu Xu, Telephone: +86-15715172289,E-mail: 523457677@qq.com.
杂志排行
中国炼油与石油化工的其它文章
- Thermal Decomposition Behavior of Terephthalate in Inert Gas
- Molecular Simulation of Competitive Adsorption on Fe(110) Between Gasoline Detergent and Deposit: I. Physical Adsorption
- Characterization and Apparent Kinetics of Polymerization of 1-Decene Catalyzed by Boron Trifluoride/Alcohol System
- Preparation of Cu-, Zn-, Co-Zeolites and Application for Adsorptive Desulfurization of Saudi Arabian Medium Crude
- Antimicrobial Degradation Performance of Novel Polyacrylamide Derivatives by Microbial Consortia for Enhanced Oil Recovery
- Hydrophobic and Magnetic Reduced Graphene Oxide Nanocomposite for Emulsified Oil Removal
